Prevalence and Antimicrobial Susceptibility of Campylobacter Species with Particular Focus on the Growth Promoting, Immunostimulant and Anti-Campylobacter jejuni Activities of Eugenol and Trans-Cinnamaldehyde Mixture in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection
2.3. Isolation and Identification of Campylobacter Species
2.4. Antimicrobial Susceptibility Testing
2.5. Conventional PCR Assay
2.6. In Vivo Assessment of the Efficacy of the Eugenol and Trans-Cinnamaldehyde Mixture
2.6.1. Plant-Derived Antimicrobials
2.6.2. Experimental Infection by XDR and the Multi-Virulent C. jejuni Strain
2.6.3. Experimental Broiler Chickens, Design, and Feeding Regime
2.6.4. Monitoring the Growth Performance of the Broilers
2.6.5. Sampling
2.6.6. Gene Expression Analysis by the Reverse Transcription Quantitative PCR Assay
2.6.7. Quantification of the Campylobacter DNA Copies by Quantitative Real-Time PCR Assay
2.7. Statistical Analysis
3. Results
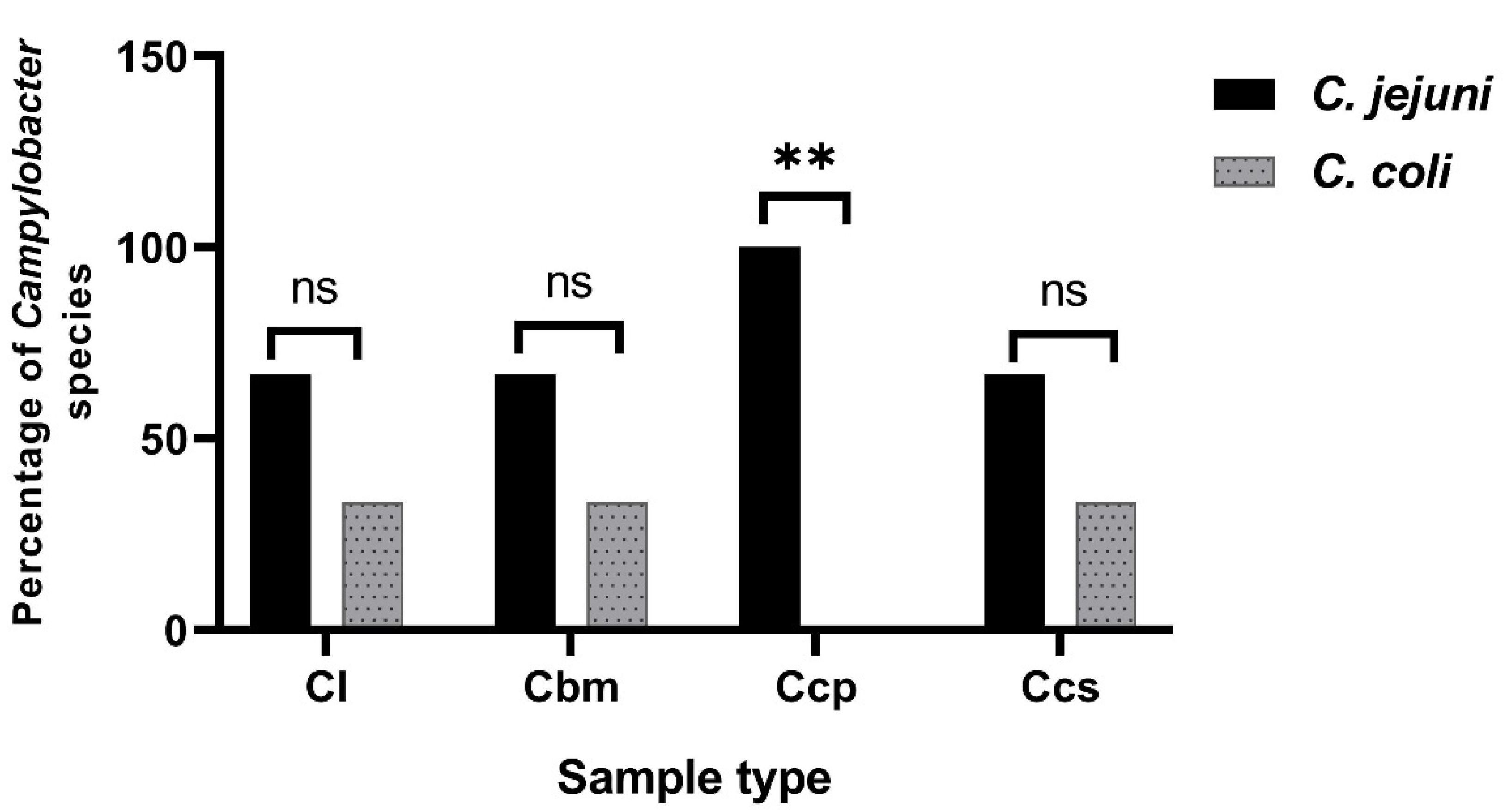
3.1. Prevalence of the Campylobacter Species in the Broiler Chickens in Sharkia Governorate, Egypt
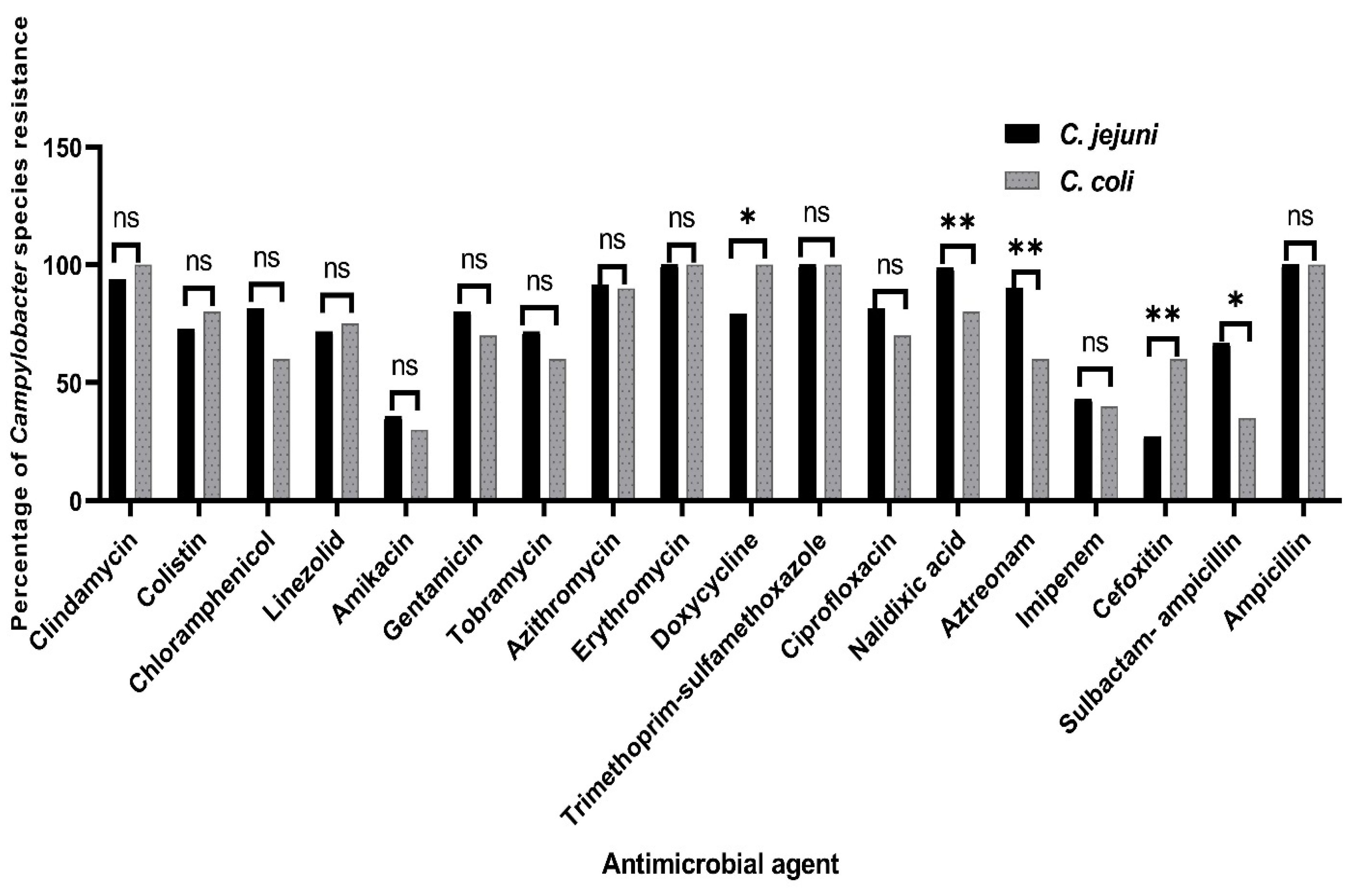
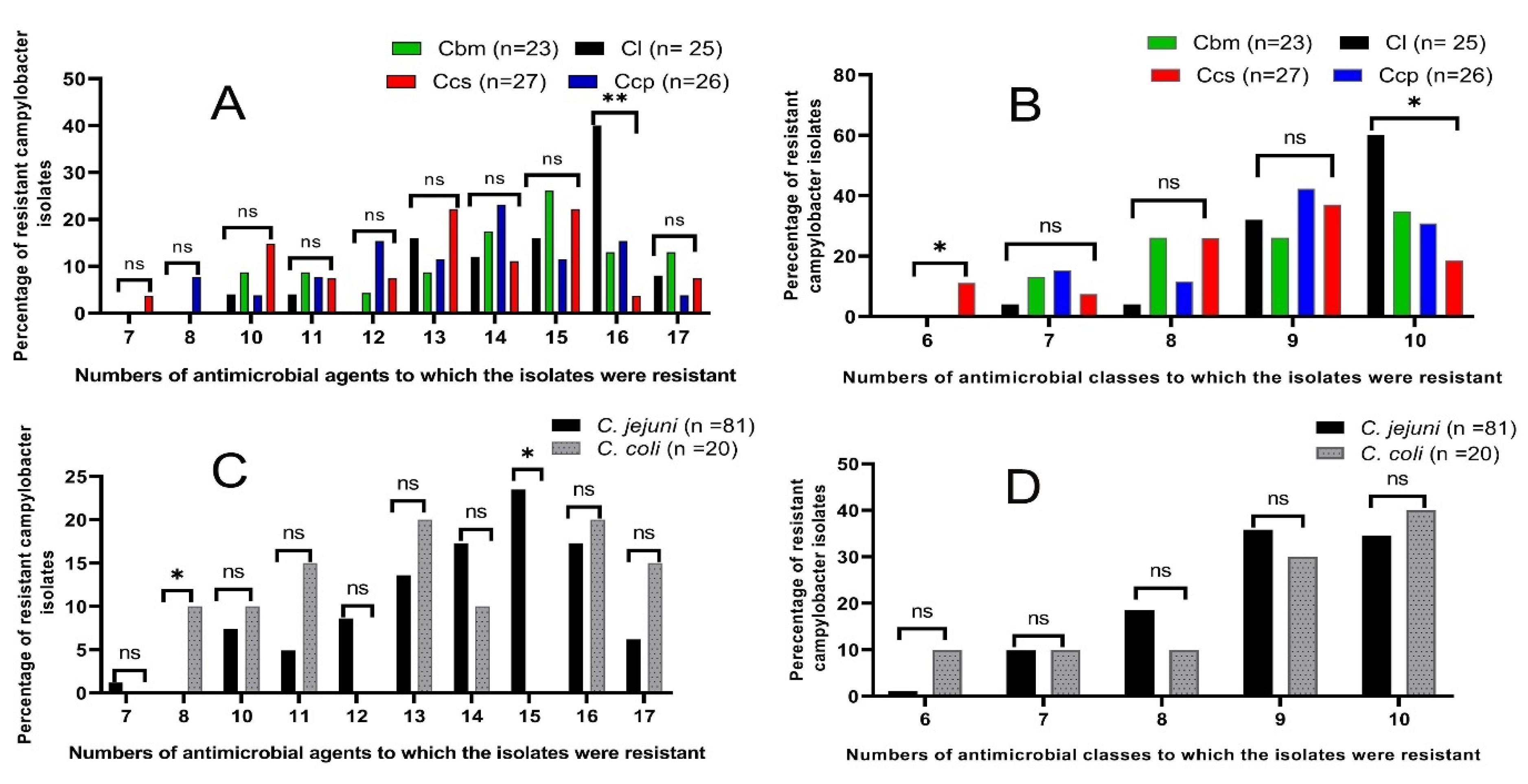
3.2. Antimicrobial Susceptibility Testing of the Campylobacter Species
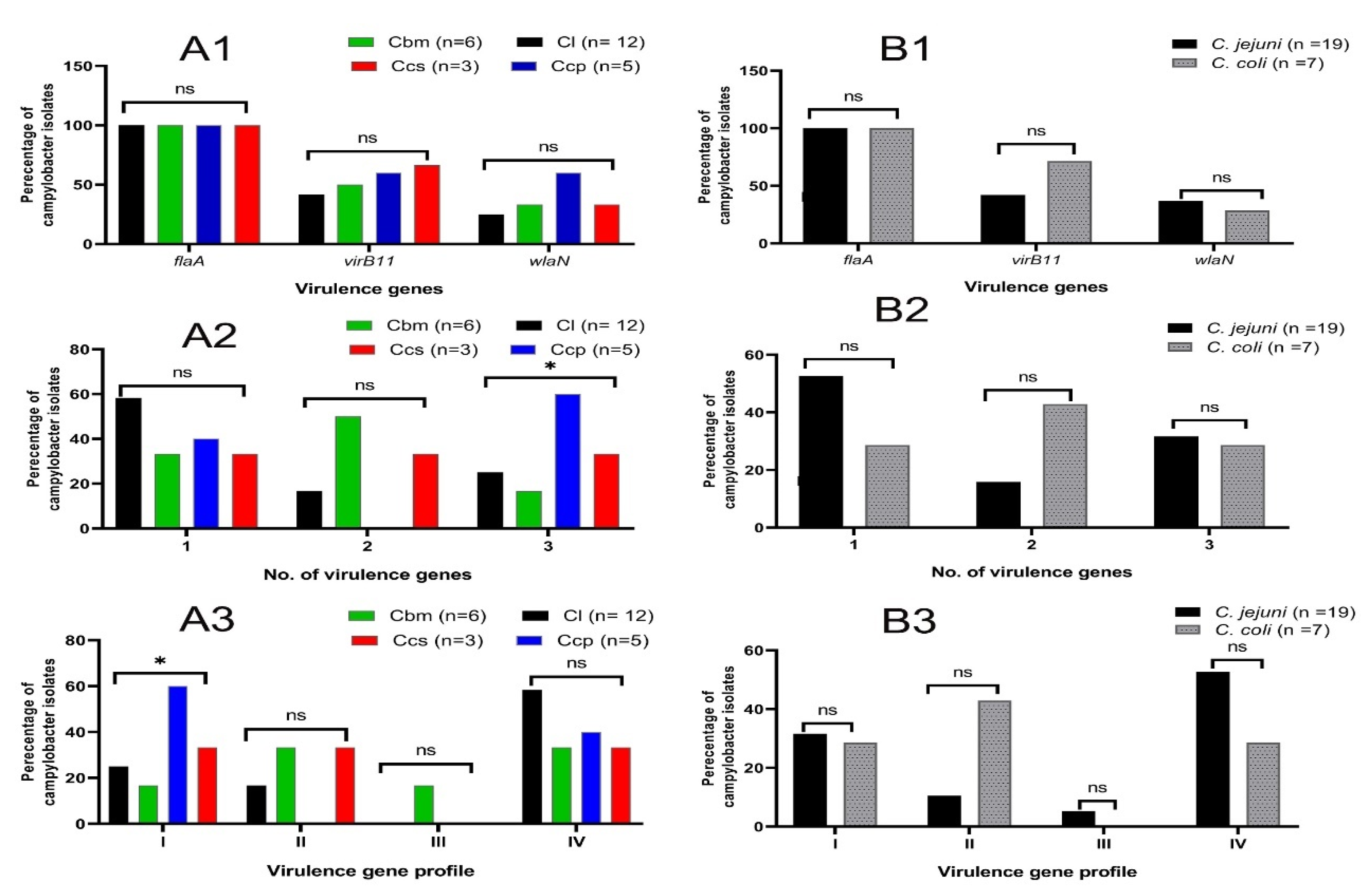
3.3. Molecular Grouping of the Campylobacter Species from Various Chicken Sources
3.4. Molecular Investigation of the Virulence-Related Genes among the Investigated Isolates
3.5. In Vivo Experimental Study
