Women’s Empowerment and Livestock Vaccination: Evidence from Peste des Petits Ruminants Vaccination Interventions in Northern Ghana
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data and Variable Description
2.4. Analytical Method
2.4.1. WELI Construction
2.4.2. Conceptual and Empirical Model
3. Results
3.1. Descriptive Analysis—Sociodemographic Characteristics of the Study Participants
3.2. Analytical Model Reliability and Validity
3.3. Model Results: The Association between PPR Vaccine Facets and Empowerment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Agency Classification | Indicators (WELI Subdimension) | Definition |
|---|---|---|
| Intrinsic Agency (Power within) | Autonomy in income | More motivated by own values than by coercion or fear of others’ disapproval: Relative Autonomy Index score >= 1 RAI score is calculated by summing responses to the three vignettes about a person’s motivation for how they use income generated from agricultural and non-agricultural activities (yes = 1; no = 0), using the following weighting scheme: 0 for vignette 1 (no alternative), 2 for vignette 2 (external motivation), 1 for vignette 3 (introjected motivation), and +3 for vignette 4 (autonomous motivation) |
| Self-efficacy | New General Self-Efficacy Scale: ‘‘Agree” or greater on average with eight self-efficacy questions | |
| Attitudes about intimate partner violence against women | Believes husband is NOT justified in hitting or beating his wife in all 5 scenarios: If: (1) She goes out without telling him (2) She neglects the children (3) She argues with him (4) She refuses to have sex with him (5) She burns the food | |
| Respect among household members | Meets ALL of the following conditions related to their spouse, the other respondent, or another household member: (1) Respondent respects relation (most of the time) (2) Relation respects respondent (most of the time) (3) Respondent trusts relation (most of the time) (4) Respondent is comfortable disagreeing with relation (most of the time) | |
| Instrumental agency (Power to) | Input in productive decisions—general: | Meets at least ONE of the following conditions for ALL of the agricultural activities they participate in: (1) Makes related decision solely (2) Makes the decision jointly and has at least some input into the decisions (3) Feels could make decision if wanted to (to at least a MEDIUM extent) |
| Input in productive decisions–livestock: | Meets at least ONE of the following conditions for ALL of the livestock activities they participate in: (1) Makes related decision solely (2) Makes the decision jointly and has at least some input into the decisions (3) Feels could make decision if wanted to (to at least a MEDIUM extent) | |
| Ownership of land and other assets | Owns, either solely or jointly, at least ONE of the following: (1) At least THREE moveable assets (equipment or consumer durables) (2) Land | |
| Access to and decisions regarding financial services | Meets at least ONE of the following conditions: (1) Belongs to a household that used a source of credit in the past year AND participated in at least ONE sole or joint decision about it (2) Belongs to a household that did not use credit in the past year but could have if wanted to from at least ONE source (3) Has access, solely or jointly, to a financial account | |
| Control over use of income | Has input in decisions related to how to use BOTH income and output from ALL of the agricultural activities they participate in AND has input in decisions related to income from ALL non-agricultural activities they participate in, unless no decision was made | |
| Work balance | Works less than 10.5 h per day: Workload = time spent in primary activity + (1/2) time spent in childcare as a secondary activity | |
| Ability to visit important locations | Meets at least ONE of the following conditions: (1) Visits at least TWO locations at least ONCE PER WEEK of (city, market, family/relative); or (2) Visits at least ONE location at least ONCE PER MONTH of (health facility, public meeting) | |
| Collective agency (Power with) | Group membership | Active member of at least ONE group |
| Membership in influential groups | Active member of at least ONE group that can influence the community to at least a MEDIUM extent |
References
- Baltenweck, I.; Enahoro, D.; Frija, A.; Tarawali, S. Why Is Production of Animal Source Foods Important for Economic Development in Africa and Asia? Anim. Front. 2020, 10, 22–29. [Google Scholar] [CrossRef]
- Gitungwa, H.; Gustafson, C.R.; Jimenez, E.Y.; Peterson, E.W.; Mwanzalila, M.; Makweta, A.; Komba, E.; Kazwala, R.R.; Mazet, J.A.K.; VanWormer, E. Female and Male-Controlled Livestock Holdings Impact Pastoralist Food Security and Women’s Dietary Diversity. One Health Outlook 2021, 3, 3. [Google Scholar] [CrossRef]
