Seasonal Expression of Gonadotropin Genes in the Pituitary and Testes of Male Plateau Zokor (Eospalax baileyi)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissues
2.2. HE Staining
2.3. Immunohistochemistry
2.4. Total RNA Isolation and First-Strand cDNA Synthesis
2.5. Primer Design
2.6. RT-PCR
2.7. Statistical Analysis
3. Results
3.1. Histology and Morphology
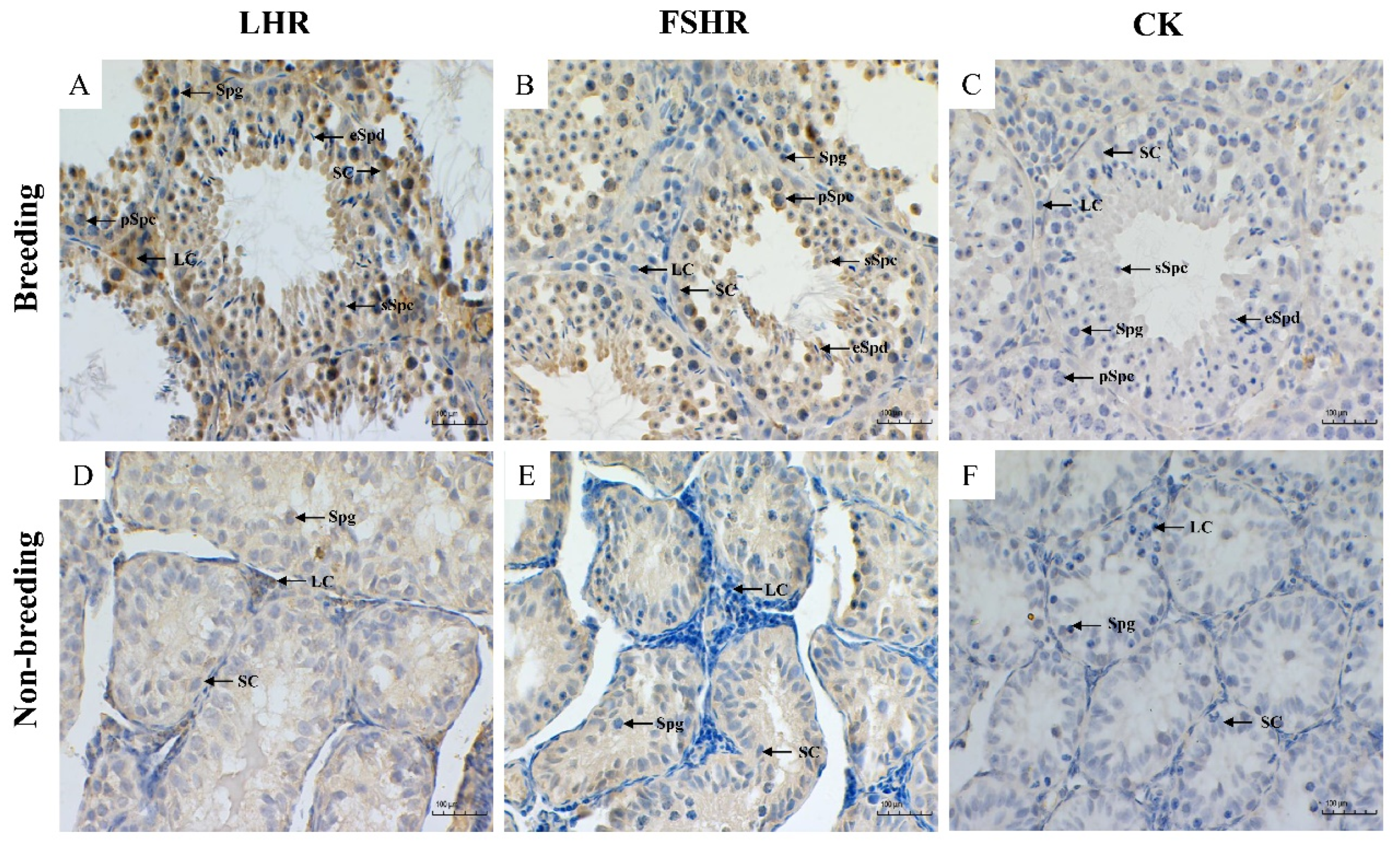
3.2. Immunohistochemical Results
3.2.1. Localization of LHR and FSHR
3.2.2. Immunopositive Evaluation of LHR and FSHR
3.3. RT-PCR Analysis
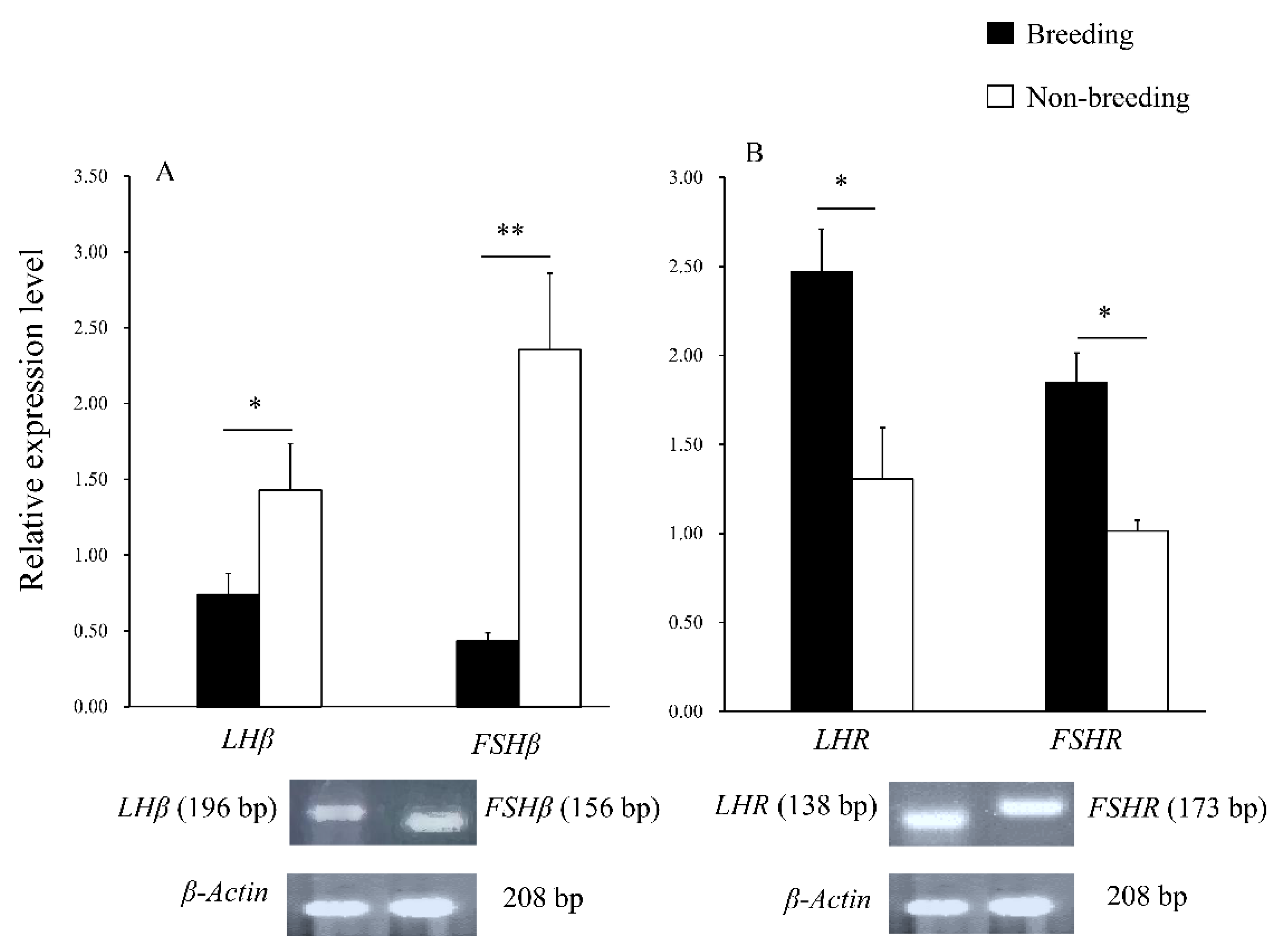
3.3.1. The Gene Expression in the Pituitary
3.3.2. The Gene Expression in Testes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henningsen, J.B.; Gauer, F.; Simonneaux, V. RFRP Neurons-the doorway to understanding seasonal reproduction in mammals. Front. Endocrinol. 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, K.A.; Lesku, J.A.; Partecke, J.; Chambers, B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. Biol. Sci. 2015, 282, 20151745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronson, F.H. Climate change and seasonal reproduction in mammals. Philos. Trans. R. Soc. B 2009, 364, 3331–3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, T.J.; Ball, G.F. Information theory and the neuropeptidergic regulation of seasonal reproduction in mammals and birds. Proc. Biol. Sci. 2011, 278, 2477–2485. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, J.U.; Bennett, N.C. The evolutionary history, population biology and social structure of African mole-rats: Family Bathyergidae. Prog. Clin. Biol. Res. 1990, 335, 97–128. [Google Scholar] [PubMed]
- Herbst, M.; Jarvis, J.; Bennett, N. A field assessment of reproductive seasonality in the threatened wild namaqua dune mole-rat (Bathyergus janetta). J. Zool. 2006, 263, 259–268. [Google Scholar] [CrossRef]
- Cooper, H.M.; Herbin, M.; Nevo, E. Ocular regression conceals adaptive progression of the visual system in a blind subterranean mammal. Nature 1993, 361, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, A.; Shimmura, T.; Nishiwaki-Ohkawa, T.; Yoshimura, T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front. Endocrinol. 2014, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Haldar, C. Photoperiodic regulation of melatonin membrane receptor (MT1R) expression and steroidogenesis in testis of adult golden hamster, Mesocricetus auratus. J. Photochem. Photobiol. B 2014, 140, 374–380. [Google Scholar] [CrossRef]
