Relationship of mRNA Expression of Selected Genes in Peripheral Blood and Synovial Fluid in Cranial Cruciate Ligament Deficient Stifles of Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection and Isolation of Total RNA
2.2.1. Blood
2.2.2. Synovial Fluid
2.3. Relative Expression of Target Genes in Quantitative Real-Time PCR (qRT-PCR)
2.4. Radiographic Procedure and Measurements
2.5. Statistical Analysis
3. Results
3.1. Animals
3.2. The Relative Expression of Selected Genes
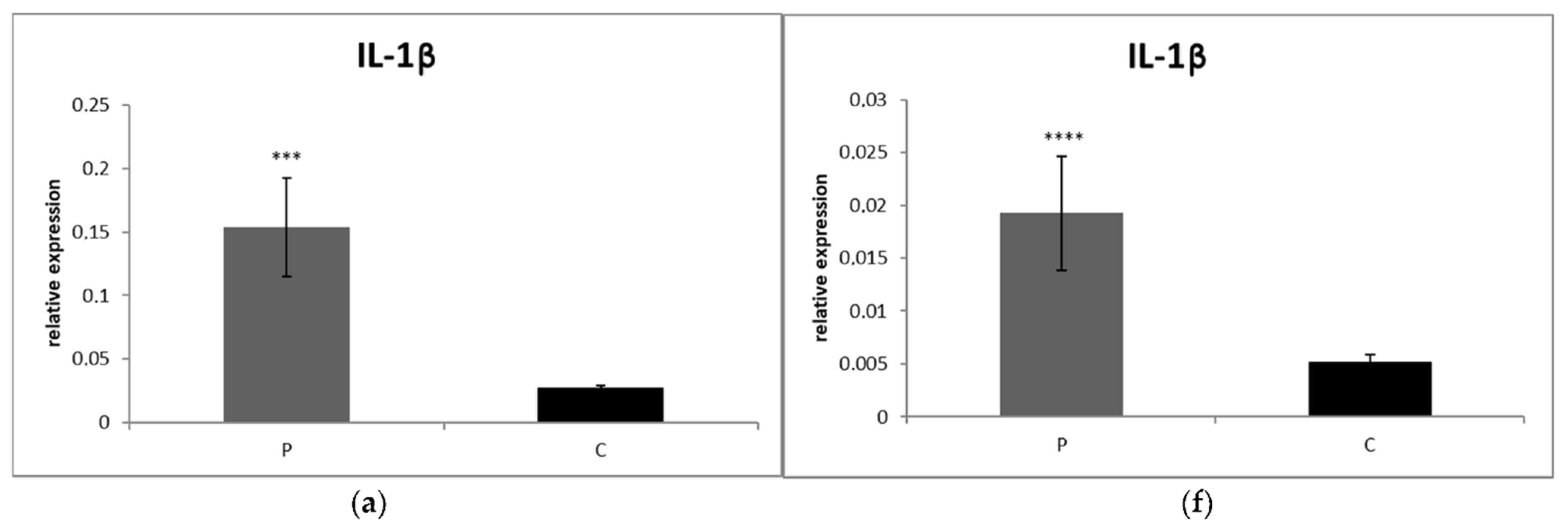
3.2.1. Blood
3.2.2. Synovial Fluid
3.3. Comparison of mRNA Expressions of Cytokines, Collagens and Elastin between Partial and Complete Ruptured Ligaments
3.4. Radiographic Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, J.L. Cranial cruciate ligament disease in dogs: Biology versus biomechanics. Vet. Surg. 2010, 39, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Manley, P.A.; Muir, P. Cranial cruciate ligament pathophysiology in dogs with cruciate disease: A review. J. Am. Anim. Hosp. Assoc. 2004, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial Cruciate Ligament Rupture in Dogs: Review on Biomechanics, Etiopathogenetic Factors and Rehabilitation. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Doom, M.; De Bruin, T.; De Rooster, H.; van Bree, H.; Cox, E. Immunopathological mechanisms in dogs with rupture of the cranial cruciate ligament. Vet. Immunol. Immunopathol. 2008, 125, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Hay, C.W.; Chu, Q.; Budsberg, S.C.; Clayton, M.K.; Johnson, K.A. Synovial fluid interleukin 6, tumor necrosis factor, and nitric oxide values in dogs with osteoarthritis secondary to cranial cruciate ligament rupture. Am. J. Vet. Res. 1997, 58, 1027–1032. [Google Scholar]
- Fujita, Y.; Hara, Y.; Nezu, Y.; Schulz, K.S.; Tagawa, M. Proinflammatory cytokine activities, matrix metalloproteinase-3 activity, and sulfated glycosaminoglycan content in synovial fluid of dogs with naturally acquired cranial cruciate ligament rupture. Vet. Surg. 2006, 35, 369–376. [Google Scholar] [CrossRef]
- De Bruin, T.; de Rooster, H.; Van Bree, H.; Duchateau, L.; Cox, E. Cytokine mRNA expression in synovial fluid of affected and contralateral stifle joints and the left shoulder joint in dogs with unilateral disease of the stifle joint. Am. J. Vet. Res. 2007, 68, 953–961. [Google Scholar] [CrossRef]
- Hasegawa, A.; Nakahara, H.; Kinoshita, M.; Asahara, H.; Koziol, J.; Lotz, M.K. Cellular and extracellular matrix changes in anterior cruciate ligaments during human knee aging and osteoarthritis. Arthritis Res. Ther. 2013, 15, R29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duerr, F.M.; Duncan, C.G.; Savicky, R.S.; Park, R.D.; Egger, E.L.; Palmer, R.H. Risk factors for excessive tibial plateau angle in large-breed dogs with cranial cruciate ligament disease. J. Am. Vet. Med. Assoc. 