Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Inclusion Criteria
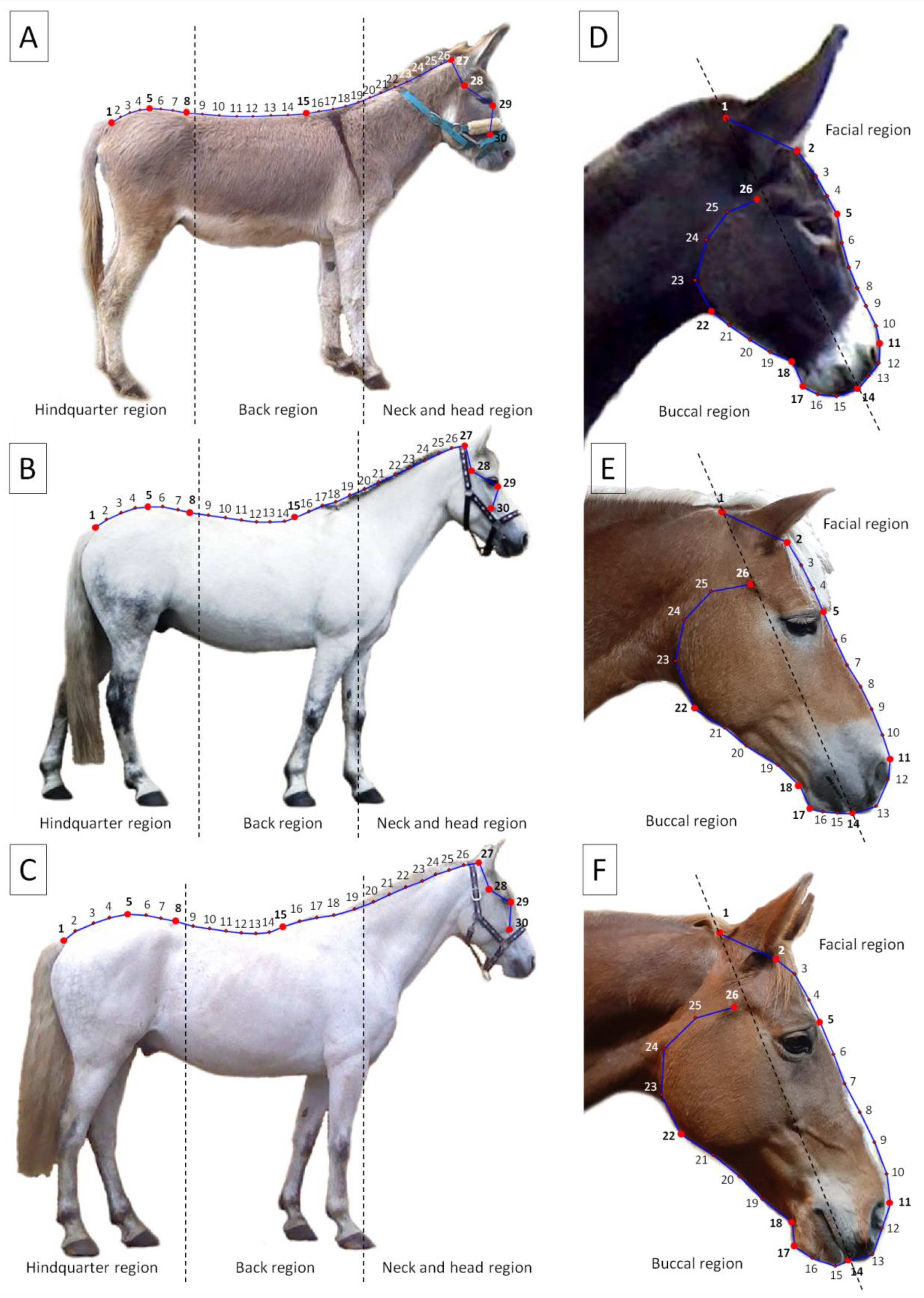
2.3. Geometric Morphometrics
2.3.1. Photographs Collection
2.3.2. Photograph Processing
2.3.3. Photographs Analysis
3. Results
3.1. Comparison of the Equids’ Dorsal Profiles
3.2. Comparison of the Equids’ Head Shapes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starkey, P.; Starkey, M. Regional and world trends in donkey populations. In Donkeys, People and Development; Starkey, P., Fielding, D., Eds.; ATNESA: Wageningen, The Netherlands, 2000; pp. 10–21. [Google Scholar]
- Norris, S.L.; Little, H.A.; Ryding, J.; Raw, Z. Global donkey and mule populations: Figures and trends. PLoS ONE 2021, 16, e0247830. [Google Scholar] [CrossRef] [PubMed]
- De Aluja, A.S. The welfare of working equids in Mexico. Appl. Anim. Behav. Sci. 1998, 59, 19–29. [Google Scholar] [CrossRef]
- Kubasiewicz, L.M.; Rodrigues, J.B.; Norris, S.L.; Watson, T.L.; Rickards, K.; Bell, N.; Judge, A.; Raw, Z.; Burden, F.A. The Welfare Aggregation and Guidance (WAG) Tool: A new method to summarize global welfare assessment data for equids. Animals 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Rayner, E.; Airikkala-Otter, I.; Susheelan, A.; Gibson, A.; Itaba, R.; Mayani, T.; Mellanby, R.J.; Gamble, L. Prevalence of skin wounds in working donkeys in Bukombe, Tanzania. Vet. Rec. 2020, 186, 284. [Google Scholar] [CrossRef]
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The current situation and trend of donkey industry in Europe. J. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Miraglia, N.; Salimei, E.; Fantuz, F. Equine milk production and valorization of marginal areas—A review. Animals 2020, 10, 353. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Martemucci, G. Lactation curve and effects of milking regimen on milk yield and quality, and udder health in Martina Franca jennies (Equus asinus). J. Anim. Sci. 2012, 90, 669–681. [Google Scholar] [CrossRef]
- Altomonte, I.; Salari, F.; Licitra, R.; Martini, M. Donkey and human milk: Insights into their compositional similarities. Int. Dairy J. 2019, 89, 111–118. [Google Scholar] [CrossRef]
- Almeida, A.; Rodrigues, J. Animal traction: New opportunities and new challenges. In Proceedings of the IX International Scientific Symposium on Farm Machinery and Processes Management in Sustainable Agriculture, Lublin, Poland, 22–24 November 2017. [Google Scholar]
- Tully, P.A.G.; Carr, N. Presenting the donkey at the seaside: A move towards tackling speciesism in the tourism industry. Ann. Leis. Res. 2020, 23, 1–18. [Google Scholar] [CrossRef]
- Colombo, E.; Manti, F.; Milani, L.; Bernardini, D. Assessing the effects of donkey-assisted therapy on adults with intellectual disabilities using the ICF framework. Soc Anim. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Burn, C.C.; Dennison, T.L.; Whay, H.R. Environmental and demographic risk factors for poor welfare in working horses, donkeys and mules in developing countries. Vet. J. 2010, 186, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Haddy, E.; Burden, F.; Proops, L. Shelter seeking behavior of healthy donkeys and mules in a hot climate. Appl. Anim. Behav. Sci. 2020, 222, 104898. [Google Scholar] [CrossRef]