- Essilfie, G.; Sebu, J.; Annim, S.K. Women’s Empowerment and Child Health Outcomes in Ghana. Afr. Dev. Rev. 2020, 32, 200–215. [Google Scholar] [CrossRef]
- Salazar, L.; Fahsbender, J.; Kim, N. Livestock Transfers, Food Security and Women’s Empowerment: Evidence from a Randomized Phased-in Program in Nicaragua; IDB Working Paper Series IDB-WP-00944; Environment, Rural Development and Risk Management Division Inter-American Development Bank: Washington, DC, USA, 2018. [Google Scholar]
- Bayeh, E. The Role of Empowering Women and Achieving Gender Equality to the Sustainable Development of Ethiopia. Pac. Sci. Rev. B Humanit. Soc. Sci. 2016, 2, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.L.; Reynolds, T.W.; Biscaye, P.; Patwardhan, V.; Schmidt, C. Economic Benefits of Empowering Women in Agriculture: Assumptions and Evidence. J. Dev. Stud. 2021, 57, 193–208. [Google Scholar] [CrossRef]
- Roth, J.A. Veterinary Vaccines and Their Importance to Animal Health and Public Health. Procedia Vaccinol. 2011, 5, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumba, H.; Teufel, N.; Baltenweck, I.; de Haan, N.; Kiara, H.; Owuor, G. Use of the Infection and Treatment Method in the Control of East Coast Fever in Kenya: Does Gender Matter for Adoption and Impact? Gend. Technol. Dev. 2020, 24, 297–313. [Google Scholar] [CrossRef]
- Seymour, G.; Doss, C.; Marenya, P.; Meinzen-Dick, R.; Passaewlli, S. Women’s Empowerment and the Adoption of Improved Maize Varieties: Evidence from Ethiopia, Kenya, and Tanzania. In Proceedings of the 2016 Agricultural & Applied Economics Association Annual Meeting, Boston, MA, USA, 31 July 31–2 August 2016. [Google Scholar]
- Mutua, E.; de Haan, N.; Tumusiime, D.; Jost, C.; Bett, B. A Qualitative Study on Gendered Barriers to Livestock Vaccine Uptake in Kenya and Uganda and Their Implications on Rift Valley Fever Control. Vaccines 2019, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- McKune, S.L.; Serra, R.; Touré, A. Gender and Intersectional Analysis of Livestock Vaccine Value Chains in Kaffrine, Senega. PLoS ONE 2021, 16, e0252045. [Google Scholar] [CrossRef] [PubMed]
- Marsh, T.L.; Yoder, J.; Deboch, T.; McElwain, T.F.; Palmer, G.H. Livestock Vaccinations Translate into Increased Human Capital and School Attendance by Girls. Sci. Adv. 2016, 2, e1601410. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Yoder, J.; de Glanville, W.; Davis, A.; Kibona, T.J.; Cleaveland, S. Relationships between Vaccinations, Herd Introductions, and Livestock Losses in Northern Tanzania. Agric. Resour. Econ. Rev. 2021, 1–19. [Google Scholar] [CrossRef]
- Oladele, I.O.; Monkhei, M. Gender Ownership Patterns of Livestock in Botswana. Livest. Res. Rural Dev. 2008, 20, 156. [Google Scholar]
- Oluka, J.; Owoyesigire, B.; Esenu, B.; Ssewannyana, E. Small Stock and Women in Livestock Production in the Teso Farming System Region of Uganda; National Agricultural Research Organisation (NARO): Entebbe, Uganda, 2002.
- Njuki, J.M.; Sanginga, P.C. (Eds.) Women, Livestock Ownership and Markets: Bridging the Gender Gap in Eastern and Southern Africa; Routledge: London, UK; New York, NY, USA, 2013; Available online: http://hdl.handle.net/10625/52269 (accessed on 2 December 2021).
- Monau, P.; Raphaka, K.; Zvinorova-Chimboza, P.; Gondwe, T. Sustainable Utilization of Indigenous Goats in Southern Africa. Diversity 2020, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Waithanji, E.; Njuki, J.; Mburu, S.; Kariuki, J.; Njeru, F. A Gendered Analysis of Goat Ownership and Marketing in Meru, Kenya. Dev. Pract. 2015, 25, 188–203. [Google Scholar] [CrossRef]
- Duku, S.; van der Zijpp, A.J.; Udo, H.M.J. Household Vulnerability and Small Ruminant Benefits in the Transitional Zone of Ghana. J. Agric. Ext. Rural Dev. 2012, 4, 98–106. [Google Scholar] [CrossRef]
- Komarek, A.M.; De Pinto, A.; Smith, V.H. A Review of Types of Risks in Agriculture: What We Know and What We Need to Know. Agric. Syst. 2020, 178, 102738. [Google Scholar] [CrossRef]
- Adams, F.; Ohene-Yankyera, K.; Aidoo, R.; Wongnaa, C.A. Economic Benefits of Livestock Management in Ghana. Agric. Food Econ. 2021, 9, 17. [Google Scholar] [CrossRef]
- Balamurugan, V.; Hemadri, D.; Gajendragad, M.R.; Singh, R.K.; Rahman, H. Diagnosis and Control of Peste Des Petits Ruminants: A Comprehensive Review. Virusdisease 2014, 25, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Njeumi, F.; Bailey, D.; Soula, J.J.; Diop, B.; Tekola, B.G. Eradicating the Scourge of Peste des Petits Ruminants from the World. Viruses 2020, 12, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folitse, R.D.; Amemor, E.; Nyarku Rejoice, E.; Emikpe, B.O.; Tasiame, W. Pattern of Peste des Petits Ruminants (PPR) Distribution in Ghana (2005–2013). Bulg. J. Vet. Med. 2017, 20, 51–57. [Google Scholar] [CrossRef]
- Utaaker, K.S.; Chaudhary, S.; Kifleyohannes, T.; Robertson, L.J. Global Goat! Is the Expanding Goat Population an Important Reservoir of Cryptosporidium? Front. Vet. Sci. 2021, 8, 648500. [Google Scholar] [CrossRef]