2007, 231, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Comerford, E.J.; Innes, J.F.; Tarton, J.F.; Bailey, A.J. Investigation of the composition, turnover, and thermal properties of cranial cruciate ligaments in dogs. Am. J. Vet. Res. 2004, 65, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Muir, P.; Manley, P.A.; Hao, Z. Collagen fragmentation in ruptured canine cranial cruciate ligament explants. Vet. J. 2006, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, Y.; Shimada, A.; Komatsu, K.; Gomi, K.; Oida, S.; Arai, T.; Fukae, M. Degradation of noncollagenous components by neutrophil elastase reduces the mechanical strength of rat periodontal ligament. J. Periodontal Res. 2008, 43, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Clegg, P.D.; Innes, J.F.; Comerford, E.J. Elastin content is high in the canine cruciate ligament and is associated with degeneration. Vet. J. 2014, 199, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Chantawiboonchai, P.; Warita, H.; Ohya, K.; Soma, K. Confocal laser scanning-microscopic observations on the three-dimensional distribution of oxytalan fibres in mouse periodontal ligament. Arch. Oral Biol. 1998, 43, 811–817. [Google Scholar] [CrossRef]
- Tashiro, K.; Sawada, T.; Inoue, S.; Yanagisawa, T. Development of oxytalan fibers in the rat molar periodontal ligament. J. Periodontal Res. 2002, 37, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Hayashi, K.; Clements, D.N.; Clegg, P.D.; Innes, J.F.; Comerford, E.J. Variation in the Quantity of Elastic Fibres with Degeneration in Canine Cranial Cruciate Ligaments from Labrador Retrievers. Vet. Comp. Orthop. Traumatol. 2017, 30, 398–402. [Google Scholar] [CrossRef]
- Comerford, E.J.; Tarlton, J.F.; Avery, N.C.; Bailey, A.J.; Innes, J.F. Distal femoral intercondylar notch dimensions and their relationship to composition and metabolism of the canine anterior cruciate ligament. Osteoarthr. Cartil. 2006, 14, 273–278. [Google Scholar] [CrossRef] [Green Version]
- De Rooster, H.; de Bruin, T.; van Bree, H. Morphologic and functional features of the canine cruciate ligaments. Vet. Surg. 2006, 35, 769–780. [Google Scholar] [CrossRef]
- Dennler, R.; Kipfer, N.M.; Tepic, S.; Hassig, M.; Montavon, P.M. Inclination of the patellar ligament in relation to flexion angle in stifle joints of dogs without degenerative joint disease. Am. J. Vet. Res. 2006, 67, 1849–1854. [Google Scholar] [CrossRef]
- Guerrero, T.G.; Geyer, H.; Hässig, M.; Montavon, P.M. Effect of conformation of the distal portion of the femur and proximal portion of the tibia on the pathogenesis of cranial cruciate ligament disease in dogs. Am. J. Vet. Res. 2007, 68, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Pozzi, A.; Kowaleski, M.P.; Lewis, D.D. Tibial osteotomies for cranial cruciate ligament insufficiency in dogs. Vet. Surg. 2008, 37, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Kipfer, N.M.; Tepic, S.; Damur, D.M.; Guerrero, T.; Hässig, M.; Montavon, P.M. Effect of tibial tuberosity advancement on femorotibial shear in cranial cruciate-deficient stifles. An in vitro study. Vet. Comp. Orthop. Traumatol. 2008, 21, 385–390. [Google Scholar] [PubMed]
- Kowaleski, M.P.; Apelt, D.; Mattoon, J.S.; Litsky, A.S. The effect of tibial plateau leveling osteotomy position on cranial tibial subluxation: An in vitro study. Vet. Surg. 2005, 34, 332–336. [Google Scholar] [CrossRef]
