Evaluation of the Taxonomic Status of Lesser Egyptian Jerboa, Jaculus jaculus: First Description of New Phylogroups in Tunisia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
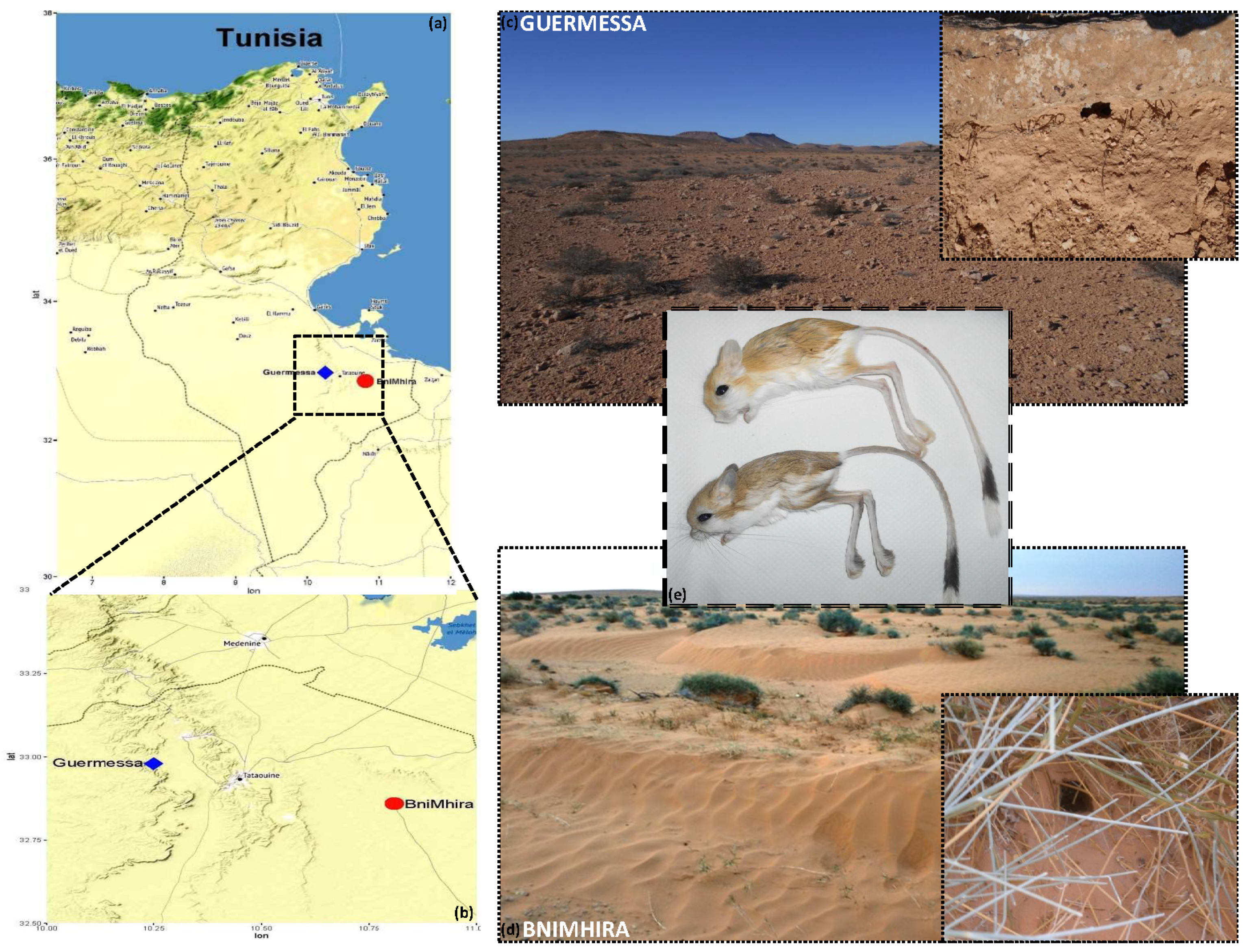
2.2. Study Sites and Sampling
2.3. Morphometric Measurements
2.4. Rodents Age Categorization
2.5. Statistical Analysis
2.6. DNA Extraction and Polymerase Chain Reaction (PCR) Conditions
2.7. DNA Sequencing
2.8. Maximum Likelihood Phylogenetic Analyses
2.9. Genetic Diversity and Polymorphism Analysis
2.10. Genetic Differentiation and Gene Flow among Rodent’s Species and Their Phylogroups
3. Results
3.1. Rodents Sampling and Morphological Measurements
3.2. Rodents Age Categorization
3.3. Phylogenetic Analyses
3.4. Genetic Diversity and Polymorphism Analysis
3.5. Genetic Differentiation and Gene Flow among Rodent Species and Their Identified Groups and Phylogroups
4. Discussion
4.1. Rodent’s Morphometry
4.2. Rodent’s Age Categorization
4.3. Rodent Species Phylogenetic and Phylogroup Description
4.4. Genetic Diversity, Polymorphism, and Differentiation among Rodent Species, Groups Belonging to Biotopes, and Phylogroups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ben Faleh, A.; Cosson, J.F.; Tatard, C.; Ben Othmen, A.; Said, K.; Granjon, L. Are there two cryptic species of the lesser jerboa Jaculus jaculus (Rodentia: Dipodidae) in Tunisia? Evidence from molecular, morphometric, and cytogenetic data. Biol. J. Linn. Soc. 2010, 99, 673–686. [Google Scholar] [CrossRef]
- Boratyński, Z.; Brito, J.C.; Campos, J.C.; Karala, M.; Mappes, T. Large spatial scale of the phenotype-environment color matching in two cryptic species of African desert jerboas (Dipodidae: Jaculus). PLoS ONE 2014, 9, e94342. [Google Scholar] [CrossRef] [PubMed]
- Ranck, G.L. The rodents of Libya: Taxonomy, ecology, and zoogeographical relationships. Bull. US Natl. Mus. 1968, 275, 220–244. [Google Scholar] [CrossRef]
- Moutinho, A.F.; Seren, N.; Pauperio, J.; Silva, T.L.; Martinez-Freiria, F.; Sotelo, G.; Faria, R.; Mappes, T.; Alves, P.C.; Brito, J.C.; et al. Evolutionary history of two cryptic species of northern African jerboas. BMC Evol. Biol. 2020, 20, 26. [Google Scholar] [CrossRef]
- Ben Faleh, A.; Ben Othmen, A.; Said, K. Taxonomy of the lesser jerboa Jaculus jaculus (Dipodidae, Rodentia) based on allozymic and morphological variation. Curr. Zool. 2010, 56, 421–431. [Google Scholar] [CrossRef]
- Ben Faleh, A.; Ben Othmen, A.; Said, K.; Granjon, L. Karyotypic variation in two species of jerboas Jaculus jaculus and Jaculus orientalis (Rodentia, Dipodidae) from Tunisia. Folia Biol. 2010, 58, 229–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ben Faleh, A.; Cornette, R.; Annabi, A.; Said, K.; Denys, C. Patterns of size and shape skull variability in Tunisian populations of Jaculus jaculus (Rodentia: Dipodidae). Acta Zool. Bulg. 2013, 65, 217–223. [Google Scholar]
