Freezability of Dog Semen after Collection in Field Conditions and Cooled Transport
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
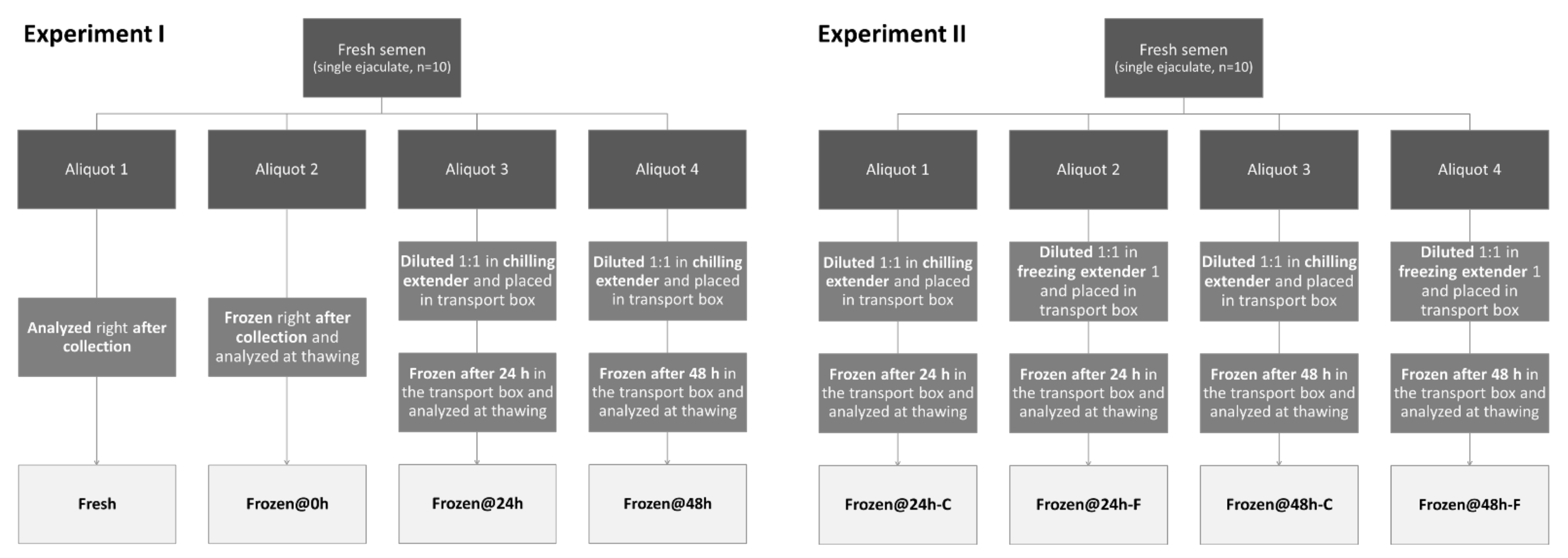
2.2. Animals, Spermatozoa Collection, and Experimental Design
- -
- One was analyzed at time zero, right after collection (Fresh);
- -
- One was frozen at time zero, right after collection, and analyzed at thawing (Frozen@0h);
- -
- One was diluted 1:1 in chilling extender [3], placed in the transport box, frozen after 24 h of storage, and analyzed at thawing (Frozen@24h);
- -
- One was diluted 1:1 in chilling extender [3], placed in the transport box, frozen after 48 h of storage, and analyzed at thawing (Frozen@48h).
- -
- One was diluted 1:1 in chilling extender [3], placed in the transport box, transported to the lab, frozen after 24 h of storage, and analyzed at thawing (Frozen@24h-C);
- -
- One was diluted 1:1 in freezing extender 1 [3], placed in the transport box, transported to the lab, frozen after 24 h of storage, and analyzed at thawing (Frozen@24h-F);
- -
- One was diluted 1:1 in chilling extender [3], placed in the transport box, transported to the lab, frozen after 48 h of storage, and analyzed at thawing (Frozen@48h-C);
- -
- One was diluted 1:1 in freezing extender 1 [3], placed in the transport box, transported to the lab, frozen after 48 h of storage, and analyzed at thawing (Frozen@48h-F).