- Van Dierendonck, M.C.; Burden, F.A.; Rickards, K.; van Loon, J.P. Monitoring acute pain in donkeys with the equine utrecht university scale for donkeys composite pain assessment (equus-donkey-compass) and the equine Utrecht University scale for donkey facial assessment of pain (Equus-Donkey-Fap). Animals 2020, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Burden, F. Practical feeding and condition scoring for donkeys and mules. Equine Vet. Educ. 2012, 24, 589–596. [Google Scholar] [CrossRef]
- McLean, A.K.; Gonzalez, F.J.N. Can scientists influence donkey welfare? Historical perspective and a contemporary view. J. Equine Vet. Sci. 2018, 65, 25–32. [Google Scholar] [CrossRef]
- Thiemann, A.K.; Buil, J.; Rickards, K.; Sullivan, R.J. A review of laminitis in the donkey. Equine Vet. Educ. 2021, 1–8. [Google Scholar] [CrossRef]
- Valle, E.; Raspa, F.; Giribaldi, M.; Barbero, R.; Bergagna, S.; Antoniazzi, S.; McLean, A.K.; Minero, M.; Cavallarin, L. A functional approach to the body condition assessment of lactating donkeys as a tool for welfare evaluation. Peer J. 2017, 5, e3001. [Google Scholar] [CrossRef]
- Maśko, M.; Wierzbicka, M.; Zdrojkowski, Ł.; Jasiński, T.; Pawliński, B.; Domino, M. Characteristics of the donkey’s dorsal profile in relation to its functional body condition assessment. Animals 2021, 11, 3095. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A.; Paul, E.S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- Sénèque, E.; Morisset, S.; Lesimple, C.; Hausberger, M. Testing optimal methods to compare horse postures using geometric morphometrics. PLoS ONE 2018, 13, e0204208. [Google Scholar] [CrossRef] [PubMed]
- Sénèque, E.; Lesimple, C.; Morisset, S.; Hausberger, M. Could posture reflect welfare state? A study using geometric morphometrics in riding school horses. PLoS ONE 2019, 14, e0211852. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, A.K.; Rickards, K.J.; Getachew, M.; Paraschou, G. Colic in the donkey. In The Equine Acute Abdomen, 3rd ed.; Blikslager, A.T., White, N.A., Moore, J.N., Mair, T.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 471–488. [Google Scholar]
- Burden, F.; Thiemann, A. Donkeys are different. J. Eq. Vet. Sci. 2015, 35, 376–382. [Google Scholar] [CrossRef]
- Hummel, H.I.; Pessanha, F.; Salah, A.A.; van Loon, T.J.; Veltkamp, R.C. Automatic pain detection on horse and donkey faces. In Proceedings of the 15th IEEE International Conference on Automatic Face and Gesture Recognition, Buenos Aires, Argentina, 16–20 November 2020. [Google Scholar]
- Ashley, F.H.; Waterman-Pearson, A.E.; Whay, H.R. Behavioral assessment of pain in horses and donkeys: Application to clinical practice and future studies. Equine Vet. J. 2005, 37, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.C.; Lindberg, A.C.; Main, D.C.J.; Whay, H.R. Assessment of the welfare of working horses, mules and donkeys, using health and behavior parameters. Prev. Vet. Med. 2005, 69, 265–283. [Google Scholar] [CrossRef]