3.5.1. Growth Performance
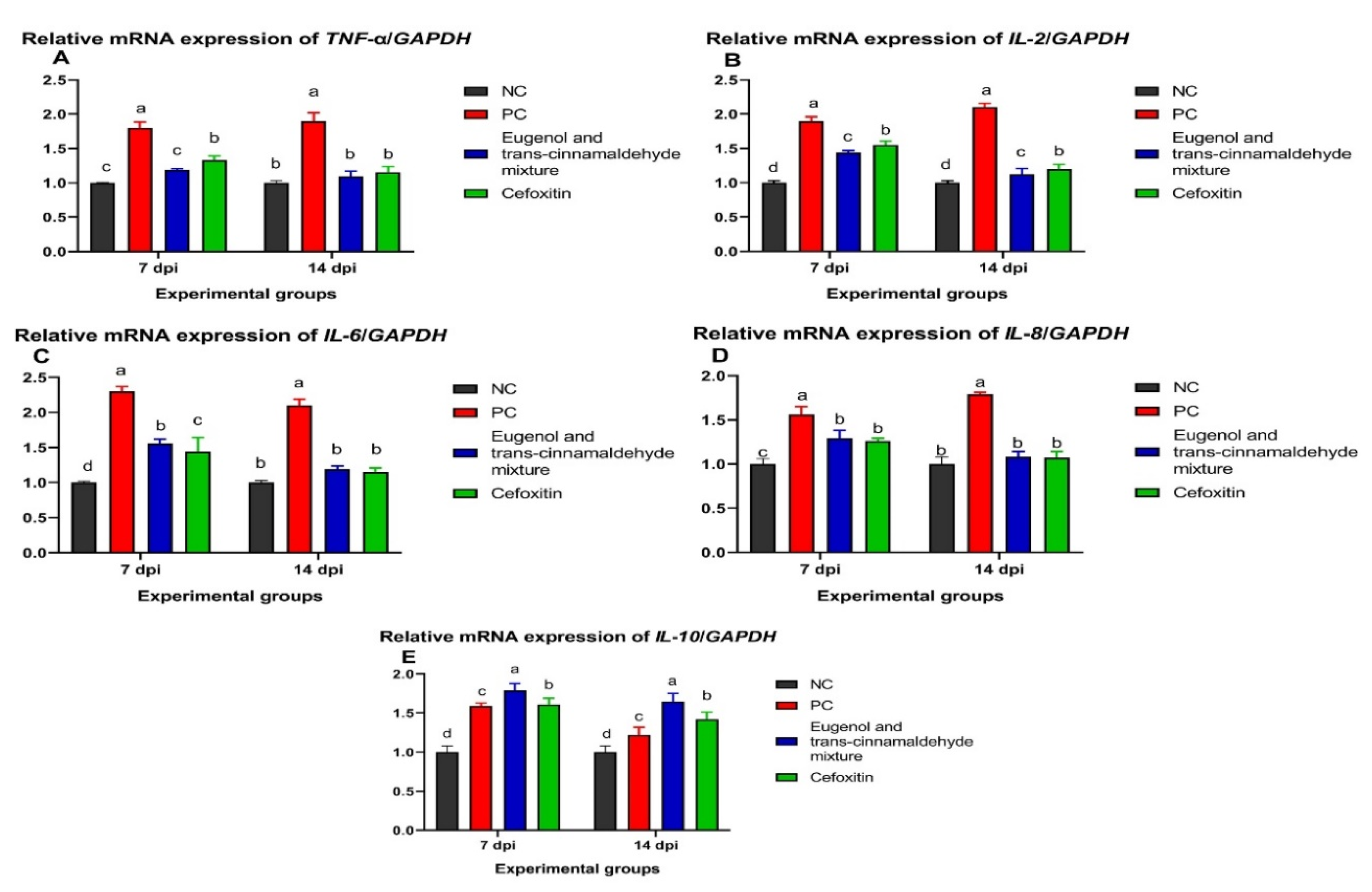
3.5.2. Analysis of Cytokines Genes Expression
3.5.3. Expression Analysis of the C. jejuni Virulence Genes via the RT-qPCR Assay
3.5.4. Quantification of the C. jejuni DNA Copies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; Abd El-Hamid, M.I.; El-Gedawy, A.A.; El-Malt, R.M.S. Campylobacter as a Major Foodborne Pathogen: A Review of Its Characteristics, Pathogenesis, Antimicrobial Resistance and Control. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 609–619. [Google Scholar]
- EFSA (European Food Safety Authority). Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008—Part B: Analysis of factors associated with Salmonella contamination of broiler carcasses. EFSA J. 2011, 9, 2017. [Google Scholar]
- EFSA (European Food Safety Authority). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Ammar, A.M.; El-Hamid, A.; Marwa, I.; El-Malt, R.M.S.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drug-resistant Campylobacter species in Egypt. Antibiotics 2021, 10, 1342. [Google Scholar] [CrossRef]
- Same, R.G.; Tamma, P.D. Campylobacter infections in children. Pediatr. Rev. 2018, 39, 533–541. [Google Scholar] [CrossRef]
- Ugarte-Ruiz, M.; Domínguez, L.; Corcionivoschi, N.; Wren, B.W.; Dorrell, N.; Gundogdu, O. Exploring the oxidative, antimicrobial and genomic properties of Campylobacter jejuni strains isolated from poultry. Res. Vet. Sci. 2018, 119, 170–175. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; El-Malt, R.M.S.; El-Gedawy, A.A.; Khalifa, E.; Elnahriry, S.S.; Abd El-Hamid, M.I. Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of Eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Animals 2021, 11, 3. [Google Scholar] [CrossRef]
- Silva, W.C.; Targino, B.N.; Gonçalves, A.G.; Silva, M.R.; Hungaro, H.M. Campylobacter: An important food safety issue. In Food Safety and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 391–430. [Google Scholar]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Med. 2009, 4, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Wieczorek, K.; Denis, E.; Osek, J. Comparative analysis of antimicrobial resistance and genetic diversity of Campylobacter from broilers slaughtered in Poland. Int. J. Food Microbiol. 2015, 210, 24–32. [Google Scholar] [CrossRef]
- Bolton, D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015, 48, 99–108. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, Q.; Chen, Y.; Li, T.; Wen, G.; Zhang, R.; Luo, L.; Lu, Q.; Ai, D.; Wang, H. Molecular epidemiology, virulence determinants and antimicrobial resistance of Campylobacter spreading in retail chicken meat in Central China. Gut Pathog. 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Sánchez, L.; Melero, B.; Rovira, J. Campylobacter in the food chain. Adv. Food Nutr. Res. 2018, 86, 215–252. [Google Scholar] [PubMed]
- Bhunia, A.K. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Springer: Cham, Switzerland, 2018. [Google Scholar]