2.3. Semen Analysis
2.3.1. Motility
2.3.2. Morphology
2.3.3. Membrane Integrity and Acrosome Status
2.3.4. Zona-Binding Assay (ZBA)
2.4. Semen-Freezing Procedure
2.5. Statistical Analysis
3. Results
3.1. Experiment I
3.2. Experiment II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linde-Forsberg, C. Achieving canine pregnancy by using frozen or chilled extended semen. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 467–485. [Google Scholar] [CrossRef]
- Linde-Forsberg, C.; Ström Holst, B.; Govette, G. Comparison of fertility data from vaginal vs intrauterine insemination of frozen-thawed dog semen: A retrospective study. Theriogenology 1999, 52, 11–23. [Google Scholar] [CrossRef]
- Linde-Forsberg, C. Regulations and recommendations for international shipment of chilled and frozen canine semen. Recent Adv. Small Anim. Reprod. 2001, 21, 1–5. [Google Scholar]
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank 2009, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Hesser, A.; Darr, C.; Gonzales, K.; Power, H.; Scanlan, T.; Thompson, J.; Love, C.; Christensen, B.; Meyers, S. Semen evaluation and fertility assessment in a purebred dog breeding facility. Theriogenology 2017, 87, 115–123. [Google Scholar] [CrossRef]
- Thomassen, R.; Sanson, G.; Krogenæs, A.; Fougner, J.A.; Andersen Berg, K.; Farstad, W. Artificial insemination with frozen semen in dogs: A retrospective study of 10 years using a non-surgical approach. Theriogenology 2006, 66, 1645–1650. [Google Scholar] [CrossRef]
- Mahiddine, F.Y.; Kim, M.J. Overview on the antioxidants, egg yolk alternatives, and mesenchymal stem cells and derivatives used in canine sperm cryopreservation. Animals 2021, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Leibo, S.P.; Songsasen, N. Cryopreservation of gametes and embryos of non-domestic species. Theriogenology 2002, 57, 303–326. [Google Scholar] [CrossRef]
- Miller, C.D. Optimizing the use of frozen–thawed equine semen. Theriogenology 2008, 70, 463–468. [Google Scholar] [CrossRef]
- Linde-Forsberg, C. Hints on dog semen freezing, cryoextenders, and frozen semen artificial insemination. In Proceedings of the Society for Theriogenology Meeting, Colorado Springs, CO, USA, 7–11 August 2002; pp. 303–320. [Google Scholar]
- Luvoni, G.C.; Colombo, M. Cold case: Small animal gametes cryobanking. Theriogenology 2020, 150, 445–451. [Google Scholar] [CrossRef]
- Hermansson, U.; Linde Forsberg, C. Freezing of stored, chilled dog spermatozoa. Theriogenology 2006, 65, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.; Batista, M.; Alamo, D.; González, F.; Niño, T.; Cabrera, F.; Gracia, A. Influence of cool storage before freezing on the quality of frozen-thawed semen samples in dogs. Reprod. Domest. Anim. 2013, 48, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ponglowhapan, S.; Chatdarong, K.; Sirivaidyapong, S.; Lohachit, C. Freezing of epididymal spermatozoa from dogs after cool storage for 2 or 4 days. Theriogenology 2006, 66, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Chatdarong, K.; Chaivechakarn, A.; Thuwanut, P.; Ponglowhapan, S. Effects of cold storage prior to freezing on superoxide dismutase, glutathione peroxidase activities, level of total reactive oxygen species and sperm quality in dogs. Reprod. Domest. Anim. 2012, 47, 274–277. [Google Scholar] [CrossRef]
- Hidalgo, M.; Portero, J.M.; Demyda-Peyrás, S.; Ortiz, I.; Dorado, J. Cryopreservation of canine semen after cold storage in a Neopor box: Effect of extender, centrifugation and storage time. Vet. Rec. 2014, 175, 20. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Morselli, M.G.; Colombo, M.; Faustini, M.; Luvoni, G.C. Morphological indices for canine spermatozoa based on the World Health Organization laboratory manual for human semen. Reprod. Domest. Anim. 2019, 54, 949–955. [Google Scholar] [CrossRef]
- England, G.; Plummer, J. Hypo-osmotic swelling of dog spermatozoa. J. Reprod. Fertil.—Suppl. 1993, 47, 261–270. [Google Scholar]