- Enahoro, D.; Galiè, A.; Abukari, Y.; Kelly, T.R.; Massawe, F.A.; Mapunda, F.; Jumba, H.; Weber, C.; Dione, M.; Kayang, B.; et al. Strategies to Upgrade Animal Health Delivery in Village Poultry Systems: Perspectives of Stakeholders from Northern Ghana and Central Zones in Tanzania. Front. Vet. Sci. 2021, 8, 609. [Google Scholar] [CrossRef]
- Meinzen-Dick, R.; Quisumbing, A.; Behrman, J.A.; Biermayr_Jenzano, P.; Wilde, V.; Noordeloos, M.; Ragasa, C.; Bientema, N. Engendering Agricultural Research; Working Paper; IFPRI: Washington, DC, USA, 2010. [Google Scholar]
- World Bank; IFPRI. Gender and Governance in Rural Services: Insights from India, Ghana and Ethiopia; The International Bank for Reconstruction and Development: Washington, DC, USA; The World Bank and International Food Policy Research Institute: Washington, DC, USA, 2010. [Google Scholar]
- Ankrah, D.A.; Freeman, C.Y.; Afful, A. Gendered Access to Productive Resources—Evidence from Small Holder Farmers in Awutu Senya West District of Ghana. Sci. Afr. 2020, 10, e00604. [Google Scholar] [CrossRef]
- Alhassan, E.; Odame, F.S. Gender Inequality in Basic Education in the Northern Region of Ghana: Household and Contextual Factors in Perspectives. Ghana J. Dev. Stud. 2015, 12, 125–141. [Google Scholar] [CrossRef] [Green Version]
- OIE; FAO. Global Strategy for the Control and Eradication of PPR; OIE (World Organisation for Animal Health): Paris, France; FAO (The Food and Agriculture Organization of the United Nations): Rome, Italy, 2015. [Google Scholar]
- Acosta, D.; Hendrickx, S.; McKune, S.L. The Livestock Vaccine Supply Chain: Why It Matters and How It Can Help Eradicate Peste Des Petits Ruminants, Based on Findings in Karamoja, Uganda. Vaccine 2019, 37, 6285–6290. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Galiè, A.; Baltenweck, I. Gender-Responsive Animal Health Research: A Framework and Checklists for ILRI Researchers; ILRI Manual ILRI Manual 48; ILRI (International Livestock Research Institute): Nairobi, Kenya, 2021. [Google Scholar]
- Zhao, H.; Njeumi, F.; Parida, S.; Benfield, C.T.O. Progress towards Eradication of Peste des Petits Ruminants through Vaccination. Viruses 2021, 13, 59. [Google Scholar] [CrossRef]
- Donadeu, M.; Nwankpa, N.; Abela-Ridder, B.; Dungu, B. Strategies to Increase Adoption of Animal Vaccines by Smallholder Farmers with Focus on Neglected Diseases and Marginalized Populations. PLoS Negl. Trop. Dis. 2019, 13, 1–17. [Google Scholar] [CrossRef]
- Omondi, I.; Baltenweck, I.; Kinuthia, E.; Kirui, L.; Njoroge-Wamwere, G.; Bett, B.; Munene, A.; Onle, S.; Dida, D.; Kiara, H. Mobile Veterinary Clinics in the Drylands of Kenya: Securing Pastoralists’ Livelihoods by Bringing Services Close. Dev. Pract. 2021, 31, 571–579. [Google Scholar] [CrossRef]
- ILRI. The Women Empowerment in Livestock Index: Vital Progress Towards Gender Equality; ILRI Solution Brief 3; ILRI (International Livestock Research Institute): Nairobi, Kenya, 2019. [Google Scholar]
- Colverson, K.E.; Harris, L.C.; Galie, A.; Moore, E.V.; Munoz, O.; McKune, S.L.; Singh, N.; Mo, R. Evolution of a Gender Tool: WEAI, WELI and Livestock Research. Glob. Food Secur. 2020, 26, 100375. [Google Scholar] [CrossRef]
- Malapit, H.; Quisumbing, A.; Meinzen-Dick, R.; Seymour, G.; Martinez, E.M.; Heckert, J.; Rubin, D.; Vaz, A.; Yount, K.M. Development of the Project-Level Women’s Empowerment in Agriculture Index (pro-WEAI). World Dev. 2019, 122, 675–692. [Google Scholar] [CrossRef]
- CARE International. Available online: https://www.care-international.org/ (accessed on 30 March 2021).
- Cowtribe. Available online: https://www.cowtribe.com/story/ (accessed on 30 March 2021).
- Quisumbing, A.; Heckert, J.; Faas, S.; Ramani, G.; Raghunathan, K.; Malapit, H.; Malapit, H.; Heckert, J.; Eissler, S.; Faas, S.; et al. The pro-WEAI for Market Inclusion Study Team. Women’s Empowerment and Gender Equality in Agricultural Value Chains: Evidence from Four Countries in Asia and Africa. Food Secur. 2021, 13, 1101–1124. [Google Scholar] [CrossRef] [PubMed]
- Awedoba, A.K. The Peoples of Northern Ghana; Ghana National Commission on Culture: Accra, Ghana, 2006.
- Dugje, I.Y.; Teli, I.A.; Larbi, A.; Gyamfi, I.; Buah, S.S.J.; Kanton, R.A.L.; Kombiok, J.M.; Kamara, A.Y.; Hoeschle-Zeledon, I. Report of Community Analyses for Sustainable Intensification of Cereal-Based Farming System in the Sudano-Sahelian Zone in Ghana; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2012. [Google Scholar]
- Amankwah, K. Enhancing Food Security in Northern Ghana Through Smallholder Small Ruminant Production and Marketing. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2013. [Google Scholar]