- García-Sánchez, L.; Melero, B.; Jaime, I.; Hänninen, M.-L.; Rossi, M.; Rovira, J. Campylobacter jejuni survival in a poultry processing plant environment. Food Microbiol. 2017, 65, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Beauvais, W.; Guitian, J. Effect of enhanced biosecurity and selected on-farm factors on Campylobacter colonization of chicken broilers. Epidemiol. Infect. 2017, 145, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Arsi, K.; Wagle, B.R.; Upadhyaya, I.; Shrestha, S.; Donoghue, A.M.; Donoghue, D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017, 8, 713. [Google Scholar] [CrossRef] [Green Version]
- Wagle, B.; Shrestha, S.; Arsi, K.; Upadhyaya, I.; Donoghue, A.; Donoghue, D. Pectin or chitosan coating fortified with eugenol reduces Campylobacter jejuni on chicken wingettes and modulates expression of critical survival genes. Poult. Sci. 2019, 98, 1461–1471. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.; Awad, N.F.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; El-Kader, A.; Shaimaa, A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; Abd El-Hamid, M.I. Supplementing Garlic Nanohydrogel Optimized Growth, Gastrointestinal Integrity and Economics and Ameliorated Necrotic Enteritis in Broiler Chickens Using a Clostridium perfringens Challenge Model. Animals 2021, 11, 2027. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Code of Federal Regulations Title 21 Part 172. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?.CFRPart1/4172 (accessed on 22 July 2020).
