Genetic Parameters of Somatic Cell Score in Florida Goats Using Single and Multiple Traits Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
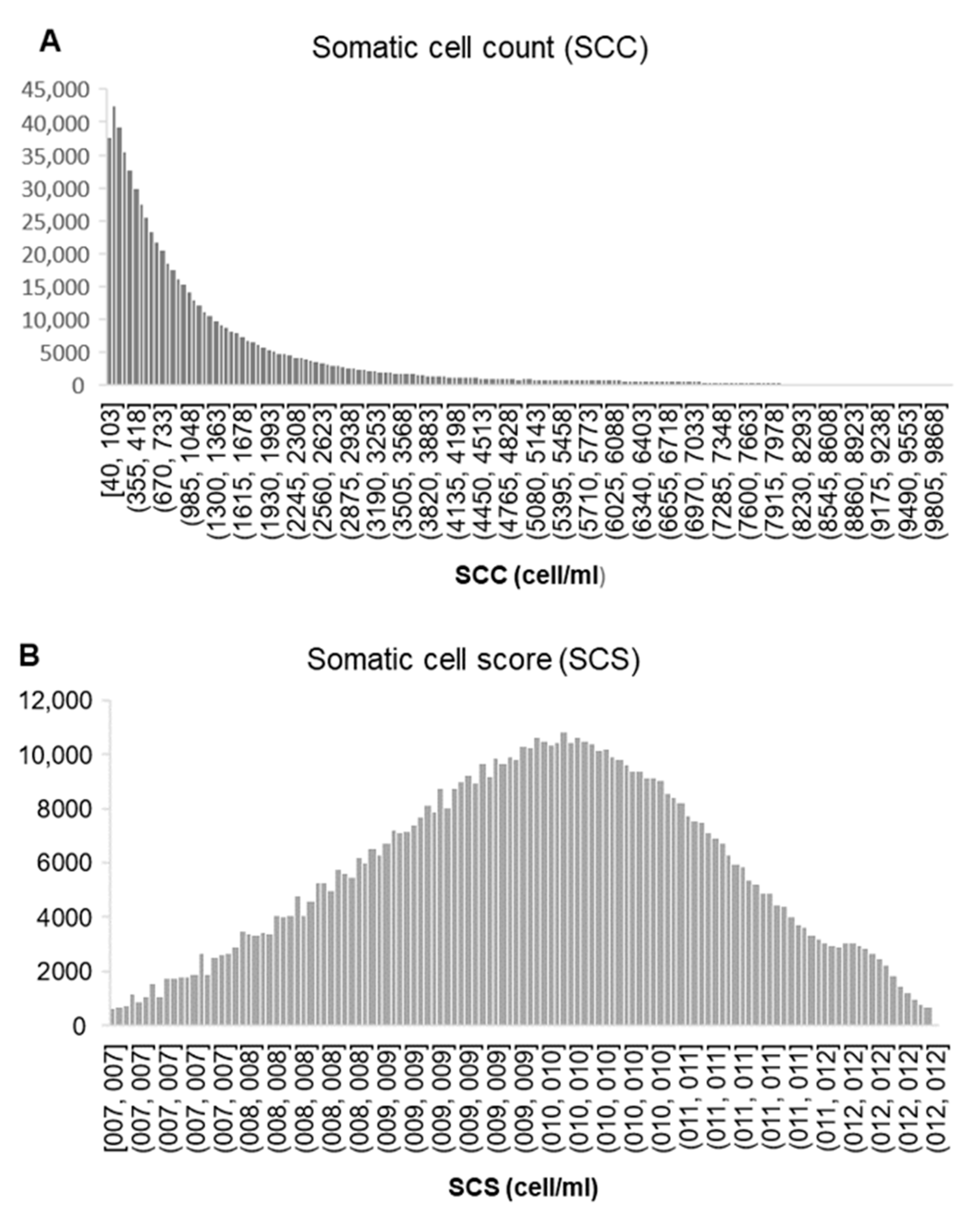
2.1. Phenotypic Data
2.2. Statistical Analysis
- Univariate approach. The parameters for SCSTD and SCSPT were estimated with the total available data (PROD1) using a single linear model (UVM), assuming that they did not vary throughout parities:
- Model 1: SCS evolution through lactation using SCSTD in each lactation
- Model 2: Evolution of the average SCS per lactation using SCSPT throughout the first three lactations.
- Multivariate approach. The parameters for SCSTD and SCSPT were estimated with the total data available from each parity (PROD2), using a multiple trait linear model (MTM), assuming that they were not the same trait across parities.
- Model 3: Evolution of SCS through lactation using SCSTD (with each SCS TD as a different trait)
- Model 4: Evolution of the average SCS per lactation throughout the different lactations (1 to 3), using SCSPT and considering them as different traits.