- Ben Faleh, A.; Granjon, L.; Tatard, C.; Boratynski, Z.; Cosson, J.F.; Said, K. Phylogeography of two cryptic species of African desert jerboas (Dipodidae: Jaculus). Biol. J. Linn. Soc. 2012, 107, 27–38. [Google Scholar] [CrossRef]
- Boratynski, Z.; Brito, J.C.; Mappes, T. The origin of two cryptic species of African desert jerboas (Dipodidae: Jaculus). Biol. J. Linn. Soc. 2012, 105, 435–445. [Google Scholar] [CrossRef]
- Shenbrot, G.; Feldstein, T.; Meiri, S. Are cryptic species of the Lesser Egyptian Jerboa, Jaculus jaculus (Rodentia, Dipodidae), really cryptic? Re-evaluation of their taxonomic status with new data from Israel and Sinai. J. Zool. Syst. Evol. Res. 2016, 54, 148–159. [Google Scholar] [CrossRef]
- Shahin, A.A.B. Genetic differentiation and relationship of the dipodids Allactaga and Jaculus (Mammalia, Rodentia) in Egypt based on protein variation. Acta Theriol. 2003, 48, 309–324. [Google Scholar] [CrossRef]
- Ben Faleh, A.; Granjon, L.; Tatard, C.; Cosson, J.F.; Said, K. Phylogeography of two cryptic species of African desert jerboas (Dipodidae: Jaculus). Biol. J. Linn. Soc. 2013, 108, 470–471. [Google Scholar]
- Ben Faleh, A.; Shahin, A.A.; Said, K. Allozyme polymorphism and genetic differentiation among populations of Jaculus jaculus and J. orientalis (Rodentia: Dipodidae) in Tunisia. Zool. Res. 2009, 3, 247–254. [Google Scholar]
- Eymann, J.; Degreef, J.; Häuser, C.; Monje, J.; Samyn, Y.; VandenSpiegel, D. Manual on Field Recording Techniques and Protocols for All Taxa Biodiversity Inventories and Monitoring; Abc Taxa: Brussels, Belgium, 2010. [Google Scholar]
- Jacques, M.-E.; McBee, K.; Elmore, D. Determining Sex and Reproductive Status of Rodents; Oklahoma Cooperative Extension Service NREM-2896; Division of Agricultural Sciences and Natural Resources, Oklahoma State University: Stillwater, OK, USA, 2015. [Google Scholar]
- Hadjoudj, M.; Manaa, A.; Derdoukh, W.; Guerzou, A.; Souttou, K.; Sekour, M.; Doumandji, S. Les Rongeurs de la Région de Touggourt; Mémoire Ing. Agro, Insti Nati Agr. El Harrach: Alger, Algeria, 2011; pp. 244–251. [Google Scholar]
- Fichet-Calvet, E.; Jomaa, I.; Ben Ismail, R.; Ashford, R.W. Leishmania major infection in the fat sand rat Psammomys obesus in Tunisia: Interaction of host and parasite populations. Ann. Trop. Med. Parasitol. 2003, 97, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Granjon, L.; Duplantier, J.-M. Les Rongeurs de l’Afrique Sahélo-Soudanienne; Muséum National d’Histoire Naturelle: Paris, France, 2009. [Google Scholar]
- Morris, P. A review of mammalian age determination methods. Mamm. Rev. 1972, 2, 69–104. [Google Scholar] [CrossRef]