- Reif, U.; Dejardin, L.M.; Probst, C.W.; DeCamp, C.E.; Flo, G.L.; Johnson, A.L. Influence of limb positioning and measurement method on the magnitude of the tibial plateau angle. Vet. Surg. 2004, 33, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Venzin, C.; Howard, J.; Rytz, U.; Spreng, D.; Schawalder, P.; Doherr, M.; Schmökel, H. Tibial plateau angle with and without cranial cruciate ligament rupture: Comparison between different dog populations and a wolf population. Vet. Comp. Orthop. Traumatol. 2004, 17, 232–236. [Google Scholar]
- Paley, D. Principles of Deformity Correction: What is Alignment and Malaligment. Improving Accuracy in Knee Arthroplasty; Jaypee Brothers. Medical Publishers Pvt. Limited: London, UK, 2012; pp. 2–4. [Google Scholar]
- Osmond, C.S.; Marcellin-Little, D.J.; Harrysson, O.L.A.; Kidd, L.B. Morphometric assessment of the proximal portion of the tibia in dogs with and without cranial cruciate ligament rupture. Vet. Radiol. Ultrasound 2006, 47, 136–141. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Griffon, D.J.; Thomas, M.W.; Constable, P.D. Morphometric characteristics of the pelvic limbs of Labrador Retrievers with and without cranial cruciate ligament deficiency. Am. J. Vet. Res. 2009, 70, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Glassman, M.; Hofmeister, E.; Weh, J.M.; Roach, W.; Torres, B.; Johnston, S.; Budsberg, S. Radiographic quantitative assessment of caudal proximal tibial angulation in 100 dogs with cranial cruciate ligament rupture. Vet. Surg. 2011, 40, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Guénégo, L.; Payot, M.; Charru, P.; Verwaerde, P. Comparison of tibial anatomical-mechanical axis angle between predisposed dogs and dogs at low risk for cranial cruciate ligament rupture. Vet. J. 2017, 225, 35–41. [Google Scholar] [CrossRef] [PubMed]
- D’Anjou, M.A.; Moreau, M.; Troncy, E.; Martel-Pelletier, J.; Abram, F.; Raynauld, J.P.; Pelletier, J.P. Osteophytosis, subchondral bone sclerosis, joint effusion and soft tissue thickening in canine experimental stifle osteoarthritis: Comparison between 1.5 T magnetic resonance imaging and computed radiography. Vet. Surg. 2008, 37, 166–177. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, S.M.; Chan, C.K.; Teo, S.H.; Singh, S.; Merican, A.; Ng, W.M.; Abbas, A.; Kamarul, T. Elevated plasma and synovial fuid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis. Knee 2020, 27, 26–35. [Google Scholar] [CrossRef]
- Piermattei, D.L.; Flo, G.L.; DeCamp, C.E. The stifle joint. In Handbook of Small Animal Orthopedics and Fracture Repair, 5th ed.; Brinker, W.O., Piermattei, D.L., Flo, G.L., DeCamp, C.E., Eds.; Saunders: Philadelphia, PA, USA, 2006; pp. 597–669. [Google Scholar]
- Karaffová, V.; Marcinková, E.; Bobíková, K.; Herich, R.; Revajová, V.; Stašová, D.; Kavuľová, A.; Levkutová, M.; Levkut, M., Jr.; Lauková, A.; et al. TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and sIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol. 2017, 62, 89–97. [Google Scholar]