- Goericke-Pesch, S.; Failing, K. Retrospective analysis of canine semen evaluations with special emphasis on the use of the hypoosmotic swelling (HOS) test and acrosomal evaluation using Spermac®. Reprod. Domest. Anim. 2013, 48, 213–217. [Google Scholar] [CrossRef]
- Cheng, F.; Fazeli, A.; Voorhout, W.; Marks, A.; Bevers, M.; Colenbrander, B. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 1996, 17, 674–682. [Google Scholar]
- Ström Holst, B.; Larsson, B.; Linde-Forsberg, C.; Rodriguez-Martinez, H. Sperm binding capacity and ultrastructure of the zona pellucida of stored canine oocytes. J. Reprod. Fertil. 2000, 119, 77–83. [Google Scholar] [CrossRef]
- Beccaglia, M.; Anastasi, P.; Chigioni, S.; Luvoni, G.C. Tris-lecithin extender supplemented with antioxidant catalase for chilling of canine semen. Reprod. Domest. Anim. 2009, 44, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Crockett, E.C.; Graham, J.K.; Bruemmer, J.E.; Squires, E.L. Effect of cooling of equine spermatozoa before freezing on post-thaw motility: Preliminary results. Theriogenology 2001, 55, 793–803. [Google Scholar] [CrossRef]
- Backman, T.; Bruemmer, J.E.; Graham, J.K.; Squires, E.L. Pregnancy rates of mares inseminated with semen cooled for 18 h and then frozen. J. Anim. Sci. 2004, 82, 690–694. [Google Scholar] [CrossRef] [PubMed]
- López-Urueña, E.; Alvarez, M.; Gomes-Alves, S.; Anel-López, L.; Martínez-Rodríguez, C.; Manrique, P.; Borragan, S.; Anel, L.; de Paz, P. Optimization of conditions for long-term prefreezing storage of brown bear sperm before cryopreservation. Theriogenology 2015, 84, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Urueña, E.; Anel-López, L.; Borragan, S.; Ortega Ferrusola, C.; Manrique, P.; de Paz, P.; Anel, L.; Alvarez, M. The use of gelatine in long-term storage (up to 48 h) at 5 °C preserves the pre-freezing and post-thawing quality of brown bear sperm. Reprod. Domest. Anim. 2016, 51, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Sharma, R.; Gupta, S.; Sharma, R. NextGen home sperm banking kit: Outcomes of offsite vs onsite collection--preliminary findings. Urology 2015, 85, 1339–1346. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.; Singh, A.; Gupta, S.; Sharma, R. Standardisation of a novel sperm banking kit-NextGen®-to preserve sperm parameters during shipment. Andrologia 2016, 48, 662–669. [Google Scholar] [CrossRef]
- Mason, S.J. Current review of artificial insemination in dogs. Vet. Clin. N. Am.—Small Anim. Pract. 2018, 48, 567–580. [Google Scholar] [CrossRef]
- Ström, B.; Rota, A.; Linde-Forsberg, C. In vitro characteristics of canine spermatozoa subjected to two methods of cryopreservation. Theriogenology 1997, 48, 247–256. [Google Scholar] [CrossRef]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef]
- Burgess, C.M.; Clutterbuck, A.L.; England, G.C.W. The effect of cryopreservation on the capacitation status and epithelial cell attachment capability of dog spermatozoa. Vet. J. 2012, 192, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Peña, A.I.; Linde-Forsberg, C.; Rodriguez-Martinez, H. In vitro capacitation of fresh, chilled and frozen-thawed dog spermatozoa assessed by the chloretetracycline assay and changes in motility patterns. Anim. Reprod. Sci. 1999, 57, 199–215. [Google Scholar] [CrossRef]
- Burgess, C.M.; Bredl, J.C.; Plummer, J.M.; England, G.C. Vital and ultrastructural changes in dog spermatozoa during cyopreservation. J. Reprod. Fertil. Suppl. 2001, 57, 357–363. [Google Scholar] [PubMed]
- Andersen, A.H.; Thinnesen, M.; Failing, K.; Goericke-Pesch, S. Effect of reduced glutathione (GSH) supplementation to Tris-egg yolk extender on chilled semen variables of dogs. Anim. Reprod. Sci. 2018, 198, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Alexopoulos, C.; Pontiki, E.; Hadjipavlou-Litina, D.; Saratsis, P.; Boscos, C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology 2007, 68, 204–212. [Google Scholar] [CrossRef]