- Higgins, D.G.; Thompson, J.D.; Gibson, T.J. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996, 266, 383–402. [Google Scholar] [CrossRef]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Tajima, F. Problems Arising in Phylogenetic Inference from Restriction-Site Data. Mol. Biol. Evol. 1987, 4, 320–323. [Google Scholar]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Curry, F.R.; Adamson, R.H.; Weinbaum, S. A model for interpreting the tracer labeling of interendothelial clefts. Ann. Biomed. Eng. 1997, 25, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.R. A new statistic for detecting genetic differentiation. Genetics 2000, 155, 2011–2014. [Google Scholar] [CrossRef]
- Hudson, R.R.; Boos, D.D.; Kaplan, N.L. A statistical test for detecting geographic subdivision. Mol. Biol. Evol. 1992, 9, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Tsompana, M.; Abad, J.; Purugganan, M.; Moyer, J.W. The molecular population genetics of the Tomato spotted wilt virus (TSWV) genome. Mol. Ecol. 2005, 14, 53–66. [Google Scholar] [CrossRef]
- Gao, F.; Lin, W.; Shen, J.; Liao, F. Genetic diversity and molecular evolution of arabis mosaic virus based on the CP gene sequence. Arch. Virol. 2016, 161, 1047–1051. [Google Scholar] [CrossRef]
- Ben Faleh, A.; Allaya, H.; Quignard, J.P.; Shahin, A.A.A.B.; Trabelsi, M. Patterns of skull variation in relation to some geoclimatic conditions in the greater jerboa Jaculus orientalis (Rodentia, Dipodidae) from Tunisia. Turkish J. Zool. 2016, 40, 900–909. [Google Scholar] [CrossRef]
- Ben Faleh, A.; Allaya, H.; Shahin, A.A.A.B. Geographic patterns of genetic variation in the greater Egyptian jerboa Jaculus orientalis (Dipodidae, Rodentia) from Tunisia. Biochem. Syst. Ecol. 2016, 68, 15–22. [Google Scholar] [CrossRef]
- Happold, D. Biology of the jerboa, Jaculus jaculus butleri (Rodentia, Dipodidae), in the Sudan. J. Zool. 1967, 151, 257–275. [Google Scholar] [CrossRef]
- Maatoug, H. Etude Morpho-Métrique des Rongeurs dans la Région du Souf. Master’s Thesis, Université El Oued, El Oued, Algeria, 2018. [Google Scholar]
- Ghawar, W.; Snoussi, M.-A.; Salem, S.; Jaouadi, K.; Zaatour, W.; Yazidi, R.; Bettaieb, J. Morphometric variation and its relation to the eyes lens weight among three species of wild rodents in Tunisia. Int. J. Fauna Biol. Stud. 2017, 4, 30–38. [Google Scholar]
- Lalis, A.; Evin, A.; Janier, M.; Koivogui, L.; Denys, C. Host evolution in Mastomys natalensis (Rodentia: Muridae): An integrative approach using geometric morphometrics and genetics. Integr. Zool. 2015, 10, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, B.M. Systematics, Distribution, and Zoogeography of Mammals of Tunisia; Texas Tech University: Lubbock, TX, USA, 1997. [Google Scholar]