- Chamberlin, J. Defining Smallholder Agriculture in Ghana: Who Are Smallholders, What Do They Do and How Are They Linked with Markets? Background Paper GSSP 0006; Ghana Strategy Support Program (GSSP): Accra, Ghana; International Food Policy Research Institute (IFPRI): Accra, Ghana, 2003; p. 44. [Google Scholar]
- Galiè, A.; Teufel, N.; Korir, L.; Baltenweck, I.; Webb Girard, A.; Dominguez-Salas, P.; Yount, K.M. The Women’s Empowerment in Livestock Index. Soc. Indic. Res. 2019, 142, 799–825. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.; Ren, Y.; Rong, K.; Zhou, J. Women’s Empowerment in Agriculture and Household Food Insecurity: Evidence from Azad Jammu & Kashmir (AJK), Pakistan. Land Use Policy 2021, 102, 105249. [Google Scholar] [CrossRef]
- Wan Afthanorhan, W.M.A. Hierarchical Component Using Reflective-Formative Measurement Model In Partial Least Square Structural Equation Modeling (Pls-Sem). Int. J. Math. Stat. Invent. 2014, 2, 33–49. [Google Scholar]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Danks, N.P.; Ray, S. An Introduction to Structural Equation Modeling. In Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R: A Workbook; Hair, J.F., Hult, G.T.M., Ringle, C.M., Sarstedt, M., Danks, N.P., Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Gerbing, D.W.; Anderson, J.C. An Updated Paradigm for Scale Development Incorporating Unidimensionality and Its Assessment. J. Mark. Res. 1988, 25, 186–192. [Google Scholar] [CrossRef]
- Elangovan, N.; Rajendran, R. Structural Equation Modeling-A Second-Generation Multivariate Analysis. In Emerging Management Paradigm in Indian Business; Department of Management, Sri Ramakrishna Institute of Technology: Coimbatore, India, 2015. [Google Scholar] [CrossRef]
- Astrachan, C.B.; Patel, V.K.; Wanzenried, G. A Comparative Study of CB-SEM and PLS-SEM for Theory Development in Family Firm Research. Innov. Establ. Res. Methods Fam. Bus. 2014, 5, 116–128. [Google Scholar] [CrossRef]
- Shook, C.L.; Ketchen, D.J.; Hult, G.T.M.; Kacmar, M. An Assessment of the Use of Structural Equation Modeling in Strategic Management Research. Strateg. Manag. J. 2004, 25, 397–404. [Google Scholar] [CrossRef]
- Haenlein, M.; Kaplan, A.M. A Beginner’s Guide to Partial Least Squares Analysis. Underst. Stat. 2004, 3, 283–297. [Google Scholar] [CrossRef]
- Diamantopoulos, A. Modelling with LISREL: A Guide for the Uninitiated. J. Mark. Manag. 1994, 10, 105–136. [Google Scholar] [CrossRef]
- Beran, T.N.; Violato, C. Structural Equation Modeling in Medical Research: A Primer. BMC Res. Notes 2010, 3, 267. [Google Scholar] [CrossRef] [Green Version]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Thiele, K.O. Mirror, Mirror on the Wall: A Comparative Evaluation of Composite-Based Structural Equation Modeling Methods. J. Acad. Mark. Sci. 2017, 45, 616–632. [Google Scholar] [CrossRef]
- Henseler, J. Bridging Design and Behavioral Research with Variance-Based Structural Equation Modeling. J. Advert. 2017, 46, 178–192. [Google Scholar] [CrossRef]
- Dijkstra, T.K.; Henseler, J. Consistent and Asymptotically Normal PLS Estimators for Linear Structural Equations. Comput. Stat. Data Anal. 2015, 81, 10–23. [Google Scholar] [CrossRef] [Green Version]
- Sarstedt, M.; Ringle, C.M.; Smith, D.; Reams, R.; Hair, J.F. Partial Least Squares Structural Equation Modeling (PLS-SEM): A Useful Tool for Family Business Researchers. Innov. Establ. Res. Methods Fam. Bus. 2014, 5, 105–115. [Google Scholar] [CrossRef]
- Henseler, J.; Ringle, C.M.; Sinkovics, R.R. The Use of Partial Least Squares Path Modeling in International Marketing. In New Challenges to International Marketing; Sinkovics, R.R., Ghauri, P.N., Eds.; Advances in International Marketing; Emerald Group Publishing Limited: Bingley, UK, 2009; Volume 20, pp. 277–319. [Google Scholar] [CrossRef] [Green Version]
- Sarstedt, M. A Review of Recent Approaches for Capturing Heterogeneity in Partial Least Squares Path Modelling. J. Model. Manag. 2008, 3, 140–161. [Google Scholar] [CrossRef]
- Vinzi, V.E.; Chin, W.W.; Henseler, J.; Wang, H. (Eds.) Handbook of Partial Least Squares: Concepts, Methods and Applications; Springer: Berlin, Germany, 2010. [Google Scholar]
- Russo, D.; Stol, K.-J. PLS-SEM for Software Engineering Research: An Introduction and Survey. ACM Comput. Surv. 2021, 54, 1–38. [Google Scholar] [CrossRef]
- StatSoft. STATISTICA Advanced; TIBCO Data Science: Palo Alto, CA, USA, 2013. [Google Scholar]
- Okello, D.; Owuor, G.; Larochelle, C.; Gathungu, E.; Mshenga, P. Gender Effect of Entrepreneurial Orientation on Dairy Farming Career Resilience in Kenya. Ournal Agric. Food Res. 2020, 6, 100213. [Google Scholar] [CrossRef]
- Becker, J.-M.; Klein, K.; Wetzels, M. Hierarchical Latent Variable Models in PLS-SEM: Guidelines for Using Reflective-Formative Type Models. Long Range Plan. 2012, 45, 359–394. [Google Scholar] [CrossRef]
- Lohmoeller, J.-B. The PLS Program Systen: Latent Variable Path Modeling with Partial Least Squares. Multivar. Behav. Res. 1988, 23, 125–127. [Google Scholar] [CrossRef]