3. Results and Discussion
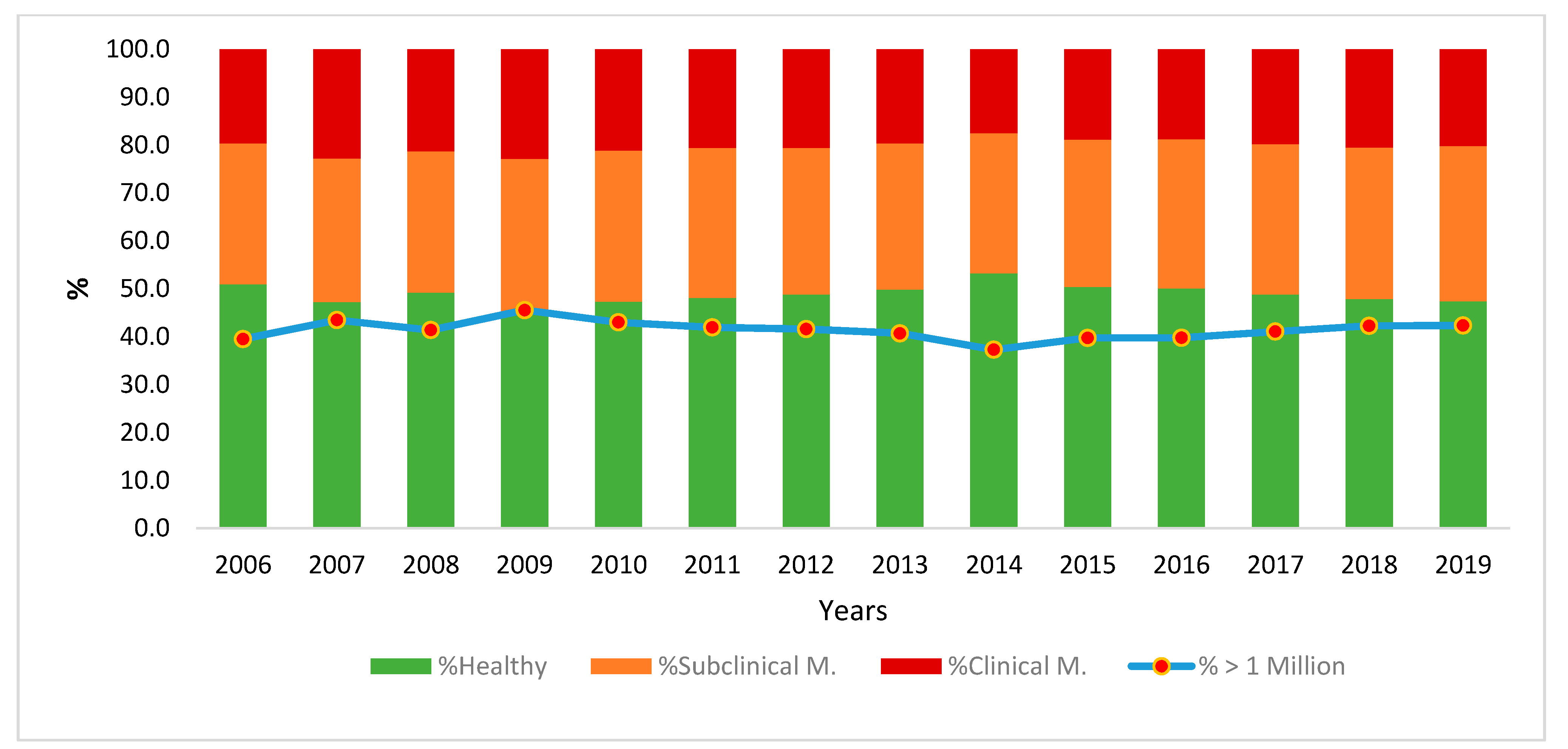
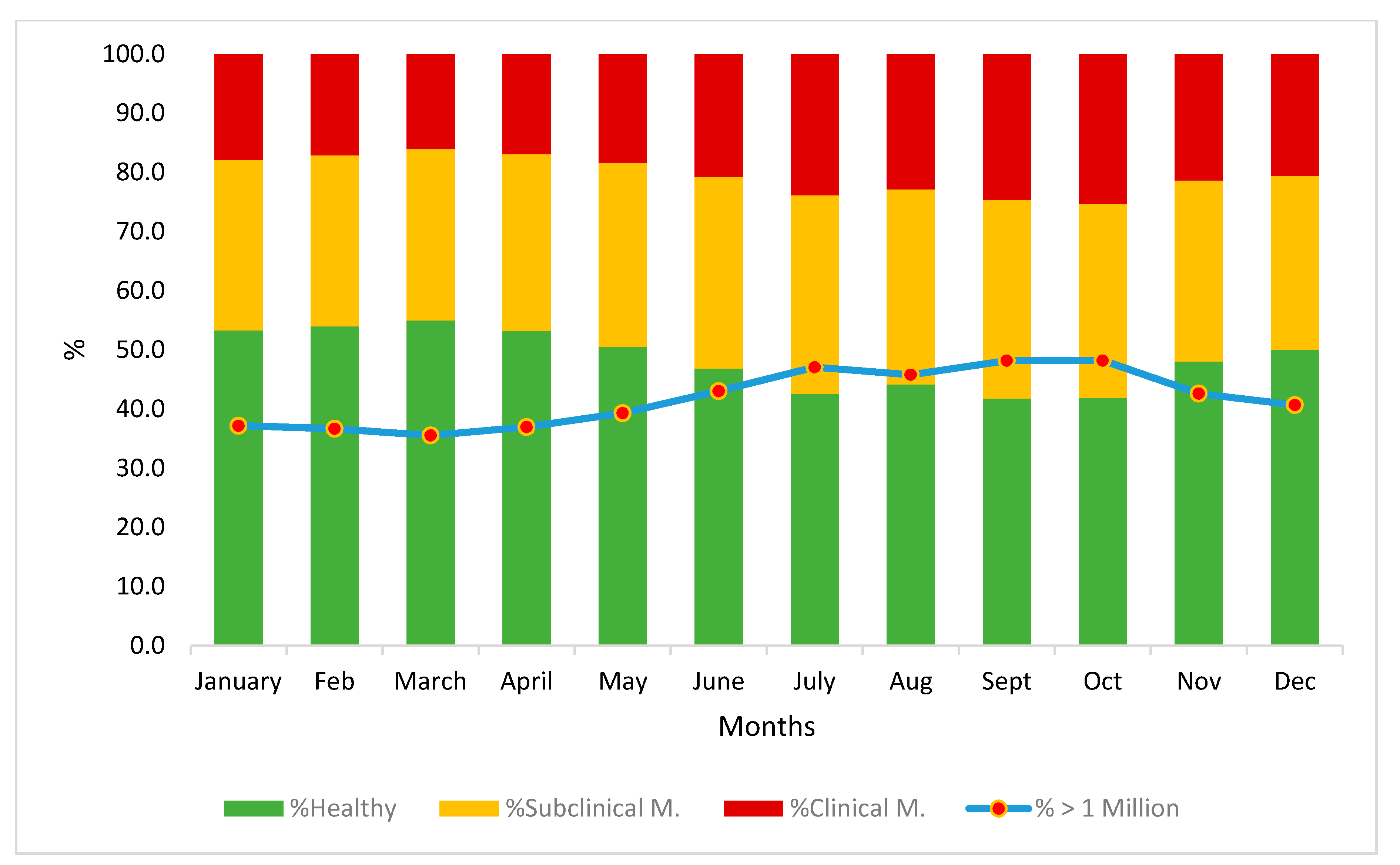
3.1. Estimating the Incidence Level of Mastitis
- 750,000 SC/mL: below this count, a goat is considered healthy