- Ansel, L.; Bentsen, A.H.; Ancel, C.; Bolborea, M.; Klosen, P.; Mikkelsen, J.D.; Simonneaux, V. Peripheral kisspeptin reverses short photoperiod-induced gonadal regression in Syrian hamsters by promoting GNRH release. Reproduction 2011, 142, 417–425. [Google Scholar] [CrossRef]
- Hut, R.A.; Beersma, D.G. Evolution of time-keeping mechanisms: Early emergence and adaptation to photoperiod. Philos. Trans. R. Soc. B 2011, 366, 2141–2154. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, N.; Tian, L.; Ren, F.; Li, Z.; Chen, Y.; Liu, L.; Hu, X.; Zhang, X.; Song, Y.; et al. Dynamic expressions of hypothalamic genes regulate seasonal breeding in a natural rodent population. Mol. Ecol. 2019, 28, 3508–3522. [Google Scholar] [CrossRef]
- Dardente, H.; Klosen, P.; Pévet, P.; Masson-Pévet, M. MT1 melatonin receptor mRNA expressing cells in the pars tuberalis of the European hamster: Effect of photoperiod. J. Neuroendocrinol. 2003, 15, 778–786. [Google Scholar] [CrossRef]
- Klosen, P.; Bienvenu, C.; Demarteau, O.; Dardente, H.; Guerrero, H.; Pévet, P.; Masson-Pévet, M. The mt1 melatonin receptor and RORbeta receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J. Histochem. Cytochem. 2002, 50, 1647–1657. [Google Scholar] [CrossRef] [Green Version]
- Hanon, E.A.; Lincoln, G.A.; Fustin, J.M.; Dardente, H.; Masson-Pévet, M.; Morgan, P.J.; Hazlerigg, D.G. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 2008, 18, 1147–1152. [Google Scholar] [CrossRef] [Green Version]
- Klosen, P.; Sébert, M.E.; Rasri, K.; Laran-Chich, M.P.; Simonneaux, V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB J. 2013, 27, 2677–2686. [Google Scholar] [CrossRef]
- Nishiwaki-Ohkawa, T.; Yoshimura, T. Molecular basis for regulating seasonal reproduction in vertebrates. J. Endocrinol. 2016, 229, R117–R127. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, T.; Hirunagi, K.; Ebihara, S.; Yoshimura, T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology 2004, 145, 4264–4267. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.C.; Kelch, R.P. Gonadotropin-releasing hormone: Role of pulsatile secretion in the regulation of reproduction. N. Engl. J. Med. 1986, 315, 1459–1468. [Google Scholar]
- Gharib, S.D.; Wierman, M.E.; Shupnik, M.A.; Chin, W.W. Molecular biology of the pituitary gonadotropins. Endocr. Rev. 1990, 11, 177–199. [Google Scholar] [CrossRef]