- Tamura, Y.; Ohta, H.; Yokoyama, N.; Lim, S.Y.; Osuga, T.; Morishita, K.; Nakamura, K.; Yamasaki, M.; Takiguchi, M. Evaluation of selected cytokine gene expression in colonic mucosa from dogs with idiopathic lymphocytic-plasmacytic colitis. J. Vet. Med. Sci. 2014, 76, 1407–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskova, Z.; Svoboda, M.; Satinska, D.; Matiasovic, J.; Leva, L.; Toman, M. Changes in leukocyte counts, lymphocyte subpopulations and the mRNA expression of selected cytokines in the peripheral blood of dogs with atopic dermatitis. Vet. Med. 2015, 60, 644–653. [Google Scholar] [CrossRef] [Green Version]
- Brachelente, C.; Cappelli, K.; Capomaccio, S.; Porcellato, I.; Silvestri, S.; Bongiovanni, L.; De Maria, R.; Supplizi, A.V.; Mechelli, L.; Sforna, M. Transcriptome Analysis of Canine Cutaneous Melanoma and Melanocytoma Reveals a Modulation of Genes Regulating Extracellular Matrix Metabolism and Cell Cycle. Sci. Rep. 2017, 7, 63–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boerkamp, K.M.; van der Kooij, M.; van Steenbeek, F.G.; van Wolferen, M.E.; Groot Koerkamp, M.J.; van Leenen, D.; Grinwis, G.C.; Penning, L.C.; Wiemer, E.A.; Rutteman, G.R. Gene expression profiling of histiocytic sarcomas in a canine model: The predisposed flatcoated retriever dog. PLoS ONE. 2013, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schunck, M.; Louton, H.; Oesser, S. The Effectiveness of Specific Collagen Peptides on Osteoarthritis in Dogs-Impact on Metabolic Processes in Canine Chondrocytes. Open J. Anim. Sci. 2017, 7, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Oh, I.Y.; Kim, K.T.; Sung, H.J. Molecular Detection of Dirofilaria immitis Specific Gene from Infected Dog Blood Sample Using Polymerase Chain Reaction. Iran J. Parasitol. 2017, 12, 433–440. [Google Scholar]
- Reif, U.; Probst, C.W. Comparison of tibial plateau angles in normal and cranial cruciate deficient stifles of labrador retrievers. Vet. Surg. 2003, 32, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Dismukes, D.I.; Tomlinson, J.L.; Fox, D.B.; Cook, J.L.; Witsberger, T.H. Radiographic measurement of canine tibial angles in the sagittal plane. Vet. Surg. 2008, 37, 300–305. [Google Scholar] [CrossRef]
- Bailey, C.J.; Smith, B.A.; Black, A.P. Geometric implications of the tibial wedge osteotomy for the treatment of cranial cruciate ligament disease in dogs. Vet. Comp. Orthop. Traumatol. 2007, 20, 169–174. [Google Scholar] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 8, 420–428. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Comerford, E.J.; Smith, K.; Hayashi, K. Update on the aetiopathogenesis of canine cranial cruciate ligament disease. Vet. Comp. Orthopaed. 2011, 242, 91–98. [Google Scholar]
- Muir, P.; Kelly, J.L.; Marvel, S.J.; Heinrich, D.A.; Schaefer, S.L.; Manley, P.A.; Tewari, K.; Singh, A.; Suresh, M.; Hao, Z.; et al. Lymphocyte populations in joint tissues from dogs with inflammatory stifle arthritis and associated degenerative cranial cruciate ligament rupture. Vet Surg. 2011, 40, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P. Arthritis and Allied Conditions. A Textbook of Rheumatology, 15th ed.; Koopman, W.J., Moreland, L.W., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 2199–2226. [Google Scholar]