- Wold, H. Soft Modeling: The Basic Design and Some Extensions. In Systems under Indirect Observation: Causality, Structure, Prediction; Joereskog, K.G., Wold, H., Eds.; North-Holland: Amsterdam, The Netherlands, 1982; Volume 2, pp. 1–54. [Google Scholar]
- Wetzels, M.; Odekerken-Schroeder, G.; van Oppen, C. Using PLS Path Modeling for Assessing Hierarchical Construct Models: Guidelines and Empirical Illustration. MIS Q. 2009, 33, 177–195. [Google Scholar] [CrossRef]
- Ringle, C.M.; Sarstedt, M.; Straub, D.W. Editor’s Comments: A Critical Look at the Use of PLS-SEM in MIS Quarterly. MIS Q. 2012, 36, iii–xiv. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Henseler, J. Modeling Reflective Higher-Order Constructs Using Three Approaches with PLS Path Modeling: A Monte Carlo Comparison. In Reputation, Responsibility, Relevance; Thyne, M., Deans, K.R., Eds.; Australian and New Zealand Marketing Academy Conference (ANZMAC): Dunedin, Newzealand, 2007; pp. 791–800. [Google Scholar]
- Fan, Y.; Chen, J.; Shirkey, G.; John, R.; Wu, S.R.; Park, H.; Shao, C. Applications of Structural Equation Modeling (SEM) in Ecological Studies: An Updated Review. Ecol. Process. 2016, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Ringle, C.M.; Wende, S.; Becker, J.-M. SmartPLS 3; Software; SmartPLS GmbH: Boenningstedt, Germany, 2015. [Google Scholar]
- Wong, K.K. Partial Least Squares Structural Equation Modeling (PLS-SEM) Techniques Using SmartPLS. Mark. Bull. 2013, 25, 1–32. [Google Scholar]
- Kock, N. Harman’s Single Factor Test in PLS-SEM: Checking for Common Method Bias. Data Anal. Perspect. J. 2020, 2, 1–6. [Google Scholar]
- Podsakoff, P.M.; MacKenzie, S.B.; Lee, J.; Podsakoff, N.P. Common Method Biases in Behavioral Research: A Critical Review of the Literature and Recommended Remedies. J. Appl. Psychol. 2003, 88, 879–903. [Google Scholar] [CrossRef] [PubMed]
- Tehseen, S.; Ramayah, T.; Sajilan, S. Testing and Controlling for Common Method Variance: A Review of Available Methods. J. Manag. Sci. 2017, 4, 146–175. [Google Scholar] [CrossRef]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Danks, N.P.; Ray, S. Evaluation of Reflective Measurement Models. In Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R: A Workbook; Hair, J.F., Hult, G.T.M., Ringle, C.M., Sarstedt, M., Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 75–90. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making Sense of Cronbach’s Alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Chen, J.; Singpurwalla, N.D. The Notion of “Composite Reliability” and Its Hierarchical Bayes Estimation. J. Am. Stat. Assoc. 1996, 91, 1474–1484. [Google Scholar] [CrossRef]
- Hair, J.F.; Sarstedt, M.; Hopkins, L.; Kuppelweiser, V.G. Partial Least Squares Structural Equilibrium Modeling (PLS-SEM) and Emerging Tool in Business Research. Eur. Bus. Rev. 2014, 26, 106–121. [Google Scholar] [CrossRef]
- Maltitz, L.V.; Bahta, Y.T. Empowerment of Smallholder Female Livestock Farmers and Its Potential Impacts to Their Resilience to Agricultural Drought. AIMS Agric. Food 2021, 6, 603–630. [Google Scholar] [CrossRef]
- Puskur, R.; Mudege, N.; Njuguna-Mungai, E.; Nchanji, E.B.; Vernooy, R.; Galiè, A.; Najjar, D. Moving Beyond Reaching Women in Seed Systems Development’. In Advancing Gender Equality through Agricultural and Environmental Research: Past, Present and Future; Pyburn, R., van Eerdewijk, A., Eds.; IFPRI: Washington, DC, USA, 2021. [Google Scholar]
- Friis-Hansen, E.; Duveskog, D. The Empowerment Route to Well-Being: An Analysis of Farmer Field Schools in East Africa. World Dev. 2012, 40, 414–427. [Google Scholar] [CrossRef]
- Seymour, G. Women’s Empowerment in Agriculture: Implications for Technical Efficiency in Rural Bangladesh. Agric. Econ. 2017, 48, 513–522. [Google Scholar] [CrossRef]
- Sharaunga, S.; Mudhara, M.; Bogale, A. Effects of ‘Women Empowerment’ on Household Food Security in Rural Kwa Zulu-Natal Province. Dev. Policy Rev. 2016, 34, 223–252. [Google Scholar] [CrossRef]
- Tsiboe, F.; Zereyesus, Y.A.; Popp, J.S.; Osei, E. The Effect of Women’s Empowerment in Agriculture on Household Nutrition and Food Poverty in Northern Ghana. Soc. Indic. Res. 2018, 138, 89–108. [Google Scholar] [CrossRef]
- Santoso, M.V.; Kerr, R.B.; Hoddinott, J.; Garigipati, J.; Olmos, S.; Young, S.L. Role of Women’s Empowerment in Child Nutrition Outcomes: A Systematic Review. Adv. Nutr. 2019, 10, 1138–1151. [Google Scholar] [CrossRef]
- Abreha, S.K.; Walelign, S.Z.; Zereyesus, Y.A. Associations between Women’s Empowerment and Children’s Health Status in Ethiopia. PLoS ONE 2020, 15, e0235825. [Google Scholar] [CrossRef]
- Jimah, K.; Fischer, G. The Role of Gender Norms in Northern Ghana’s Small Ruminant Value Chains and Implications for Transformative Interventions. 2021. Available online: https://cgspace.cgiar.org/handle/10568/117083 (accessed on 20 January 2022).