- Pisano, J.; Condamine, F.L.; Lebedev, V.; Bannikova, A.; Quere, J.P.; Shenbrot, G.I.; Pages, M.; Michaux, J.R. Out of Himalaya: The impact of past Asian environmental changes on the evolutionary and biogeographical history of Dipodoidea (Rodentia). J. Biogeogr. 2015, 42, 856–870. [Google Scholar] [CrossRef]
- Corbet, G.B. The Mammals of the Palaearctic Region: A Taxonomic Review; British Museum (Natural History): London, UK, 1978. [Google Scholar]
- Mohammadi, S.; Ebrahimi, E.; Moghadam, M.S.; Bosso, L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol. Inform. 2019, 52, 7–13. [Google Scholar] [CrossRef]
- Stumpf, M.P. Haplotype diversity and SNP frequency dependence in the description of genetic variation. Eur. J. Hum. Genet. 2004, 12, 469–477. [Google Scholar] [CrossRef] [PubMed]

| Mean | SD | Minimum | Maximum | IQR | |
|---|---|---|---|---|---|
| Weight (g) | 42.52 | 9.42 | 25 | 65 | 35–50.5 |
| Head and body length (mm) | 108.13 | 8.89 | 90 | 128 | 100.75–115 |
| Tail length (mm) | 177.58 | 9.09 | 160 | 196 | 170–184.25 |
| Ear length (mm) | 21.36 | 1.58 | 18 | 24 | 20–22.25 |
| Hind feet length (mm) | 62.5 | 1.87 | 59 | 67 | 61–64 |
| ELW (mg) | 75.26 | 28.47 | 44 | 148 | 56.25–99.5 |
| J. jaculus | J. hirtipes | p * | |
|---|---|---|---|
| Number of specimens | 21 | 20 | |
| Mean of body measurements (±SD) | |||
| Weight (g) | 41.29 ± 9.20 | 44.60 ± 9.35 | 0.26 |
| Head and body length (mm) | 106.71 ± 7.77 | 109.10 ± 9.19 | 0.37 |
| Tail length (mm) | 176.38 ± 9.10 | 178.55 ± 9.04 | 0.449 |
| Ear length (mm) | 20.52 ± 1.40 | 22.25 ± 1.20 | <10−3 |
| Hind feet length (mm) | 63.29 ± 1.79 | 62 ± 1.65 | 0.022 |
| Mean of ELW (±SD) (mg) | 79.19 ± 31.05 | 73.1 ± 27.28 | 0.509 |
| Number | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Juveniles (mg) | 14 | 51.43 | 4.65 | 44 | 58 |
| Adults (mg) | 32 | 85.69 | 28.26 | 59 | 148 |
| n | H | Hd ± SD | S | π ± SD | K | Tajima’s D | Fu’s FS | |
|---|---|---|---|---|---|---|---|---|
| Sequences from the present study | ||||||||
| 41 | 38 | 0.995 ± 0.007 | 141 | 0.071 ± 0.002 | 49.052 | 1.032 | −6.648 | |
| According to species identification | ||||||||
| J. jaculus | 21 | 20 | 0.995 ± 0.016 | 82 | 0.021 ± 0.002 | 17.347 | −1.212 | −6.445 |
| J. hirtipes | 20 | 18 | 0.984 ± 0.024 | 68 | 0.022 ± 0.002 | 17.273 | −0.455 | −4.038 |
| According to the biotope | ||||||||
| BNIMHIRA | ||||||||
| J. jaculus | 13 | 12 | 0.987 ± 0.035 | 62 | 0.020 ± 0.002 | 16.923 | −0.866 | −1.904 |
| J. hirtipes | 13 | 13 | 1 ± 0.03 | 67 | 0.026 ± 0.004 | 19.012 | −0.597 | −3.384 |
| GUERMESSA | ||||||||
| J. jaculus | 8 | 8 | 1 ± 0.063 | 54 | 0.022 ± 0.002 | 19.571 | −0.506 | −0.949 |