- 750,000–2 million SC/mL: moderate inflammation; subclinical mastitis
- 2 million SC/mL: above this count, a goat has severe inflammation; clinical mastitis.
3.2. Preliminary Analysis of Environmental Effects on SCS
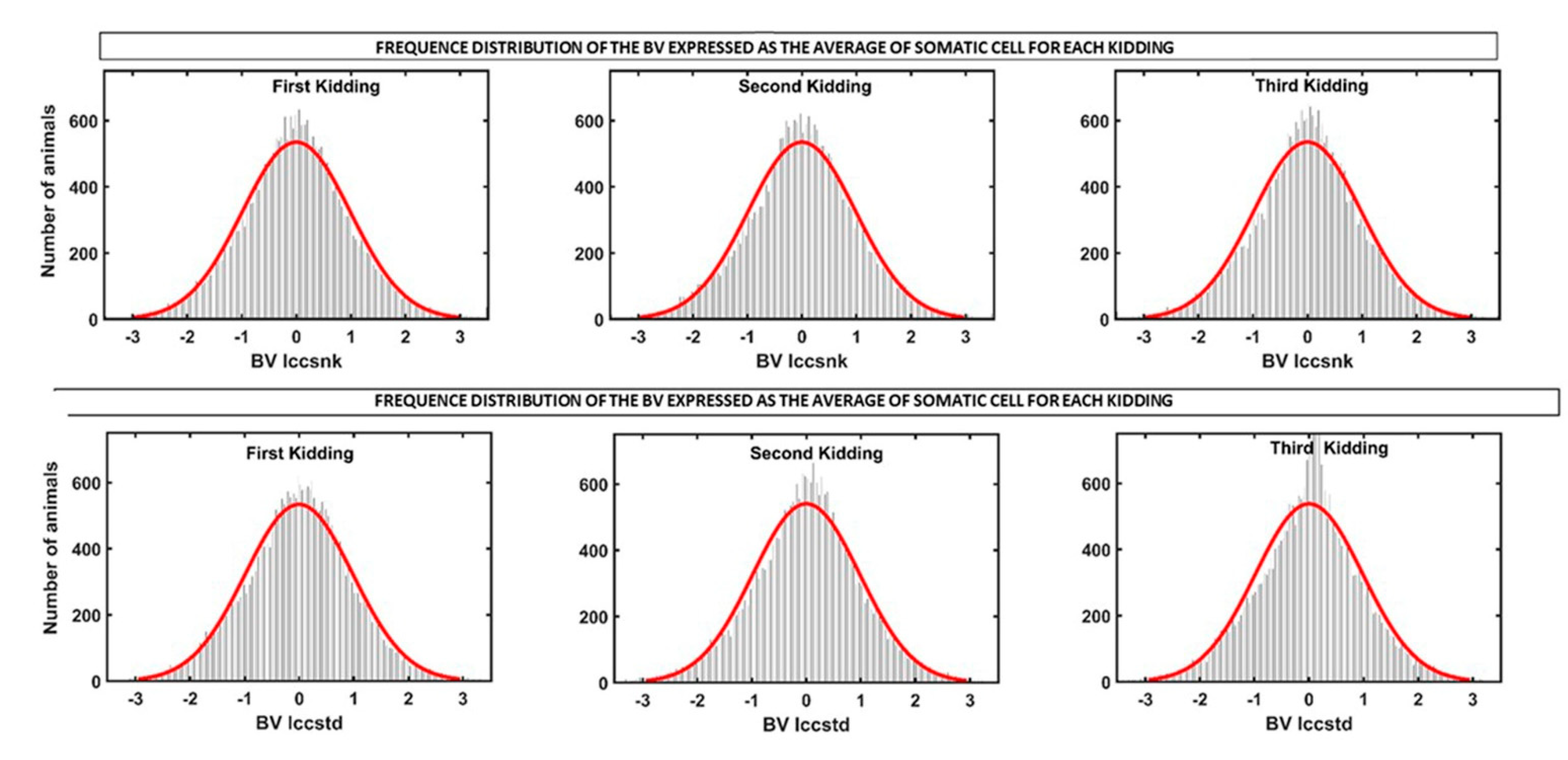
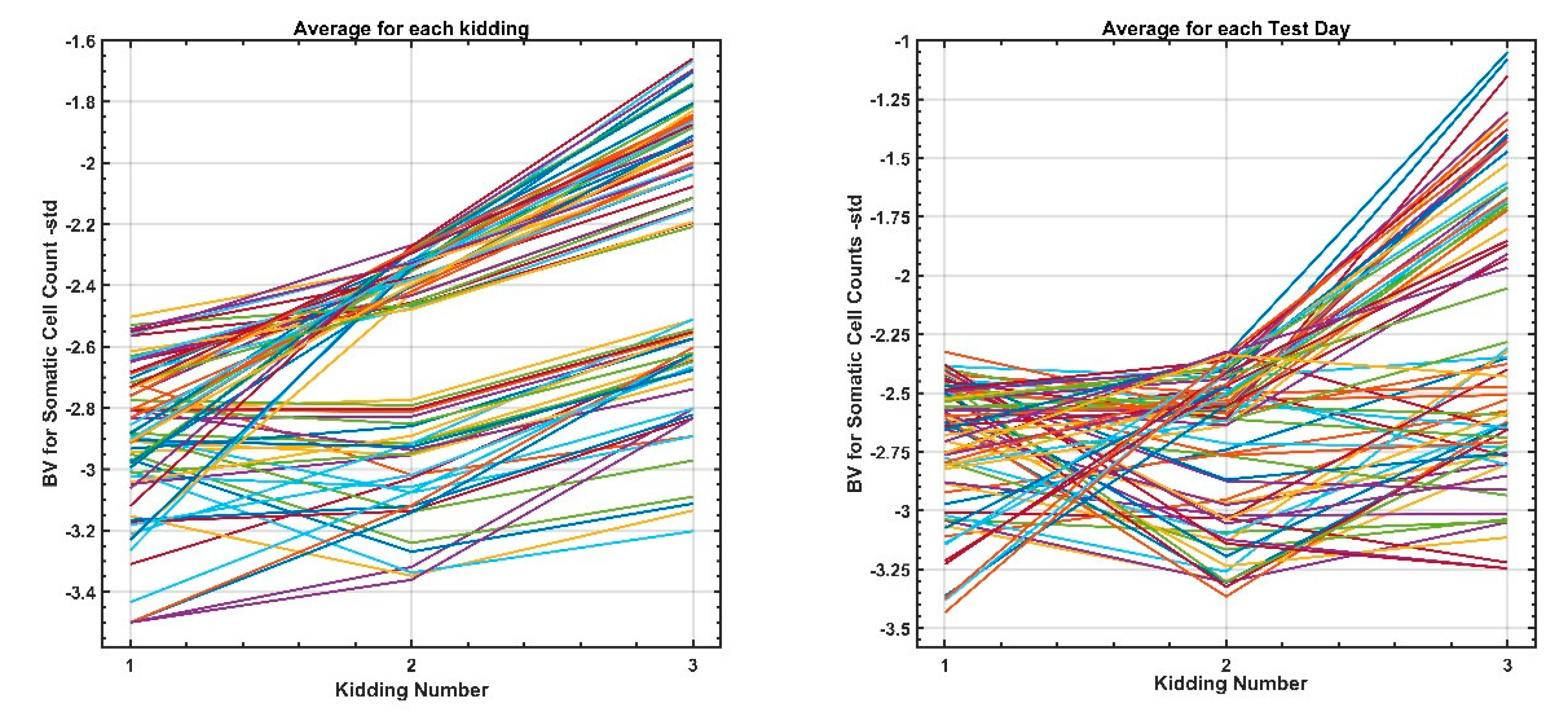