- Bernard, D.J.; Merzlyak, I.Y.; Horton, T.H.; Turek, F.W. Differential regulation of pituitary gonadotropin subunit messenger ribonucleic acid levels in photostimulated Siberian hamsters. Biol. Reprod. 2000, 62, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Dong, Y.; Matzuk, M.M.; Kumar, T.R. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc. Natl. Acad. Sci. USA 2004, 101, 17294–17299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zi, X.; Huang, L.; Wang, Y.; Lu, J. Comparative messenger RNA expression of FSHβ, LHβ, FSHR, LHR, and ERβ in high and low prolific goat breeds. Anim. Biotechnol. 2013, 24, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Zhang, T.; Abudureyimu, A.; Liu, J.; Zhang, G.; Ma, Z. Protein and mRNA expression of gonadotropin-releasing hormone receptor in yaks during estrus. Rev. Bras. Zootec. 2018, 47, e20160360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, F.; Zhu, M.; Wang, J.; Sheng, X.; Yuan, Z.; Han, Y.; Watanabe, G.; Taya, K.; Weng, Q. Seasonal expressions of follicle-stimulating hormone receptor and luteinizing hormone receptor in the scented gland of the male muskrat (Ondatra zibethicus). Am. J. Physiol.-Reg. Ingetr. Comp. Physiol. 2017, 312, R569–R574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraishi, K.; Matsuyama, H. Gonadotoropin actions on spermatogenesis and hormonal therapies for spermatogenic disorders. Endocr. J. 2017, 64, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della-Maria, J.; Gerard, A.; Franck, P.; Gerard, H. Effects of androgen-binding protein (ABP) on spermatid Tnp1 gene expression in vitro. Mol. Cell. Endocrinol. 2002, 198, 131–141. [Google Scholar] [CrossRef]
- Stanton, P.G. Regulation of the blood-testis barrier. Semin. Cell. Dev. Biol. 2016, 59, 166–173. [Google Scholar] [CrossRef]
- Su, J.; Aryal, A.; Nan, Z.; Ji, W. Climate change-induced range expansion of a subterranean rodent: Implications for rangeland management in Qinghai-Tibetan plateau. PLoS ONE 2015, 10, e0138969. [Google Scholar] [CrossRef]
- Kang, Y.; Su, J.; Yao, B.; Wang, C.; Zhang, D.; Ji, W. Interspecific skull variation at a small scale: The genus Eospalax exhibits functional morphological variations related to the exploitation of ecological niche. J. Zool. Syst. Evol. Res. 2021, 59, 902–917. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, J. Burrowing rodents as ecosystem engineers: The ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal. Rev. 2003, 33, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Ji, W.; Li, H.; Yao, T.; Wang, J.; Nan, Z. Zokor disturbances indicated positive soil microbial responses with carbon cycle and mineral encrustation in alpine grassland. Ecol. Eng. 2020, 144, 105702. [Google Scholar] [CrossRef]