- Schmidli, M.R.; Fuhrer, B.; Kurt, N.; Senn, D.; Drögemüller, M.; Rytz, U.; Spreng, D.E.; Forterre, S. Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease. BMC Vet. Res. 2018, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.D.; Miao, W.H.; Zhang, Y.Y.; Zou, M.J.; Yan, X.F. Inhibition of miR-126 protects chondrocytes from IL-1β induced inflammation via upregulation of Bcl-2. Bone Jt. Res. 2018, 7, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.M.; Winyard, P.G.; Ryan, B.; Harries, L.W.; Haigh, R.; Viner, N.; Eggleton, P. Extracellular calreticulin is present in the joints of patients with rheumatoid arthritis and inhibits FasL (CD95L)-mediated apoptosis of T cells. Arthritis Rheum. 2010, 62, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Malek, S.; Weng, H.-Y.; Martinson, S.A.; Rochat, M.C.; Be’raud, R.; Riley, C.B. Evaluation of serum MMP-2 and MMP-3, synovial fluid IL-8, MCP-1, and KC concentrations as biomarkers of stifle osteoarthritis associated with naturally occurring cranial cruciate ligament rupture in dogs. PLoS ONE 2020, 15, e0242614. [Google Scholar] [CrossRef]
- Garner, B.C.; Stoker, A.M.; Kuroki, K.; Evans, R.; Cook, C.R.; Cook, J.L. Using animal models in osteoarthritis biomarker research. J. Knee Surg. 2011, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Qi, C.; Liu, Y.; Gao, H.; Zhao, D.; Jiang, Y. Increased frequency of peripheral blood follicular helper T cells and elevated serum IL 21 levels in patients with knee osteoarthritis. Mol. Med. Rep. 2017, 15, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, C.E.; Smale, S.; Carty, S.M.; Nicholas, A.; Sarah, N.L.; Rhian, M.G.; Peter, J.R.; Simon, A.J.; Nicholas, T.; Anwen, S.W. Interferon-gamma inhibits interleukin-1beta-induced matrix metalloproteinase production by synovial fibroblasts and protects articular cartilage in early arthritis. Arthritis Res. Ther. 2010, 12, R49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starling, E.H. On the Absorption of Fluids from the Connective Tissue Spaces. J. Physiol. 1896, 19, 312–326. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Li, Y.Q.; Chen, C.; Cai, W.; Zeng, Y.L. The role of PPARγ in advanced glycation end products induced inflammatory response in human chondrocytes. PLoS ONE 2015, 10, 0125776. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, Y.; Huang, Y.; Liu, S.; Lu, H.; Sun, T. Cadherin-11 involves in synovitis and increases the migratory and invasive capacity of fibroblast-like synoviocytes of osteoarthritis. Int. Immunopharmacol. 2015, 26, 153–161. [Google Scholar] [CrossRef]