- Regan, F.H.; Hockenhull, J.; Pritchard, J.C.; Waterman-Pearson, A.E.; Whay, H.R. Behavioral Repertoire of working donkeys and consistency of behavior over time, as a preliminary step towards identifying pain-related behaviors. PLoS ONE 2014, 9, e101877. [Google Scholar] [CrossRef]
- Grint, N.J.; Murrell, J.C.; Whay, H.R. Investigating the opinions of donkey owners and veterinary surgeons towards pain and analgesia in donkeys. Equine Vet. Educ. 2015, 27, 365–371. [Google Scholar] [CrossRef]
- Bussieres, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadore, J.M.; Desmaizieres, M.; Cuvelliez, S.G.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef]
- Graubner, C.; Gerber, V.; Doherr, M.; Spadavecchia, C. Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet. J. 2011, 188, 178–183. [Google Scholar] [CrossRef]
- Van Loon, J.P.; van Dierendonck, M.C. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for composite pain assessment (EQUUS-COMPASS) and the Equine Utrecht University scale for facial assessment of pain (EQUUS-FAP): A scale-construction study. Vet. J. 2015, 206, 356–364. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef] [PubMed]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Merkies, K.; Paraschou, G.; McGreevy, P.D. Morphometric characteristics of the skull in horses and donkeys—A pilot study. Animals 2020, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Martin Jr, B.B.; Klide, A.M. Physical examination of horses with back pain. Vet. Clin. North. Am. Equine Pract. 1999, 15, 61–70. [Google Scholar] [CrossRef]
- Davidson, E.J. Lameness evaluation of the athletic horse. Vet. Clin. Equine Pract. 2018, 34, 181–191. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Jerbi, H.; Rejeb, A.; Erdoğan, S.; Pérez, W. Anatomical and morphometric study of gastrointestinal tract of donkey (Equus africanus asinus). J. Morphol. Sci. 2014, 31, 18–22. [Google Scholar] [CrossRef][Green Version]
- Jamdar, M.; Ema, A. A note on the vertebral formula of the donkey. Br. Vet. J. 1982, 138, 209–211. [Google Scholar] [CrossRef]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. The neck, back, and vertebral column of the horse. In Dyce, Sack, and Wensing’s Textbook of Veterinary Anatomy, 5th ed.; Singh, B., Ed.; Saunders: Philadelphia, PA, USA, 2017. [Google Scholar]
- Burnham, S.L. Anatomical differences of the donkey and mule. In Proceedings of the Annual Convention of the AAEP 2002, Orlando, FL, USA, 4–8 December 2002. [Google Scholar]
- McLean, A.N.; Christensen, J.W. The application of learning theory in horse training. Appl. Anim. Behav. Sci. 2017, 190, 18–27. [Google Scholar] [CrossRef]
- Grint, N.J.; Beths, T.; Yvorchuk-St Jean, K.; Whay, H.R.; Murrell, J.C. Analysis of behaviors observed during mechanical nociceptive threshold testing in donkeys and horses. J. Equine Vet. Sci. 2017, 50, 102–109. [Google Scholar] [CrossRef]