- An, X.; Wang, Y.; Li, Y.; Jia, G.; Yang, Q. Morphological features and regulation of seasonal spermatogenesis in plateau zokor (Eospalax baileyi). Acta Theriol. Sin. 2020, 40, 435–445. [Google Scholar]
- Zhang, Y. The biology and ecology of plateau zokors (Eospalax fontanierii). In Subterranean Rodents: News from Underground; Begall, S., Burda, H., Schleich, C.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 237–249. [Google Scholar]
- Su, J.; Peng, R.; Nan, Z.; Ji, W.; Cai, Z. Age determination and composition analyses of plateau zokor (Eospalax baileyi) in Gannan meadow. Chin. J. Zool. 2018, 53, 46–54. [Google Scholar]
- Yin, Y.; Demolf, W.C.; Morgentaler, A. Experimental cryptorchidism inducestesticular germ cell apoptosis by p53-dependent and independent pathways in mice. Biol. Reprod. 1998, 58, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Apfelbeck, B.; Mortega, K.G.; Flinks, H.; Illera, J.C.; Helm, B. Testosterone, territorial response, and song in seasonally breeding tropical and temperate stonechats. BMC Evol. Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken-Palmer, C.; Hou, R.; Burrell, C.; Zhang, Z.; Wang, C.; Spindler, R.; Wildt, D.E.; Ottinger, M.A.; Howard, J. Protracted reproductive seasonality in the male giant panda (Ailuropoda melanoleuca) reflected by patterns in androgen profiles, ejaculate characteristics, and selected behaviors. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, K.; Broda, D.; Romerowicz-Misielak, M.; Nowak, S.; Koziorowski, M. Melatonin concentration in peripheral blood and melatonin receptors (MT1 and MT2) in the testis and epididymis of male roe deer during active spermatogenesis. Theriogenology 2020, 149, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Teerds, K.J.; De Rooij, D.G.; Rommerts, F.F.; Wensing, C.J. The regulation of the proliferation and differentiation of rat Leydig cell precursor cells after EDS administration or daily HCG treatment. J. Androl. 1988, 9, 343–351. [Google Scholar] [CrossRef]
- Mendis-Handagama, S.M.; Watkins, P.A.; Gelber, S.J.; Scallen, T.J. The effect of chronic luteinizing hormone treatment on adult rat Leydig cells. Tissue Cell 1998, 30, 64–73. [Google Scholar] [CrossRef]