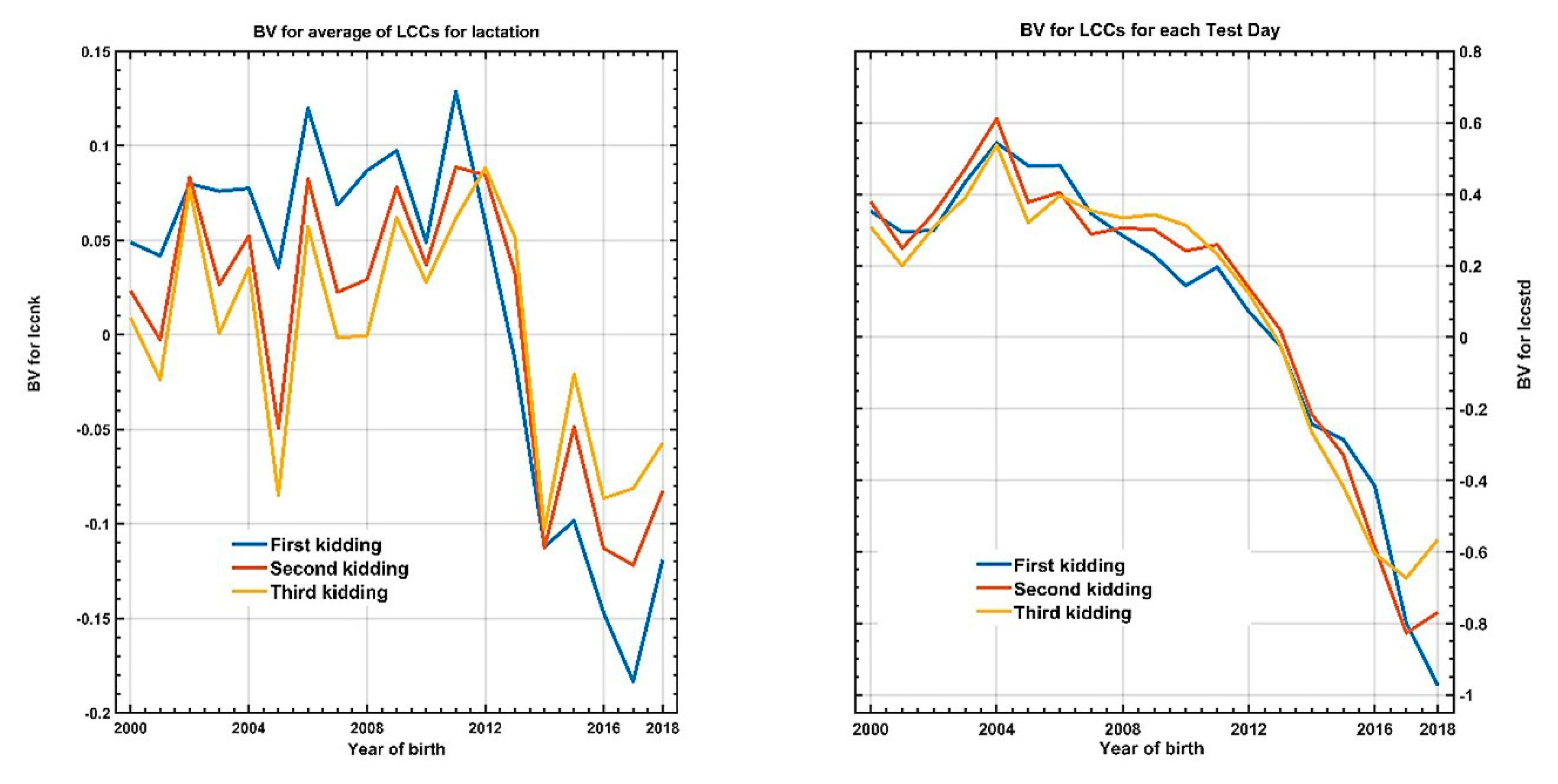
3.3. Genetic Analysis of SCS throughout Lactation and between Lactations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ARCA. Sistema Nacional De Información De Razas. Raza Caprina Florida, Ministerio De Agricultura, Alimentación Y Medio Ambiente. Gobierno De España. 2021. Available online: https://www.mapa.gob.es/es/ganaderia/temas/zootecnia/razas-ganaderas/razas/catalogo-razas/caprino/florida/default.aspx (accessed on 1 August 2021).