- Corrales-Hernández, A.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Roldan-Santiago, P.; Rodríguez-Salinas, S.; Yáñez-Pizaña, A.; de la Cruz, L.; González-Lozano, M.; Mora-Medina, P. Physiological responses in horses, donkeys and mules sold at livestock markets. Int. J. Vet. Sci. Med. 2018, 6, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Davis, E. Donkey and mule welfare. Vet. Clin. N. Am.-Eq. Pract. 2019, 35, 481–491. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.K.; Navas González, F.J.; Canisso, I.F. Donkey and mule behavior. Vet. Clin. N. Am.-Eq. Pract. 2019, 35, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.A.; Farghali, H.A.; Abdelnaby, E.A.; Emam, I.A. Gross anatomical, radiographic and doppler sonographic approach to the infra-auricular parotid region in donkey (Equus asinus). J. Equine Vet. Sci. 2020, 88, 102968. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303. [Google Scholar] [CrossRef]
- Brooks, S.A.; Makvandi-Nejad, S.; Chu, E.; Allen, J.J.; Streeter, C.; Gu, E.; McCleery, B.; Murphy, B.A.; Bellone, R.; Sutter, N.B. Morphological variation in the horse: Defining complex traits of body size and shape. Anim. Genet. 2010, 41, 159–165. [Google Scholar] [CrossRef]




| Centroid Size | SS | MS | df | F | p |
| Equid | 3,444,784.26 | 1,722,437.12 | 2 | 29.67 | <0.0001 |
| D vs. P 1 | 169,763.49 | 169,763.48 | 1 | 1.99 | 0.171 |
| D vs. H 2 | 3,144,525.96 | 3,144,525.92 | 1 | 99.42 | <0.0001 |
| P vs. H 3 | 148,290.19 | 57,035.77 | 1 | 32.49 | <0.0001 |
| Shape | SS | MS | df | F | p |
| Equid | 0.040 | 0.0004 | 112 | 12.20 | <0.0001 |
| D vs. P 1 | 0.032 | 0.0005 | 56 | 19.97 | <0.0001 |
| D vs. H 2 | 0.022 | 0.0004 | 56 | 12.14 | <0.0001 |
| P vs. H 3 | 0.009 | 0.00005 | 168 | 3.92 | 0.065 |
| Donkeys | Ponies | ||
|---|---|---|---|
| Ponies | MD | 18.65 | |
| PD | 0.068 | ||
| Horses | MD | 22.16 | 9.53 |
| PD | 0.056 | 0.027 | |
| Centroid Size | SS | MS | df | F | p |
| Equid | 429,195.33 | 214,597.67 | 2 | 30.62 | <0.0001 |
| D vs. P 1 | 26,212.04 | 26,212.04 | 1 | 2.51 | 0.125 |
| D vs. H 2 | 397,797.56 | 397,797.56 | 1 | 89.86 | <0.0001 |
| P vs. H 3 | 219,783.39 | 219,783.39 | 1 | 35.62 | <0.0001 |
| Shape | SS | MS | df | F | p |
| Equid | 0.092 | 0.0010 | 96 | 12.45 | <0.0001 |
| D vs. P 1 | 0.065 | 0.0014 | 48 | 16.34 | <0.0001 |
| D vs. H 2 | 0.056 | 0.0011 | 48 | 13.73 | <0.0001 |
| P vs. H 3 | 0.002 | 0.00003 | 48 | 0.93 | 0.617 |
| Donkeys | Ponies | ||
|---|---|---|---|
| Ponies | MD | 15.44 | |
| PD | 0.096 | ||
| Horses | MD | 17.77 | 7.93 |
| PD | 0.090 | 0.050 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maśko, M.; Wierzbicka, M.; Zdrojkowski, Ł.; Jasiński, T.; Sikorska, U.; Pawliński, B.; Domino, M. Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics. Animals 2022, 12, 931. https://doi.org/10.3390/ani12070931
Maśko M, Wierzbicka M, Zdrojkowski Ł, Jasiński T, Sikorska U, Pawliński B, Domino M. Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics. Animals. 2022; 12(7):931. https://doi.org/10.3390/ani12070931
Chicago/Turabian StyleMaśko, Małgorzata, Małgorzata Wierzbicka, Łukasz Zdrojkowski, Tomasz Jasiński, Urszula Sikorska, Bartosz Pawliński, and Małgorzata Domino. 2022. "Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics" Animals 12, no. 7: 931. https://doi.org/10.3390/ani12070931
APA StyleMaśko, M., Wierzbicka, M., Zdrojkowski, Ł., Jasiński, T., Sikorska, U., Pawliński, B., & Domino, M. (2022). Comparison of Donkey, Pony, and Horse Dorsal Profiles and Head Shapes Using Geometric Morphometrics. Animals, 12(7), 931. https://doi.org/10.3390/ani12070931