- Vigier, M.; Weiss, M.; Perrard, M.H.; Godet, M.; Durand, P. The effects of FSH and of testosterone on the completion of meiosis and the very early steps of spermiogenesis of the rat: An in vitro study. J. Mol. Endocrinol. 2004, 33, 729–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle–stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlström, T.; Huhtaniemi, I.; Hovatta, O.; Seppälä, M. Localization of luteinizing hormone, follicle-stimulating hormone, prolactin, and their receptors in human and rat testis using immunohistochemistry and radioreceptor assay. J. Clin. Endocrinol. Metab. 1983, 57, 825–830. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, M.; Zhang, F.; Sheng, X.; Zhang, H.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal expression of luteinizing hormone receptor and follicle stimulating hormone receptor in testes of the wild ground squirrels (Citellus dauricus Brandt). ACTA Histochem. 2017, 119, 727–732. [Google Scholar] [CrossRef]
- Boufermes, R.; Richard, N.; Le Moguen, K.; Amirat, Z.; Khammar, F.; Kottler, M.L. Seasonal expression of KiSS-1 and the pituitary gonadotropins LHβ and FSHβ in adult male Libyan jird (Meriones libycus). Anim. Reprod. Sci. 2014, 147, 56–63. [Google Scholar] [CrossRef]
- Kishi, H.; Itoh, M.; Wada, S.; Yukinari, Y.; Tanaka, Y.; Nagamine, N.; Jin, W.; Watanabe, G.; Taya, K. Inhibin is an important factor in the regulation of FSH secretion in the adult male hamster. Am. J. Physiol. Endoc. Metab. 2000, 278, E744–E751. [Google Scholar] [CrossRef]
- Kano, Y.; Nakano, T.; Kumakura, M.; Wasa, T.; Suzuki, M.; Yamauchi, K.; Tanaka, S. Seasonal expression of LHbeta and FSHbeta in the male newt pituitary gonadotrophs. Gen. Comp. Endocrinol. 2005, 141, 248–258. [Google Scholar] [CrossRef]
- Misrahi, M.; Beau, I.; Meduri, G.; Bouvattier, C.; Atger, M.; Loosfelt, H.; Ghinea, N.; Hai, M.V.; Bougnères, P.F.; Milgrom, E. Gonadotropin receptors and the control of gonadal steroidogenesis: Physiology and pathology. Bailliere Clin. Endoc. 1998, 12, 35–66. [Google Scholar] [CrossRef]
- Lei, Z.M.; Mishra, S.; Ponnuru, P.; Li, X.; Yang, Z.; Rao, C. Testicular phenotype in luteinizing hormone receptor knockout animals and the effect of testosterone replacement therapy. Biol. Reprod. 2004, 71, 1605–1613. [Google Scholar] [CrossRef]
- Pintus, E.; Ros-Santaella, J.L.; Garde, J.J. Beyond testis size: Links between Spermatogenesis and sperm traits in a seasonal breeding mammal. PLoS ONE 2015, 10, e0139240. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.M.; Gunsalus, G.L.; Lamperti, A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988, 122, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Vigier, M.; Hue, D.; Perrard-Sapori, M.H.; Marret, C.; Avallet, O.; Durand, P. Pre- and postmeiotic expression of male germ cell-specific genes throughout 2-week cocultures of rat germinal and Sertoli cells. Biol. Reprod. 