| J. hirtipes | 7 | 5 | 0.857 ± 0.137 | 37 | 0.018 ± 0.004 | 16.190 | 0.413 | 2.704 |
| Tunisian sequences retrieved from the GenBank | ||||||||
| 41 | 35 | 0.988 ± 0.01 | 170 | 0.053 ± 0.004 | 48.559 | 0.691 | −3.132 | |
| Whole Dataset | ||||||||
| 82 | 67 | 0.991 ± 0.005 | 182 | 0.065 ± 0.002 | 43.329 | 0.109 | −14.946 | |
| According to the species identification | ||||||||
| J. jaculus | 50 | 44 | 0.99 ± 0.008 | 151 | 0.022 ± 0.002 | 17.779 | −1.893 * | −21.532 |
| J. hirtipes | 32 | 26 | 0.98 ± 0.015 | 75 | 0.022 ± 0.002 | 15.792 | −0.653 | −6.682 |
| According to the phylogroups | ||||||||
| J. jaculus | ||||||||
| Phylogroup 1 | 9 | 8 | 0.972 ± 0.064 | 50 | 0.016 ± 0.002 | 15.388 | −0.917 | −0.190 |
| Phylogroup 2 | 41 | 36 | 0.987 ± 0.012 | 123 | 0.018 ± 0.002 | 14.793 | −1.990 * | −17.345 |
| J. hirtipes | ||||||||
| Phylogroup 1 | 12 | 12 | 1 ± 0.034 | 51 | 0.016 ± 0.004 | 12.757 | −1.190 | −4.063 |
| Phylogroup 2 | 20 | 15 | 0.963 ± 0.028 | 24 | 0.005 ± 0.0007 | 4.715 | −1.168 | −6.981 |
| Comparison | Ks * | Kst * | Z * | Snn | Fst |
|---|---|---|---|---|---|
| J. jaculus vs. J. hirtipes Clade 1 vs. Clade 2 | 2.4471 a | 0.2716 a | 6.4423 a | 1 a | 0.812 |
| J. jaculus phylogroups | 2.5164 a | 0.0803 a | 5.8349 a | 1 a | 0.4618 |
| Phylogroup 1 vs. Phylogroup 2 | |||||
| J. hirtipes phylogroups | 1.7876 a | 0.2762 a | 4.6813 a | 1 a | 0.6961 |
| Phylogroup 1 vs. Phylogroup 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghawar, W.; Chaouch, M.; Ben Abderrazak, S.; Snoussi, M.A.; Salem, S.; Chouchen, S.; Bouaoun, A.; Ben Salah, A.; Bettaieb, J. Evaluation of the Taxonomic Status of Lesser Egyptian Jerboa, Jaculus jaculus: First Description of New Phylogroups in Tunisia. Animals 2022, 12, 758. https://doi.org/10.3390/ani12060758
Ghawar W, Chaouch M, Ben Abderrazak S, Snoussi MA, Salem S, Chouchen S, Bouaoun A, Ben Salah A, Bettaieb J. Evaluation of the Taxonomic Status of Lesser Egyptian Jerboa, Jaculus jaculus: First Description of New Phylogroups in Tunisia. Animals. 2022; 12(6):758. https://doi.org/10.3390/ani12060758
Chicago/Turabian StyleGhawar, Wissem, Melek Chaouch, Souha Ben Abderrazak, Mohammed Ali Snoussi, Sadok Salem, Said Chouchen, Amor Bouaoun, Afif Ben Salah, and Jihene Bettaieb. 2022. "Evaluation of the Taxonomic Status of Lesser Egyptian Jerboa, Jaculus jaculus: First Description of New Phylogroups in Tunisia" Animals 12, no. 6: 758. https://doi.org/10.3390/ani12060758
APA StyleGhawar, W., Chaouch, M., Ben Abderrazak, S., Snoussi, M. A., Salem, S., Chouchen, S., Bouaoun, A., Ben Salah, A., & Bettaieb, J. (2022). Evaluation of the Taxonomic Status of Lesser Egyptian Jerboa, Jaculus jaculus: First Description of New Phylogroups in Tunisia. Animals, 12(6), 758. https://doi.org/10.3390/ani12060758