- Menéndez-Buxadera, A.; Romero, F.; González, O.; Arrebola, F.; Molina, A. Estimación De Parámetros Genéticos Para La Producción De Leche Y Sus Componentes En La Raza Caprina Papoya Mediante Técnicas De Regresión Aleatoria; ITEA: Zaragoza, Spain, 2008; Volume 104, pp. 127–132. [Google Scholar]
- Menéndez-Buxadera, A.; Molina, A.; Arrebola, F.; Gil, M.; Serradilla, J. Random regression analysis of milk yield and milk composition in the first and second lactations of Murciano-Granadina goats. J. Dairy Sci. 2010, 93, 2718–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, A.; Muñoz, E.; Díaz, C.; Menéndez-Buxadera, A.; Ramón, M.; Sánchez, M.; Carabaño, M.J.; Serradilla, J.M. Goat genomic selection: Impact of the integration of genomic information in the genetic evaluations of the Spanish Florida goats. Small Rumin. Res. 2018, 163, 72–75. [Google Scholar] [CrossRef]
- Ziadi, C.; Molina, A.; Muñoz-Mejías, E.; Sánchez, M.; López, M.; González-Casquet, O. Selection Criteria for Improving Fertility in Spanish Goat Breeds: Estimation of Genetic Parameters and Designing Selection Indices for Optimal Genetic Responses. Animals 2021, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Ziadi, C.; Muñoz-Mejías, E.; Rodríguez, M.S.; López, M.D.; González-Casquet, O.; Alcalá, A.M. Genetic analysis of litter size and number of kids born alive across parities in Spanish goat breeds using a random regression model. Ital. J. Anim. Sci. 2021, 20, 94–101. [Google Scholar] [CrossRef]
- Capoferri, R.; Cremonesi, P.; Castiglioni, B.; Pisoni, G.; Roccabianca, P.; Riva, F.; Filipe, J.; del Corvo, M.; Stella, A.; Williams, J.L.; et al. Comparison of the response of mammary gland tissue from two divergent lines of goat with high and low milk somatic cell scores to an experimental Staphylococcus aureus infection. Vet. Immunol. Immunopathol. 2021, 234, 110208. [Google Scholar] [CrossRef]
- Boutinaud, M.; Jammes, H. Potential uses of milk epithelial cells: A review. Reprod. Nutr. Dev. 2002, 42, 133–147. [Google Scholar] [CrossRef]
- Bergonier, D.; de Crémoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [Green Version]
- Persson, Y.; Olofsson, I. Direct and indirect measurement of somatic cell count as indicator of intramammary infection in dairy goats. Acta Vet. Scand. 2011, 53, 15. [Google Scholar] [CrossRef] [Green Version]
- Knuth, R.M.; Stewart, W.C.; Taylor, J.B.; Yeoman, C.J.; Bisha, B.; Page, C.M.; Rowley, C.M.; Lindsey, B.C.; Van Emon, M.L.; Murphy, T.W. Subclinical mastitis in sheep: Etiology and association with milk somatic cell count and ewe productivity in three research flocks in the Western United States. Transl. Anim. Sci. 2019, 3 (Suppl. 1), 1739–1743. [Google Scholar] [CrossRef] [Green Version]
- Miles, A.M.; Huson, H.J. Graduate Student Literature Review: Understanding the genetic mechanisms underlying mastitis. J. Dairy Sci. 2021, 104, 1183–1191. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Reglamento (CE) Nº 853/2004. Diario Oficial de la Unión Europea. 2004, Volume L 139/55, pp. 1–151. Available online: https://boe.es/doue/2004/139/L00055-00205.pdf (accessed on 21 February 2022).
- Martin, P.; Barkema, H.W.; Brito, L.F.; Narayana, S.G.; Miglior, F. Symposium review: Novel strategies to genetically improve mastitis resistance in dairy cattle. J. Dairy Sci. 2018, 101, 2724–2736. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Boichard, D.; Rupp, R. Invited review: Low milk somatic cell count and susceptibility to mastitis. J. Dairy Sci. 2018, 101, 6703–6714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholtens, M.; Lopez-Villalobos, N.; Garrick, D.J.; Blair, H.T. Heritability of longevity in New Zealand dairy goats. N. Z. J. Anim. Sci. Prod. 2018, 78, 11–15. [Google Scholar]
- Arnal, M.; Larroque, H.; Leclerc, H.; Ducrocq, V.; Robert-Granié, C. Estimation of genetic parameters for dairy traits and somatic cell score in the first 3 parities using a random regression test-day model in French Alpine goats. J. Dairy Sci. 2020, 103, 4517–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cremoux, R. Principes Du Dépistage Des Infections De La Mamelle Par Un Test Fondé Sur Les Concentrations Cellulaires. 2012. Available online: http://idele.fr/no_cache/recherche/publication/idelesolr/recommends/principes-du-depistage-des-infections-de-la-mamelle-par-un-test-fonde-sur-les-concentrations-cellu.html (accessed on 4 February 2021).