1997, 57, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hue, D.; Staub, C.; Perrard-Sapori, M.H.; Weiss, M.; Nicolle, J.C.; Vigier, M.; Durand, P. Meiotic differentiation of germinal cells in three-week cultures of whole cell population from rat seminiferous tubules. Biol. Reprod. 1998, 59, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Meachem, S.J.; Wreford, N.G.; Stanton, P.G.; Robertson, D.M.; McLachlan, R.I. Follicle-stimulating hormone is required for the initial phase of spermatogenic restoration in adult rats following gonadotropin suppression. J. Androl. 1998, 19, 725–735. [Google Scholar] [PubMed]
- Sluka, P.; O’Donnell, L.; Bartles, J.R.; Stanton, P.G. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J. Endocrinol. 2006, 189, 381–395. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer Sequence | Temperature (°C) | Size (bp) |
|---|---|---|---|
| LHβ | F: 5′-CAACCCTGGCTGCAGAGAAT-3′ | 53.5 | 196 |
| R: 5′-GGGCCACAGGAAAGGAGAC-3′ | |||
| FSHβ | F: 5′-CAGGCTACTGCTACACCAGG-3′ | 53.5 | 156 |
| R: 5′-CAGTGGCTACTGGGTACGTG-3′ | |||
| LHR | F: 5′-ATGCCTTTGACAACCTCCTCA-3′ | 54 | 138 |
| R: 5′-GGGTCTGGATGCCTGTGTTA-3′ | |||
| FSHR | F: 5′-GCTGAGGCCTTCCAGAATCTT-3′ | 54 | 173 |
| R: 5′-AAACTCAGTCCCATGAAGGAAT-3′ | |||
| β–Actin | F: 5′-TTGTGCGTGACATCAAAGAG-3′ | 53.5 | 208 |
| R: 5′-ATGCCAGAAGATTCCATACC-3′ |
| Gene Name | Rat (%) | Mouse (%) | Marmot (%) | Human (%) |
|---|---|---|---|---|
| LHβ | 83.7 | 82.7 | 84.5 | 73.0 |
| FSHβ | 84.1 | 85.7 | 87.4 | 83.6 |
| LHR | 92.7 | 95.2 | 90.1 | 88.0 |
| FSHR | 88.2 | 85.9 | 90.4 | 87.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, K.; Yao, B.; Kang, Y.; Bao, M.; Tan, Y.; Pu, Q.; Su, J. Seasonal Expression of Gonadotropin Genes in the Pituitary and Testes of Male Plateau Zokor (Eospalax baileyi). Animals 2022, 12, 725. https://doi.org/10.3390/ani12060725
An K, Yao B, Kang Y, Bao M, Tan Y, Pu Q, Su J. Seasonal Expression of Gonadotropin Genes in the Pituitary and Testes of Male Plateau Zokor (Eospalax baileyi). Animals. 2022; 12(6):725. https://doi.org/10.3390/ani12060725
Chicago/Turabian StyleAn, Kang, Baohui Yao, Yukun Kang, Mingfang Bao, Yuchen Tan, Qiangsheng Pu, and Junhu Su. 2022. "Seasonal Expression of Gonadotropin Genes in the Pituitary and Testes of Male Plateau Zokor (Eospalax baileyi)" Animals 12, no. 6: 725. https://doi.org/10.3390/ani12060725