- Yen, T.B.; Chang, S.T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- On, S. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 1996, 9, 405–422. [Google Scholar] [CrossRef]
- Bauer, A. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, R.; Dahiya, S.; Sayal, P. Evaluation of multiple antibiotic resistance (MAR) index and Doxycycline susceptibility of Acinetobacter species among inpatients. Indian J. Microbiol. Res. 2016, 3, 299. [Google Scholar] [CrossRef]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [Green Version]
- Shin, E.-J.; Lee, Y.-H. Comparison of three different methods for Campylobacter isolation from porcine intestines. J. Microbiol. Biotechnol. 2009, 19, 647–650. [Google Scholar] [PubMed]
- Datta, S.; Niwa, H.; Itoh, K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003, 52, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning. A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Talukder, K.A.; Aslam, M.; Islam, Z.; Azmi, I.J.; Dutta, D.K.; Hossain, S.; Nur-E-Kamal, A.; Nair, G.B.; Cravioto, A.; Sack, D.A. Prevalence of virulence genes and cytolethal distending toxin production in Campylobacter jejuni isolates from diarrheal patients in Bangladesh. J. Clin. Microbiol. 2008, 46, 1485–1488. [Google Scholar] [CrossRef] [Green Version]
- Aviagen, W. Ross 308: Broiler’s Management and Nutrition Specification; AOAC: Rockville, ML, USA, 2018. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Rockville, ML, USA, 2012. [Google Scholar]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Arisha, A.H.; Abd El-Aziz, R.M.; Sherief, W.R.; Adil, S.H.; El Sayed, R.; Metwally, A.E. Impact of feeding anaerobically fermented feed supplemented with acidifiers on its quality and growth performance, intestinal villi and enteric pathogens of mulard ducks. Livest. Sci. 2020, 242, 104299. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S.; Shahin, S.E.; Omar, A.E.; Mohammed, H.A.; Mahmoud, H.I.; Ibrahim, D. Effects of graded levels of microbial fermented or enzymatically treated dried brewer’s grains on growth, digestive and nutrient transporter genes expression and cost effectiveness in broiler chickens. BMC Vet. Res. 2020, 16, 424. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Metwally, A.S.; Nassan, M.A.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A.T. Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals 2021, 11, 1038. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Shahin, S.; Sherief, W.; Farag, M.; Nassan, M. Impact of fermented or enzymatically fermented dried olive pomace on growth, expression of digestive enzymes and glucose transporters genes, oxidative stability of frozen meat and economic efficiency of broiler chickens. Front. Vet. Sci. 2021, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.; Solyman, S.; Azab, M.; Mahmoud, N.; Hanora, A. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016, 62, 55–61. [Google Scholar] [PubMed]
- Abd El-Hamid, M.I.; Bendary, M. Comparative phenotypic and genotypic discrimination of methicillin resistant and susceptible Staphylococcus aureus in Egypt. Cell. Mol. Biol. 2015, 61, 101–112. [Google Scholar]
- Abd El-Hamid, M.I.; Awad, N.F.; Hashem, Y.M.; Abdel-Rahman, M.A.; Abdelaziz, A.M.; Mohammed, I.A.; Abo-Shama, U.H. In vitro evaluation of various antimicrobials against field Mycoplasma gallisepticum and Mycoplasma synoviae isolates in Egypt. Poult. Sci. 2019, 98, 6281–6288. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M.; Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance among methicillin resistant S. aureus recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar]
- Ammar, A.M.; Attia, A.M.; Abd El-Hamid, M.I.; El-Shorbagy, I.M.; Abd El-Kader, S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016, 62, 7–15. [Google Scholar]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.A.; Elsohaby, I.; Mohammad Ghaith, D.; Alshareef, W.A. What is behind phylogenetic analysis of hospital-, community- and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar]
- Awad, N.F.S.; Abd El-Hamid, M.I.; Hashem, Y.M.; Erfan, A.M.; Abdelrahman, B.A.; Mahmoud, H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: An in vivo perspective. J. Appl. Microbiol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Ammar, A.M.; Attia, A.M.; Abd El-Aziz, N.K.; Abd El Hamid, M.I.; El-Demerdash, A.S. Class 1 integron and associated gene cassettes mediating multiple-drug resistance in some food borne pathogens. Int. Food Res. J. 2016, 23, 332–339. [Google Scholar]
- Ammar, A.M.; Abd El-Hamid, M.I.; Eid, S.E.A.; El Oksh, A.S. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they? Rev. Med. Vet. 2015, 166, 304–314. [Google Scholar]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Samir, M.; El-Naenaeey, E.-S.Y.; Abo Remela, E.M.; Mosbah, R.A.; Bendary, M.M. Genetic diversity of Campylobacter jejuni isolated from avian and human sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, M.; Ahmed, H.; El-Gedawy, A.; Saad, A. Molecular identification of C. jejuni and C. coli in chicken and humans, at Zagazig, Egypt, with reference to the survival of C. jejuni in chicken meat at refrigeration and freezing temperatures. Int. Food Res. J. 2014, 21, 1801. [Google Scholar]