- Xu, L.; Sun, C.; Zhang, S.; Xu, X.; Zhai, L.; Wang, Y.; Wang, S.; Liu, Z.; Cheng, H.; Xiao, M.; et al. Sam68 promotes NF-κB activation and apoptosis signaling in articular chondrocytes during osteoarthritis. Inflamm. Res. 2015, 64, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, B.; Li, Y.; Zhang, S.; Li, Z. Blocking of the P2X7 receptor inhibits the activation of the MMP-13 and NF-κB pathways in the cartilage tissue of rats with osteoarthritis. Int. J. Mol. Med. 2016, 38, 1922–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özler, K.; Aktaş, E.; Atay, Ç.; Yılmaz, B.; Arıkan, M.; Güngör, Ş. Serum and knee synovial fluid matrixmetalloproteinase-13 and tumor necrosis factor-alpha levels in patients with late stage osteoarthritis. Acta Orthop. Traumatol. Turc. 2016, 50, 670–673. [Google Scholar] [CrossRef]
- Klein-Wieringa, I.R.; Kloppenburg, M.; Bastiaansen-Jenniskens, Y.M.; Yusuf, E.; Kwekkeboom, J.C.; El-Bannoudi, H.; Nelissen, R.G.; Zuurmond, A.; Stojanovic-Susulic, V.; Van Osch, G.J. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann. Rheum. Dis. 2011, 705, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Clements, D.N.; Carter, S.D.; Innes, J.F.; Ollier, W.E.; Day, P.J. Gene expression profiling of normal and ruptured canine anterior cruciate ligaments. Osteoarthr. Cartil. 2008, 16, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breshears, L.A.; Cook, J.L.; Stoker, A.M.; Fox, D.B.; Luther, J.K. The effect of uniaxial cyclic tensile load on gene expression in canine cranial cruciate ligamentocytes. Vet. Surg. 2010, 39, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Kanno, N.; Harada, Y.; Yogo, T.; Tagawa, M.; Hara, Y. Histological and immunohistological analysis of degenerative changes in the cranial cruciate ligament in a canine model of excessive tibial plateau angle. Vet. Comp. Orthop. Traumatol. 2015, 28, 240–249. [Google Scholar] [PubMed]
- Zachos, T.A.; Arnoczky, S.P.; Lavagnino, M.; Tashman, S. The effect of cranial cruciate ligament insufficiency on caudal cruciate ligament morphology: An experimental study in dogs. Vet. Surg. 2002, 31, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Abramowitch, S.D.; Kilger, R.; Liang, R. Biomechanics of knee ligaments: Injury, healing, and repair. J. Biomech. 2006, 39, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kielty, C.M.; Heagerty, A.M.; Canfield, A.E.; Shuttleworth, C.A. Upregulation of collagen VIII following porcine coronary artery angioplasty is related to smooth muscle cell migration not angiogenesis. Int. J. Exp. Pathol. 2001, 82, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Vracko, R.; Thorning, D.; Frederickson, R.G. Spatial arrangements of microfibrils in myocardial scars: Application of antibody to fibrillin. J. Mol. Cell Cardiol. 1990, 22, 749–757. [Google Scholar] [CrossRef]
- Fricke, M.; Langer, C.; Brunner, E.; Sakai, L.Y.; Füzesi, L.; Reinhardt, D.P.; Quondamatteo, F. Fibrillin-1 in incisional hernias: An immunohistochemical study in scar and non-scar regions of human skin and muscle fasciae. J. Anat. 2008, 212, 674–685. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Maria Borzi, R.; Goldring, M.B. Goldring. NF-κB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Guénégo, L.; Serri, P.; Charru, P.; Verwaerde, P. Comparison of the Tibial Anatomical-Mechanical Axis Angle and Patellar Positions between Labrador Retrievers and Golden Retrievers with and without Cranial Cruciate Ligament Rupture. J. Vet. Sci. Res. 2020, 5, 000199. [Google Scholar]