- Diiro, G.M.; Seymour, G.; Kassie, M.; Muricho, G.; Muriithi, B.W. Women’s Empowerment in Agriculture and Agricultural Productivity: Evidence from Rural Maize Farmer Households in Western Kenya. PLoS ONE 2018, 13, e0197995. [Google Scholar] [CrossRef] [Green Version]
- Nchanji, E.B.; Mutua, M.; Odhiambo, C.; Nchanji, Y.K.; Karanja, D. Deconstructing Leisure Time and Workload: Case of Women Bean Producers in Kenya. Agric. Food Secur. 2021, 10, 12. [Google Scholar] [CrossRef]
- Oladele, I.O.; Antwi, M.A.; Kolawole, A.E. Factors Affecting Livestock Farmers Perception of Risk of Disease in along Villages along South Africa and Namibia. J. Anim. Vet. Adv. 2013, 12, 173–176. [Google Scholar] [CrossRef]
- Vattaa, A.F.; Lindberg, A.L.E. Managing Anthelmintic Resistance in Small Ruminant Livestock of Resource-Poor Farmers in South Africa. J. S. Afr. Vet. Assoc. 2006, 77, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Durr-e-Shahwar; Ahmad, K.A.; Naveed, F.; Babar, S. Obstacles and Challenges for Women Empowerment in Agriculture: The Case of Rural Punjab, Pakistan. Pak. J. Agric. Sci. 2021, 58, 1075–1080. [Google Scholar]
- Meinzen-Dick, R.; Myers, E.C.; Quisumbing, A. How Empowered Are Women in African Agriculture? Case Study 6. In Gender Equality in Rural Africa: From Commitments to Outcomes; ReSAKSS 2019 Annual Trends and Outlook Report; Quisumbing, A., Meinzen-Dick, R., Njuki, J.M., Eds.; IFPRI: Washington, DC, USA, 2019. [Google Scholar]
- Kristjanson, P.; Waters-Bayer, A.; Johnson, N.; Tipilda, A.; Njuki, J.M.; Baltenweck, I.; Grace, D.; MacMillan, S. Livestock and Women’s Livelihoods: A Review of the Recent Evidence; Discussion Paper 20; International Livestock Research Institute: Nairobi, Kenya, 2010; p. 30. [Google Scholar]
- Galiè, A.; Mulema, A.; Mora Benard, M.A.; Onzere, S.N.; Colverson, K.E. Exploring Gender Perceptions of Resource Ownership and Their Implications for Food Security among Rural Livestock Owners in Tanzania, Ethiopia, and Nicaragua. Agric. Food Secur. 2015, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Serra, R.; Ludgate, N.; Fiorillo Dowhaniuk, K.; McKune, S.L.; Russo, S. Beyond the Gender of the Livestock Holder: Learnings from Intersectional Analyses of PPR Vaccine Value Chains in Nepal, Senegal, and Uganda. Animals 2022, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Bollen, K.A.; Pearl, J. Eight Myths About Causality and Structural Equation Models. In Handbook of Causal Analysis for Social Research; Morgan, S.L., Ed.; Springer: Cham, Switzerland, 2013; Chapter 15; pp. 301–328. [Google Scholar]
- Waithanji, E.; Wanyoike, S.; Liani, M. The Role of Gender and Other Socio-Economic Factors in the Adoption of the Contagious Bovine Pleuropneumonia (CBPP) Vaccine; ILRI Discussion Paper 29; ILRI (International Livestock Research Institute): Nairobi, Kenya, 2015. [Google Scholar]

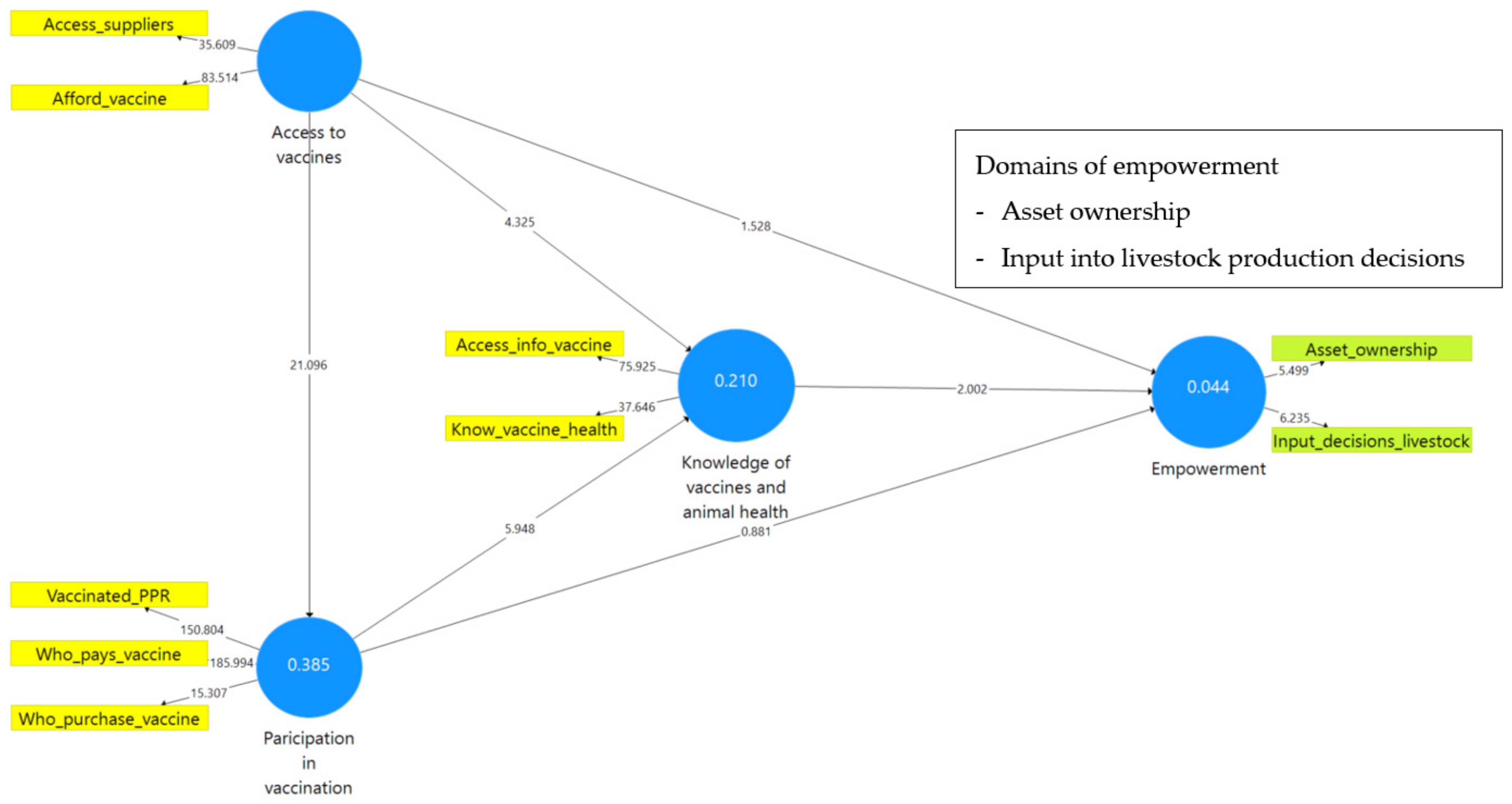
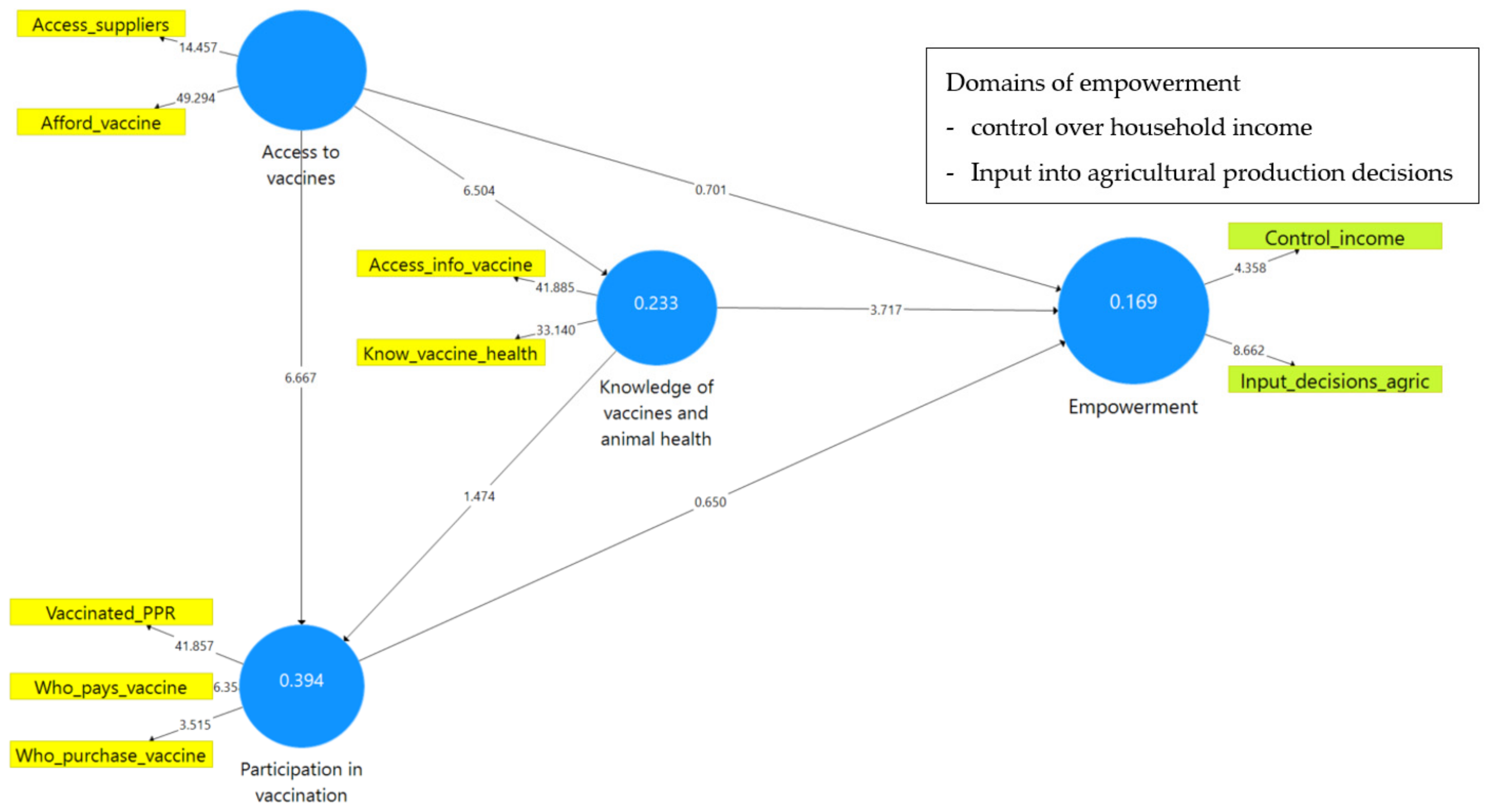



| The Vaccine Module Questions Were Classified into Questions That Observe | Variable Description | Variable Name (Used in the PLS-SEM Model) | |
|---|---|---|---|
| Knowledge and attitude about vaccines and animal health | Where to buy | Knowing where to get PPR vaccines | Know_where_get |
| Level of knowledge (low, medium, high) | Know government’s role in vaccinations | Know_regulation_govt_role | |