- Khalifa, N.O.; Afify, J.S.; Rabie, N.S. Zoonotic and molecular characterizations of Campylobacter jejuni and Campylobacter coli isolated from beef cattle and children. Glob. Vet. 2013, 11, 585–591. [Google Scholar]
- Nguyen, T.N.M.; Hotzel, H.; Njeru, J.; Mwituria, J.; El-Adawy, H.; Tomaso, H.; Neubauer, H.; Hafez, H.M. Antimicrobial resistance of Campylobacter isolates from small scale and backyard chicken in Kenya. Gut Pathog. 2016, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.; Sekar, M.; Gunaseelan, L.; Gawande, M.; Suganya, G.; Malar, P.A.S.; Karthikeyan, A. Molecular identification of Campylobacter jejuni and coli from chicken, calves and dogs to determine its potential threat on human being. Vet. World. 2015, 8, 1420–1423. [Google Scholar] [CrossRef] [Green Version]
- El-Aziz, A.; Norhan, K.; Ammar, A.M.; Hamdy, M.M.; Gobouri, A.A.; Azab, E.; Sewid, A.H. First report of aacC5-aadA7Δ4 Gene Cassette array and phage tail tape measure protein on Class 1 Integrons of Campylobacter Species isolated from animal and human sources in Egypt. Animals 2020, 10, 2067. [Google Scholar] [CrossRef]
- Gharbi, M.; Béjaoui, A.; Ben Hamda, C.; Jouini, A.; Ghedira, K.; Zrelli, C.; Hamrouni, S.; Aouadhi, C.; Bessoussa, G.; Ghram, A. Prevalence and antibiotic resistance patterns of Campylobacter spp. isolated from broiler chickens in the North of Tunisia. BioMed Res. Int. 2018, 2018, 7943786. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Dong, J.; Cheng, Y.; Lu, Q.; Luo, Q.; Wen, G.; Liu, G.; Shao, H. Genotypic diversity, antimicrobial resistance and biofilm-forming abilities of Campylobacter isolated from chicken in Central China. Gut Pathog. 2017, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Chatur, Y.A.; Brahmbhatt, M.N.; Modi, S.; Nayak, J.B. Fluoroquinolone resistance and detection of topoisomerase gene mutation in Campylobacter jejuni isolated from animal and human sources. Int. J. Curr. Microbiol. App. Sci 2014, 3, 773–783. [Google Scholar]
- Wei, B.; Cha, S.-Y.; Yoon, R.-H.; Kang, M.; Roh, J.-H.; Seo, H.-S.; Lee, J.-A.; Jang, H.-K. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from retail chicken and duck meat in South Korea. Food Control 2016, 62, 63–68. [Google Scholar] [CrossRef]
- Samad, A.; Abbas, F.; Ahmed, Z.; Akbar, A.; Naeem, M.; Sadiq, M.B.; Ali, I.; Bugti, F.S.; Achakzai, S.K. Prevalence, antimicrobial susceptibility, and virulence of Campylobacter jejuni isolated from chicken meat. J. Food Saf. 2019, 39, e12600. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; El-Sayed, M.; Ali, A.R.; Abdallah, H.; Arnaout, M.I.; El-Mowalid, G.A. Marjoram extract down-regulates the expression of Pasteurella multocida adhesion, colonization and toxin genes: A potential mechanism for its antimicrobial activity. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 101–108. [Google Scholar] [CrossRef] [PubMed]
- El-Hamid, A.; Marwa, I.; El-Naenaeey, E.-S.Y.; Hegazy, W.A.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising antibiofilm agents: Recent breakthrough against biofilm producing methicillin-resistant Staphylococcus aureus. Antibiotics 2020, 9, 667. [Google Scholar] [CrossRef]
- Elmowalid, G.A.; Abd El-Hamid, M.I.; Abd El-Wahab, A.M.; Atta, M.; Abd El-Naser, G.; Attia, A.M. Garlic and ginger extracts modulated broiler chicks innate immune responses and enhanced multidrug resistant Escherichia coli O78 clearance. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101334. [Google Scholar] [CrossRef]
- Girgis, S.A.; Rashad, S.S.; Othman, H.B.; Bassim, H.H.; Kassem, N.N.; El-Sayed, F.M. Multiplex PCR for identification and differentiation of Campylobacter species and their antimicrobial susceptibility pattern in Egyptian patients. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 861–875. [Google Scholar]
- Oh, J.-Y.; Kwon, Y.-K.; Wei, B.; Jang, H.-K.; Lim, S.-K.; Kim, C.-H.; Jung, S.-C.; Kang, M.-S. Epidemiological relationships of Campylobacter jejuni strains isolated from humans and chickens in South Korea. J. Microbiol. 2017, 55, 13–20. [Google Scholar] [CrossRef]
- Kelly, C.; Gundogdu, O.; Pircalabioru, G.; Cean, A.; Scates, P.; Linton, M.; Pinkerton, L.; Magowan, E.; Stef, L.; Simiz, E. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog. Dis. 2017, 14, 341–349. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; Abd El-Hamid, M.I. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef]