- Kawakami, E.; Vandevoort, C.A.; Mahi-Brown, C.A.; Overstreet, J.W. Induction of acrosome reactions of canine sperm by homologous zona pellucida. Biol. Reprod. 1993, 48, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Burkman, L.J.; Coddington, C.C.; Franken, D.R.; Kruger, T.F.; Rosenwaks, Z.; Hodgen, G.D. The hemizona assay (HZA): Development of a diagnostic test for the binding of human spermatozoa to the human hemizona pellucida to predict fertilization potential. Fertil. Steril. 1988, 49, 688–697. [Google Scholar] [CrossRef]
| MAI | TZI | SDI | Intact Acrosomes (%) | Intact Membranes (%) | |
|---|---|---|---|---|---|
| Fresh | 1.16 ± 0.28 a | 0.99 ± 0.42 a | 0.43 ± 0.28 a | 89.91 ± 10.51 a | 89.87 ± 7.67 a |
| Frozen@0h | 1.59 ± 0.11 b | 1.51 ± 0.11 b | 1.48 ± 0.18 b | 52.27 ± 18.22 b | 65.37 ± 17.44 b |
| Frozen@24h | 1.61 ± 0.26 b | 1.56 ± 0.24 b | 1.52 ± 0.28 b | 42.1 ± 25.22 b | 62.41 ± 21.69 b |
| Frozen@48h | 1.55 ± 0.23 b | 1.48 ± 0.18 b | 1.47 ± 0.24 b | 51.1 ± 16.86 b | 62.02 ± 16.05 b |
| Motility Pre-Freezing (%) | Motility at Thawing (%) | Motility 6 h Post-Thawing (%) | |
|---|---|---|---|
| Frozen@0h | 86 ± 13.5 a | 45.5 ± 19.5 a | 11.3 ± 10.41 a |
| Frozen@24h | 73 ± 17.67 a, b | 41.3 ± 23.69 a | 8.3 ± 15.15 a |
| Frozen@48h | 58.1 ± 23.68 b | 27 ± 18.74 a | 4.78 ± 4.32 a |
| Spermatozoa Per Oocyte (n) | Spermatozoa Per “Bound Oocyte” 1 (n) | Oocytes with Bound Spermatozoa (%) | |
|---|---|---|---|
| Fresh | 9.18 ± 7.93 | 30.05 ± 60.17 | 56.57 ± 33.57 |
| Frozen@0h | 7.77 ± 10.02 | 10.96 ± 11.71 | 55.5 ± 30.95 |
| Frozen@24h | 7.12 ± 10.19 | 10.13 ± 13.73 | 48.11 ± 28.43 |
| Frozen@48h | 10.71 ± 9.2 | 13.68 ± 9.05 | 64.44 ± 33.41 |
| MAI | TZI | SDI | Intact Acrosomes (%) | Intact Membranes (%) | |
|---|---|---|---|---|---|
| Frozen@24h-C | 1.4 ± 0.36 | 1.30 ± 0.28 | 1.03 ± 0.6 | 62.77 ± 20.26 | 54.48 ± 14.95 |
| Frozen@24h-F | 1.43 ± 0.29 | 1.33 ± 0.32 | 1.12 ± 0.54 | 67.62 ± 18.13 | 57.92 ± 12.72 |
| Frozen@48h-C | 1.48 ± 0.26 | 1.39 ± 0.2 | 1.37 ± 0.34 | 63.29 ± 21.66 | 50.78 ± 19.38 |
| Frozen@48h-F | 1.48 ± 0.17 | 1.38 ± 0.13 | 1.35 ± 0.25 | 59.97 ± 12.68 | 58.66 ± 14.09 |
| Motility Pre-Freezing (%) | Motility at Thawing (%) | Motility 6 h Post-Thawing (%) | |
|---|---|---|---|
| Frozen@24h-C | 69 ± 21.83 a | 47 ± 16.36 a | 24.9 ± 23.67 a |
| Frozen@24h-F | 68 ± 18.74 a | 44 ± 21.19 a, b | 26.2 ± 20.79 a |
| Frozen@48h-C | 57.1 ± 28.47 a | 23 ± 15.49 b | 8.44 ± 12.42 a |
| Frozen@48h-F | 54.1 ± 24.79 a | 33.6 ± 21.44 a, b | 10.22 ± 10.97 a |
| Spermatozoa Per Oocyte (n) | Spermatozoa Per “Bound Oocyte” 1 (n) | Oocytes with Bound Spermatozoa (%) | |
|---|---|---|---|
| Frozen@24h-C | 21.79 ± 22.82 | 24.79 ± 24.3 | 79.22 ± 18.76 |
| Frozen@24h-F | 7.28 ± 11.32 | 8.78 ± 11.84 | 55.75 ± 36.02 |
| Frozen@48h-C | 7.75 ± 6.22 | 9.85 ± 6.69 | 77.67 ± 27.53 |
| Frozen@48h-F | 11.91 ± 11.24 | 14.13 ± 11.23 | 75 ± 22.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, M.; Morselli, M.G.; Franchi, G.; Schäfer-Somi, S.; Luvoni, G.C. Freezability of Dog Semen after Collection in Field Conditions and Cooled Transport. Animals 2022, 12, 816. https://doi.org/10.3390/ani12070816
Colombo M, Morselli MG, Franchi G, Schäfer-Somi S, Luvoni GC. Freezability of Dog Semen after Collection in Field Conditions and Cooled Transport. Animals. 2022; 12(7):816. https://doi.org/10.3390/ani12070816
Chicago/Turabian StyleColombo, Martina, Maria Giorgia Morselli, Giulia Franchi, Sabine Schäfer-Somi, and Gaia Cecilia Luvoni. 2022. "Freezability of Dog Semen after Collection in Field Conditions and Cooled Transport" Animals 12, no. 7: 816. https://doi.org/10.3390/ani12070816
APA StyleColombo, M., Morselli, M. G., Franchi, G., Schäfer-Somi, S., & Luvoni, G. C. (2022). Freezability of Dog Semen after Collection in Field Conditions and Cooled Transport. Animals, 12(7), 816. https://doi.org/10.3390/ani12070816