| Knowing vaccine regulations | Know_regulation | ||
| Knowing who can vaccinate | Know_regulation_vaccinate | ||
| Knowing about animal health | Know_vaccine_health | ||
| Vaccines use | Knows the best time to administer PPR vaccines | Know_vaccine_administer | |
| Attitude | Would like to access suppliers; | Aspire_access_suppliers | |
| Think vaccines can prevent PPR | Attitude_vaccines_can | ||
| Impact of vaccinations | Level of severity of past infection in the herd, by extent of loss of animals | Impact_loss_animals | |
| Access to information about vaccines and animal health | Attending training and seminars on animal health | Attend_training | |
| Access to training/seminars on small ruminants’ health | Access_info_training | ||
| Access to information on PPR vaccination | Access_info_vaccine | ||
| Participation in vaccinations | Purchasing | Who participated in purchasing PPR vaccines | Who_purchase_vaccine |
| Woman participated in purchasing PPR vaccines, either alone or with others | Woman_purchase_vaccine | ||
| Man participated in purchasing PPR vaccines, either alone or with others | Man_purchase_vaccine | ||
| Paying for vaccination | Man paid for vaccine | Man_pays_vaccine | |
| Woman paid for vaccine | Woman_pays_vaccine | ||
| Both man and woman paid for PPR vaccine | Man_woman_pays_vaccine | ||
| Who pays for PPR vaccines | Who_pays_vaccine | ||
| Woman pays for PPR vaccines either singly or with men | Woman_s_j_pays_vaccine | ||
| Man pays for PPR vaccines, either singly or with woman | Man_s_j_pays_vaccine | ||
| Vaccinating | One’s goats have been vaccinated against PPR | Vaccinated_PPR | |
| Woman actively participates in the process of vaccinating animals against PPR | Woman_vaccinate_PPR | ||
| Man actively participates in the process of vaccinating animals against PPR | Man_vaccinate_PPR | ||
| Access to vaccine | Physical access | Access to PPR vaccine suppliers/vaccinators | Access_suppliers |
| Access to cold chain for PPR vaccine supplies | Access_cold_chain | ||
| Affordability | Ability to pay for PPR vaccine/vaccination | Afford_vaccine | |
| Respondent and Household Characteristics | Women Value (n = 465) | Men Value (n = 92) | t-Test | |
|---|---|---|---|---|
| Household structure | Age of respondent age (mean age) | 44.14 | 46.34 | 1.47, df = 555 |
| Household size (mean number or persons) | 6.44 | 7.65 | 4.48, df = 555 *** | |
| Percentage of respondents who felt the livestock species (out of the species kept in the household) to be most important for their household’s livelihood | Small ruminants (sheep and/or goats—local or improved breeds) | 59.35 | 30.43 | - |
| Chickens (local or improved breeds) | 10.54 | 17.39 | - | |
| Large ruminants e.g., cattle (beef or dual-purpose—local or improved breeds) | 18.71 | 30.43 | - | |
| Large ruminants e.g., cattle (dairy—local or improved breeds) | 10.75 | 19.57 | - | |
| Pigs (and/or others—local or improved) | 0.65 | 2.17 | - | |
| Percentage of respondents who felt the livestock species (out of the species kept in the household) to be most important for their own livelihood | Small ruminants (sheep and/or goats—local or improved breeds) | 46.45 | 53.26 | - |
| Chickens (local or improved breeds) | 42.58 | 32.61 | - | |
| Large ruminants e.g., cattle (beef or dual-purpose—local or improved breeds) | 0.86 | 8.70 | - | |