- Ibrahim, D.; Sewid, A.H.; Arisha, A.H.; Abd El-Fattah, A.H.; Abdelaziz, A.M.; Al-Jabr, O.A.; Kishawy, A.T. Influence of Glycyrrhiza glabra Extract on Growth, Gene Expression of Gut Integrity, and Campylobacter jejuni Colonization in Broiler Chickens. Front. Vet. Sci. 2020, 7, 612063. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K.; Kumar, P.; Samanta, I.; Pradhan, S.; Samanta, A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-G.; Fang, W.-L.; Wei, J.; Wang, T.; Wang, N.; Ma, J.-L.; Shi, M. The involvement of NLRX1 and NLRP3 in the development of nonalcoholic steatohepatitis in mice. J. Chin. Med. Assoc. 2013, 76, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Lillehoj, E.P.; Min, W.; Bravo, D.M. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nutr. 2013, 110, 840–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatun, J.; Loh, T.C.; Foo, H.L.; Akit, H.; Khan, K.I. Growth performance, cytokine expression, and immune responses of broiler chickens fed a dietary palm oil and sunflower oil blend supplemented with L-Arginine and varying concentrations of vitamin E. Front. Vet. Sci. 2020, 7, 619. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Zhou, H.; Tian, F.; Ni, Y. Antimicrobial activities and in vitro properties of cold-adapted Lactobacillus strains isolated from the intestinal tract of cold water fishes of high latitude water areas in Xinjiang, China. BMC Microbiol. 2019, 19, 247. [Google Scholar] [CrossRef] [Green Version]
- Szott, V.; Reichelt, B.; Alter, T.; Friese, A.; Roesler, U. In vivo efficacy of carvacrol on Campylobacter jejuni prevalence in broiler chickens during an entire fattening period. Eur. J. Microbiol. Immunol. 2020, 10, 131–138. [Google Scholar] [CrossRef]
- Grilli, E.; Vitari, F.; Domeneghini, C.; Palmonari, A.; Tosi, G.; Fantinati, P.; Massi, P.; Piva, A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: From in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013, 114, 308–317. [Google Scholar] [CrossRef]


| Specificity (Target Gene) | Primer Sequence (5’-3’) | Amplified Product (bp) | Denaturation Temperature | References |
|---|---|---|---|---|
| Genus Campylobacter (23S rRNA) | F: TATACCGGTAAGGAGTGCTGGAG | 650 | 95 °C | [28,32] |
| R: ATCAATTAACCTTCGAGCACCG | ||||
| Campylobacter jejuni (mapA) | F: CTATTTTATTTTTGAGTGCTTGTG | 589 | 95 °C | [29,33] |
| R: GCTTTATTTGCCATTTGTTTTATTA | ||||
| Campylobacter coli (ceuE) | F: AATTGA AAATTG CTCCAACTATG | 462 | 95 °C | [29,33] |
| R: TGATTT TATTATTTGTAGCAGCG | ||||
| FlaA (flaA) | F: AATAAAAATGCTGATAAAACAGGTG | 855 | 94 °C | [30,34] |
| R: TACCGAACCAATGTCTGCTCTGATT | ||||
| VirB (virB11) | F: TCTTGTGAGTTGCCTTACCCCTTTT | 494 | 94 °C | [30,34] |
| R: CCTGCGTGTCCTGTGTTATTTACCC | ||||
| WlaN (wlaN) | F: TTAAGAGCAAGATATGAAGGTG | 672 | 94 °C | [32,35] |
| R: CCATTTGAATTGATATTTTTG |
| Ingredient (%) | Starter (Days 1–10) | Grower (Days 11–20) | Finisher (Days 21–37) |
|---|---|---|---|
| Yellow corn | 60.10 | 64.00 | 65.70 |
| Soybean oil | 1.57 | 2.50 | 4.20 |
| Soybean meal | 34.5 | 29.3 | 25.90 |
| Common salt | 0.3 | 0.3 | 0.3 |
| Calcium diphasic phosphate | 1.2 | 1.2 | 1.2 |
| Calcium carbonate | 1.1 | 1.1 | 1.1 |
| Premix * | 0.8 | 0.8 | 0.8 |
| Choline chloride | 0.2 | 0.2 | 0.2 |
| DL-methionine 99% | 0.17 | 0.17 | 0.17 |
| L-lysine HCL 78% | 0.33 | 0.33 | 0.33 |
| Anti-mycotoxin | 0.1 | 0.1 | 0.1 |
| Calculated composition | |||
| ME (kcal/kg) | 3001 | 3100 | 3222 |
| CF (%) | 2.64 | 2.50 | 2.46 |
| EE (%) | 4.07 | 5.14 | 6.83 |
| CP (%) | 23.01 | 21.02 | 19.57 |
| Available P (%) | 0.49 | 0.44 | 0.42 |
| Ca (%) | 1.02 | 1.04 | 1.03 |
| Methionine (%) | 0.500 | 0.44 | 0.46 |
| Lysine (%) | 1.41 | 1.28 | 1.18 |
| Target Gene | Primer Sequence (5’-3’) | Accession No. |
|---|---|---|
| TNF-α | F: CTGCACTTCAGGGTGATCG | XM_008262537.2 |
| R: CTACGTGGGCTAGAGGCTTG | ||
| IL-2 | F: GCTTATGGAGCATCTCTATCATCA | XM_015276098.2 |
| R: GGTGCACTCCTGGGTCTC | ||
| IL-6 | F: GCCAACCCTACAACAAGA | NC_013678 |
| R: AGAGCCACAACGACTGAC | ||
| IL-8 | F: CTCTCTTGGCAACCTTCCTG | KT216053.1 |
| R: TTGCACAGTGAGGTCCACTC | ||
| IL-10 | F: AAAAGCTAAAAGCCCCAGGA | NM001082045.1 |
| R: CGGGAGCTGAGGTATCAGAG | ||
| GAPDH | F: TGTTTGTGATGGGCGTGAA | NC_013676.1 |
| R: CCTCCACAATGCCGAAGT |
| Sample Type (Symbol, No.) | Total No. of Campylobacter Isolates (%) * | No. of Campylobacter spp. (%) * | |
|---|---|---|---|
| C. jejuni | C. coli | ||
| Chicken luncheon meats (Clm, 30) | 0 | 0 | 0 |
| Chicken livers (Cl, 30) | 25 (83.3) | 19 (63.3) | 6 (20) |
| Chicken breast meats (Cbm, 30) | 23 (76.7) | 20 (66.7) | 3 (10) |
| Chicken cecal parts (Ccp, 30) | 26 (86.7) | 21 (70) | 5 (16.7) |
| Chicken cloacal swabs (Ccs, 30) | 27 (90) | 21 (70) | 6 (20) |
| Total (150) | 101 (67.3) | 81 (54) | 20 (13.3) |
| Antimicrobial Class | Antimicrobial Agent | No. of Resistant Campylobacter Isolates from Various Chicken Sources (%) | Total No. of Campylobacter Isolates (%) (n = 101) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Liver (n = 25) | Breast Meats (n = 23) | Cecal Parts (n = 26) | Cloacal Swabs (n = 27) | ||||
| Lincosamide | Clindamycin | 24 (96) | 20 (87) | 25 (96.2) | 27 (100) | 96 (95) | 0.18 |