- Paape, M.; Poutrel, B.; Contreras, A.; Marco, J.C.; Capuco, A. Milk Somatic Cells and Lactation in Small Ruminants. J. Dairy Sci. 2001, 84, E237–E244. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Shook, G.E. A Lactation Measure of Somatic Cell Count. J. Dairy Sci. 1987, 70, 2666–2672. [Google Scholar] [CrossRef]
- Schaeffer, L.R. Cumulative permanent environmental effects for repeated records animal models. J. Anim. Breed. Genet. 2011, 128, 95–99. [Google Scholar] [CrossRef]
- Togashi, K.; Lin, C. Selection for Milk Production and Persistency Using Eigenvectors of the Random Regression Coefficient Matrix. J. Dairy Sci. 2006, 89, 4866–4873. [Google Scholar] [CrossRef]
- The MathWorks, Inc. MATLAB and Statistics Toolbox Release; The MathWorks, Inc.: Natick, MA, USA, 2019. [Google Scholar]
- Raynal-Ljutovac, K.; Pirisi, A.; de Cremoux, R.; Gonzalo, C. Somatic cells of goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin. Res. 2007, 68, 126–144. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Thiéry, R.; Vautor, E.; Le Loir, Y. Mastitis impact on technological properties of milk and quality of milk products—A review. Dairy Sci. Technol. 2011, 91, 247–282. [Google Scholar] [CrossRef] [Green Version]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent advances in exploiting goat’s milk: Quality, safety and production aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Mehdid, A.; Olives, A.M.-D.; Fernández, N.; Rodríguez, M.; Peris, C. Effect of stress on somatic cell count and milk yield and composition in goats. Res. Vet. Sci. 2019, 125, 61–70. [Google Scholar] [CrossRef]
- Jimenez-Granado, R. El Recuento de Células Somáticas y Factores Productivos en Caprino de Leche. Master’s Thesis, Córdoba University, Córdoba, Spain, October 2011. [Google Scholar]
- Margatho, G.; Rodríguez-Estévez, V.; Medeiros, L.; Simoes, J. Seasonal variation of Serrana goat milk contents in mountain grazing system for cheese manufacture. Rev. Méd. Vét. 2018, 169, 166–172. [Google Scholar]
- Delgado-Pertiñez, M.; Alcalde, M.; Guzmán-Guerrero, J.; Castel, J.; Mena, Y.; Caravaca, F. Effect of hygiene-sanitary management on goat milk quality in semi-extensive systems in Spain. Small Rumin. Res. 2003, 47, 51–61. [Google Scholar] [CrossRef]
- Garcia-Hernandez, R.; Newton, G.; Horner, S.; Nuti, L.C. Effect of photoperiod on milk yield and quality, and reproduction in dairy goats. Livest. Sci. 2007, 110, 214–220. [Google Scholar] [CrossRef]
- Jiménez-Granado, R.; Sánchez-Rodríguez, M.; Arce, C.; Rodriguez-Estevez, V. Factors affecting somatic cell count in dairy goats: A review. Span. J. Agric. Res. 2014, 12, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Apodaca-Sarabia, C.A.; Lopez-Villalobos, N.; Blair, H.T.; Prosser, C.G. Genetic parameters for somatic cell score in dairy goats estimated by random regression. In New Zealand Society of Animal Production; CAB Direct: Glasgow, UK, 2009; pp. 206–209. [Google Scholar]
- Scholtens, M.R.; Lopez-Villalobos, N.; Garrick, D.; Blair, H.; Lehnert, K.; Snell, R. Genetic parameters for total lactation yields of milk, fat, protein, and somatic cell score in New Zealand dairy goats. Anim. Sci. J. Nihon Chikusan Gakkaiho 2019, 91, e13310. [Google Scholar] [CrossRef]
- Rzewuska, K.; Jamrozik, J.; Zarnecki, A.; Strabel, T. Genetic parameters of test-day somatic cell scores for the first three lactations of Polish Holstein-Friesian cattle. Czech J. Anim. Sci. 2011, 56, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Bagnicka, E.; Łukaszewicz, M.; Ådnøy, T. Genetic parameters of somatic cell score and lactose content in goat’s milk. J. Anim. Feed Sci. 2016, 25, 210–215. [Google Scholar] [CrossRef]
- Barrón-Bravo, O.; Gutiérrez-Chávez, A.; Angel, C.; Montaldo, H.; Shepard, L.; Valencia-Posadas, M. Losses in milk yield, fat and protein contents according to different levels of somatic cell count in dairy goats. Small Rumin. Res. 2013, 113, 421–431. [Google Scholar] [CrossRef]
- Anniss, F.; McDougall, S. Prevalence of bacterial infections and somatic cell count in early postpartum milking goats. In New Zealand Society of Animal Production; CAB Direct: Glasgow, UK, 2000; pp. 284–286. Available online: https://www.cabdirect.org/cabdirect/abstract/20000405775 (accessed on 2 December 2021).