| Genes | Stability Measure M value |
|---|---|
| GAPDH | 0.333 |
| RPL13A | 0.404 |
| TBP | 0.495 |
| Primer | Sequence 5′–3′ | References |
|---|---|---|
| IL 1β Fw | CTGTGTGATGAAGGATGGAA | [36] |
| IL-1β Rev | AATCGCTTTTCCATCTTCCT | |
| IL-8 Fw | GAACCGCAATCCTACTTTTG | |
| IL-8 Rev | GATCATTCAACCCAGCATTG | |
| IL-10 Fw | TGCATGGCTCAGCACTGCTCTGTTG | [37] |
| IL-10 Rev | AGTGGGTGCAGTCGTCCTCAAGTAG | |
| TNF-α Fw | TCTCGAACCCCAAGTGACAAG | [36] |
| TNF-α Rev | CAACCCATCTGACGGCACTA | |
| IFN-γ Fw | AAGATCAGCTGAGTCCTTTG | |
| IFN-γ Rev | AAATCACGCAAAGCTGAAAA | |
| Col1a1 Fw | AGAGCATGACCGACGGATTC | [38] |
| Col1a1 Rev | ACGCTGTTCTTGCAGTGGTA | |
| Col1a3 Fw | CTGAAGGAAACAGCAAATTC | [39] |
| Col1a3 Rev | ATTCCCCAGTGTGTTTAGTG | |
| ELN Fw | GGCCTGGGAATTGGTGGTAA | [40] |
| ELN Rev | CTCTTCCGGCCACAGGATTT | |
| GAPDH Fw | CATGTTTGTGATGGGCGTGAA | [41] |
| GAPDH Rev | GATGACTTTGGCTAGAGGAGC |
| Group | No. of Stifles | MMT | Sex (M/F) | RS (M/F) | Age (y) | BW (kg) |
|---|---|---|---|---|---|---|
| CrCL R | 50 | 22 | 22/28 | 12/18 | 5.9 ± 2.3 | 32.3 ± 10.8 |
| Complete | 27 | 17 | 13/14 | 8/10 | 6 ± 2 | 30.5 ± 11 |
| Partial | 23 | 5 | 9/14 | 7/5 | 5.9 ± 2.9 | 35.5 ± 10.1 |
| Control | 15 | - | 9/8 | - | 8.2 ± 3.8 | 22 ± 12.8 |
| Parameter | PB | SF |
|---|---|---|
| IL 10 | 0.30 | 0.21 |
| IL-1b | 0.38 | 0.46 |
| IL-8 | 0.06 | 0.43 |
| TNF-α | 0.48 | 0.42 |
| IFN-γ | 0.49 | 0.26 |
| Col 1 | - | 0.18 |
| Col 3 | - | 0.22 |
| ELN | - | 0.09 |
| Group | AMA | TPA | ||
|---|---|---|---|---|
| Mean ± SD | Median; IQR | Mean ± SD | Median; IQR | |
| CrCL R | 2.99 ± 1.03 | 2.9; 1.17 | 24.96 ± 2.84 | 25.45; 3.93 |
| Complete | 3.02 ± 1.20 | 2.55; 1.48 | 25.01 ± 3.24 | 25.69; 4.35 |
| Partial | 2.8 ± 0.6 | 2.95; 1.02 | 25.23 ± 2.49 | 25; 4.26 |
| Control | 1.81 ± 0.29 | 1.94; 0.45 | 24.91 ± 1.15 | 25.05; 1.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ševčík, K.; Karaffová, V.; Hluchý, M.; Ševčíková, M.; Ševčíková, Z.; Ledecký, V. Relationship of mRNA Expression of Selected Genes in Peripheral Blood and Synovial Fluid in Cranial Cruciate Ligament Deficient Stifles of Dogs. Animals 2022, 12, 754. https://doi.org/10.3390/ani12060754
Ševčík K, Karaffová V, Hluchý M, Ševčíková M, Ševčíková Z, Ledecký V. Relationship of mRNA Expression of Selected Genes in Peripheral Blood and Synovial Fluid in Cranial Cruciate Ligament Deficient Stifles of Dogs. Animals. 2022; 12(6):754. https://doi.org/10.3390/ani12060754
Chicago/Turabian StyleŠevčík, Karol, Viera Karaffová, Marián Hluchý, Marieta Ševčíková, Zuzana Ševčíková, and Valent Ledecký. 2022. "Relationship of mRNA Expression of Selected Genes in Peripheral Blood and Synovial Fluid in Cranial Cruciate Ligament Deficient Stifles of Dogs" Animals 12, no. 6: 754. https://doi.org/10.3390/ani12060754
APA StyleŠevčík, K., Karaffová, V., Hluchý, M., Ševčíková, M., Ševčíková, Z., & Ledecký, V. (2022). Relationship of mRNA Expression of Selected Genes in Peripheral Blood and Synovial Fluid in Cranial Cruciate Ligament Deficient Stifles of Dogs. Animals, 12(6), 754. https://doi.org/10.3390/ani12060754