| Polypeptides | Colistin | 19 (76) | 17 (73.9) | 22 (84.6) | 17 (63) | 75 (74.3) | 0.348 |
| Phenicols | Chloramphenicol | 24 (96) | 16 (69.6) | 18 (69.2) | 20 (74.1) | 78 (77.2) | 0.076 |
| Oxazolidones | Linezolid | 23 (92) | 17 (73.9) | 21 (80.8) | 12 (44.4) | 73 (72.3) | 0.001 ** |
| Aminoglycosides | Amikacin | 9 (36) | 9 (39.1) | 7 (26.9) | 10 (37) | 35 (34.7) | 0.821 |
| Gentamicin | 21 (84) | 21 (91.3) | 16 (61.5) | 21 (77.8) | 79 (78.2) | 0.07 | |
| Tobramycin | 19 (76) | 15 (65.2) | 20 (76.9) | 16 (59.3) | 70 (69.3) | 0.458 | |
| Macrolides | Azithromycin | 23 (92) | 22 (95.7) | 23 (88.5) | 24 (88.9) | 92 (91.1) | 0.871 |
| Erythromycin | 25 (100) | 23 (100) | 26 (100) | 27 (100) | 101 (100) | NA | |
| Tetracyclines | Doxycycline | 23 (92) | 18 (78.3) | 20 (76.9) | 23 (85.2) | 84 (83.2) | 0.472 |
| Sulphonamides | Trimethoprim-sulfamethoxazole | 25 (100) | 23 (100) | 26 (100) | 27 (100) | 101 (100) | NA |
| Quinolones | Ciprofloxacin | 20 (80) | 19 (82.6) | 21 (80.8) | 20 (74.1) | 80 (79.2) | 0.903 |
| Nalidixic acid | 25 (100) | 23 (100) | 23 (88.5) | 25 (92.6) | 96 (95) | 0.04 * | |
| Beta-lactams | Aztreonam | 18 (72) | 21 (91.3) | 21 (80.8) | 25 (92.6) | 85 (84.2) | 0.015 * |
| Imipenem | 14 (56) | 11 (47.8) | 7 (26.9) | 11 (40.7) | 43 (42.6) | 0.195 | |
| Cefoxitin | 13 (52) | 8 (34.8) | 8 (30.8) | 5 (18.5) | 34 (33.7) | 0.047 * | |
| Sulbactam-ampicillin | 18 (72) | 17 (73.9) | 13 (50) | 13 (48.1) | 61 (60.4) | 0.114 | |
| Ampicillin | 25 (100) | 23 (100) | 26 (100) | 27 (100) | 101 (100) | NA | |
| MAR Index | No. of Antimicrobials to Which the Isolates Were Resistant * | No. of AMC | No. of Resistant Campylobacter Isolates from Different Chicken Sources (%) | Total No. of Campylobacter Isolates (%) (n = 101) | The Character of Resistant Strains | |||
|---|---|---|---|---|---|---|---|---|
| Liver (n = 25) | Breast Meats (n = 23) | Cecal Parts (n = 26) | Cloacal Swabs (n = 27) | |||||
| 0.39 | 7 | 7 | - | - | - | 1 (3.7) | 1 (1) | MDR |
| 0.44 | 8 | 7 | - | - | 2 (7.7) | - | 2 (2) | |
| 0.56 | 10 | 6 | - | - | - | 3 (11.1) | 3 (3) | |
| 7 | 1 (4) | 2 (8.7) | 1 (3.8) | 1 (3.7) | 5 (5) | |||
| 0.61 | 11 | 8 | 1 (4) | 2 (8.7) | 1 (3.8) | 1 (3.7) | 5 (5) | |
| 9 | - | - | 1 (3.8) | 1 (3.7) | 2 (2) | |||
| 0.67 | 12 | 7 | - | 1 (4.3) | 1 (3.8) | - | 2 (2) | |
| 8 | - | - | 1 (3.8) | 2 (7.4) | 3 (3) | |||
| 9 | - | - | 2 (7.7) | - | 2 (2) | |||
| 0.72 | 13 | 8 | - | - | - | 2 (7.4) | 2 (2) | |
| 9 | 2 (8) | 2 (8.7) | 3 (11.5) | 2 (7.4) | 9 (8.9) | |||
| 10 | 2 (8) | - | - | 2 (7.4) | 4 (4) | |||
| 0.78 | 14 | 8 | - | - | 1 (3.8) | - | 1 (1) | |
| 9 | 1 (4) | - | 3 (11.5) | 2 (7.4) | 6 (5.9) | |||
| 10 | 2 (8) | 4 (17.4) | 2 (7.7) | 1 (3.7) | 9 (8.9) | |||
| 0.83 | 15 | 8 | - | 1 (4.3) | - | 2 (7.4) | 3 (3) | |
| 9 | 2 (8) | 4 (17.4) | 2 (7.7) | 4 (14.8) | 12 (11.9) | |||
| 10 | 2 (8) | 1 (4.3) | 1 (3.8) | - | 4 (4) | |||
| 0.89 | 16 | 8 | - | 3 (13) | - | - | 3 (3) | XDR |
| 9 | 3 (12) | - | - | 1 (3.7) | 4 (4) | |||
| 10 | 7 (28) | - | 4 (15.4) | - | 11 (10.9) | |||
| 0.94 | 17 | 10 | 2 (8) | 3 (13) | 1 (3.8) | 2 (7.4) | 8 (7.9) | |
| Experimental Group | p-Value | SEM | ||||
|---|---|---|---|---|---|---|
| NC | PC | Eugenol and Trans-Cinnamaldehyde Mixture | Cefoxitin | |||
| BW, g/bird | 2372 a | 1873 c | 2355 ab | 2363 a | 0.03 | 5.44 |
| BWG, g/bird | 2327 a | 1830 c | 2311 ab | 2319 a | 0.04 | 6.74 |
| FI, g/bird | 3843 b | 3998 a | 3884 b | 4075 a | 0.001 | 7.60 |
| FCR | 1.65 c | 2.18 a | 1.68 bc | 1.76 b | 0.001 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljazzar, A.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; El-Gharreb, W.R.; Abdel-Raheem, S.M.; Ibrahim, A.M.; Abdelaziz, A.M.; Ibrahim, D. Prevalence and Antimicrobial Susceptibility of Campylobacter Species with Particular Focus on the Growth Promoting, Immunostimulant and Anti-Campylobacter jejuni Activities of Eugenol and Trans-Cinnamaldehyde Mixture in Broiler Chickens. Animals 2022, 12, 905. https://doi.org/10.3390/ani12070905
Aljazzar A, Abd El-Hamid MI, El-Malt RMS, El-Gharreb WR, Abdel-Raheem SM, Ibrahim AM, Abdelaziz AM, Ibrahim D. Prevalence and Antimicrobial Susceptibility of Campylobacter Species with Particular Focus on the Growth Promoting, Immunostimulant and Anti-Campylobacter jejuni Activities of Eugenol and Trans-Cinnamaldehyde Mixture in Broiler Chickens. Animals. 2022; 12(7):905. https://doi.org/10.3390/ani12070905
Chicago/Turabian StyleAljazzar, Ahmed, Marwa I. Abd El-Hamid, Rania M. S. El-Malt, Waleed Rizk El-Gharreb, Sherief M. Abdel-Raheem, Abdelazim M. Ibrahim, Adel M. Abdelaziz, and Doaa Ibrahim. 2022. "Prevalence and Antimicrobial Susceptibility of Campylobacter Species with Particular Focus on the Growth Promoting, Immunostimulant and Anti-Campylobacter jejuni Activities of Eugenol and Trans-Cinnamaldehyde Mixture in Broiler Chickens" Animals 12, no. 7: 905. https://doi.org/10.3390/ani12070905