| Large ruminants e.g., cattle (dairy—local or improved breeds) | 1.51 | 5.43 | - | |
| Pigs (and/or others—local or improved) | 8.60 | 0.00 | - | |
| Constructs | Variable Name in the Data | Women | Men | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λ | CA | rho_A | CR | AVE | Λ | CA | rho_A | CR | AVE | ||
| Access to vaccines | Access_suppliers | 0.82 | 0.66 | 0.70 | 0.85 | 0.74 | 0.81 | 0.69 | 0.76 | 0.86 | 0.76 |
| Afford_vaccine | 0.90 | 0.92 | |||||||||
| Knowledge of vaccines and animal health | Access_info_vaccine | 0.93 | 0.74 | 0.80 | 0.88 | 0.79 | 0.91 | 0.79 | 0.79 | 0.91 | 0.83 |
| Know_vaccine_health | 0.85 | 0.91 | |||||||||
| Participation in vaccination | Vaccinated_PPR | 0.94 | 0.81 | 0.89 | 0.88 | 0.73 | 0.92 | 0.72 | 0.84 | 0.84 | 0.65 |
| Who_pays_vaccine | 0.95 | 0.92 | |||||||||
| Who_purchase_vaccine | 0.64 | 0.53 | |||||||||
| WELI | Asset_ownership | 0.73 | 0.26 | 0.26 | 0.73 | 0.57 | - | - | - | - | - |
| Input_decisions_livestock | 0.78 | - | |||||||||
| Input_decisions_agric | - | - | - | - | - | 0.84 | 0.45 | 0.46 | 0.78 | 0.64 | |
| Control_income | - | 0.77 | |||||||||
| Hypotheses | Original Sample (O) (n = 465) | Sample Mean (M) (n = 5000) | Standard Deviation (STDEV) | p-Values |
|---|---|---|---|---|
| Access to vaccines <> knowledge of vaccines and animal health | 0.22 | 0.22 | 0.05 | 0.000 |
| Access to vaccines <> participation in vaccination | 0.62 | 0.62 | 0.03 | 0.000 |
| Access to vaccines <> empowerment | 0.09 | 0.09 | 0.06 | 0.126 |
| Knowledge of vaccines and animal health <> participation in vaccination | 0.29 | 0.29 | 0.05 | 0.000 |
| Knowledge of vaccines and animal health <> empowerment | 0.11 | 0.12 | 0.06 | 0.045 |
| Participation in vaccination <> empowerment | 0.06 | 0.06 | 0.07 | 0.378 |
| Hypotheses | Original Sample (O) (n = 97) | Sample Mean (M) (n = 1000) | Standard Deviation (STDEV) | p-Values |
|---|---|---|---|---|
| Access to vaccines <> knowledge of vaccines and animal health | 0.48 | 0.48 | 0.07 | 0.000 |
| Access to vaccines <> participation in vaccination | 0.55 | 0.56 | 0.08 | 0.000 |
| Access to vaccines <> empowerment | 0.09 | 0.10 | 0.13 | 0.483 |
| Knowledge of vaccines and animal health <> participation in vaccination | 0.14 | 0.14 | 0.09 | 0.141 |
| Knowledge of vaccines and animal health <> empowerment | 0.32 | 0.33 | 0.09 | 0.00 |
| Participation in vaccination <> empowerment | 0.06 | 0.05 | 0.10 | 0.516 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omondi, I.; Galiè, A.; Teufel, N.; Loriba, A.; Kariuki, E.; Baltenweck, I. Women’s Empowerment and Livestock Vaccination: Evidence from Peste des Petits Ruminants Vaccination Interventions in Northern Ghana. Animals 2022, 12, 717. https://doi.org/10.3390/ani12060717
Omondi I, Galiè A, Teufel N, Loriba A, Kariuki E, Baltenweck I. Women’s Empowerment and Livestock Vaccination: Evidence from Peste des Petits Ruminants Vaccination Interventions in Northern Ghana. Animals. 2022; 12(6):717. https://doi.org/10.3390/ani12060717
Chicago/Turabian StyleOmondi, Immaculate, Alessandra Galiè, Nils Teufel, Agnes Loriba, Eunice Kariuki, and Isabelle Baltenweck. 2022. "Women’s Empowerment and Livestock Vaccination: Evidence from Peste des Petits Ruminants Vaccination Interventions in Northern Ghana" Animals 12, no. 6: 717. https://doi.org/10.3390/ani12060717