- Arnal, M.; Larroque, H.; Leclerc, H.; Ducrocq, V.; Robert-Granié, C. Genetic parameters for first lactation dairy traits in the Alpine and Saanen goat breeds using a random regression test-day model. Genet. Sel. Evol. 2019, 51, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rupp, R.; Clément, V.; Piacere, A.; Robert-Granié, C.; Manfredi, E. Genetic parameters for milk somatic cell score and relationship with production and udder type traits in dairy Alpine and Saanen primiparous goats. J. Dairy Sci. 2011, 94, 3629–3634. [Google Scholar] [CrossRef] [Green Version]
- Murphy, T.W.; Berger, Y.M.; Holman, P.W.; Baldin, M.; Burgett, R.L.; Thomas, D.L. Estimates of genetic parameters, genetic trends, and inbreeding in a crossbred dairy sheep research flock in the United States. J. Anim. Sci. 2017, 95, 4300–4309. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Cho, C.I.; Choi, T.J.; Park, B.; Choi, J.G.; Choy, Y.H.; Lee, S.S.; Cho, K.H. Estimation of Genetic Parameters for Somatic Cell Scores of Holsteins Using Multi-trait Lactation Models in Korea. Asian-Austral. J. Anim. Sci. 2015, 28, 303–310. [Google Scholar] [CrossRef]
- Via, S.; Gomulkiewicz, R.; Gerdien, D.J.; Samuel, M.S.; Carl, S.; Peter, T. Adaptive phenotypic plasticity: Consensus and controversy. Trends Ecol. Evol. 1995, 10, 212–217. [Google Scholar] [CrossRef]
- De Jong, G.; Bijma, P. Selection and phenotypic plasticity in evolutionary biology and animal breeding. Livest. Prod. Sci. 2002, 78, 195–214. [Google Scholar] [CrossRef]
- Rupp, R.; Bergonier, D.; Dion, S.; Hygonenq, M.C.; Aurel, M.R.; Robert-Granié, C.; Foucras, G. Response to somatic cell count-based selection for mastitis resistance in a divergent selection experiment in sheep. J. Dairy Sci. 2009, 92, 1203–1219. [Google Scholar] [CrossRef] [Green Version]
- Rupp, R.; Huau, C.; Caillat, H.; Fassier, T.; Bouvier, F.; Pampouille, E.; Clément, V.; Palhière, I.; Larroque, H.; Tosser-Klopp, G.; et al. Divergent selection on milk somatic cell count in goats improves udder health and milk quality with no effect on nematode resistance. J. Dairy Sci. 2019, 102, 5242–5253. [Google Scholar] [CrossRef] [Green Version]
- Savegnago, R.; Rosa, G.; Valente, B.; Herrera, L.; Carneiro, R.; Sesana, R.; El Faro, L.; Munari, D. Estimates of genetic parameters and eigenvector indices for milk production of Holstein cows. J. Dairy Sci. 2013, 96, 7284–7293. [Google Scholar] [CrossRef] [Green Version]
- Boligon, A.A.; Vicente, I.S.; Vaz, R.Z.; Campos, G.; Souza, F.R.P.; Carvalheiro, R.; Albuquerque, L. Principal component analysis of breeding values for growth and reproductive traits and genetic association with adult size in beef cattle1. J. Anim. Sci. 2016, 94, 5014–5022. [Google Scholar] [CrossRef]

| Parity | Total | |||
|---|---|---|---|---|
| First | Second | Third | ||
| No. of goats | 25,430 | 19,268 | 12,599 | 27,749 |
| No. of sires | 941 | 900 | 807 | 941 |
| No. of dams | 15,215 | 12,150 | 8,406 | 16,243 |
| No. of TD records | 145,816 | 118,039 | 76,799 | 340,654 |
| Average SCC (×103) | 972.44 (3.02) | 1249.45 (3.71) | 1483.02 (4.69) | 1193.96 (2.14) |
| Model 1. Test Day Average | Model 2. Average Total Lactations | |
|---|---|---|
| Genetic effect variance | 0.2577 (0.013) | 0.4678 (0.011) |
| Permanent effect variance | 0.2522 (0.010) | 0.3511 (0.017) |
| Residual variance | 0.5389 (0.005) | 1.4785 (0.004) |
| Heritability | 0.246 (0.011) | 0.204 (0.005) |
| Repeatability | 0.486 (0.005) | 0.356 (0.003) |
| Number of records | 57,297 | 340,654 |
| Model 3. Average Each Parity | Model 4. Test Day Average | |||||
|---|---|---|---|---|---|---|
| Genetic Var First parity | 0.257 (0.016) | 0.696 (0.020) | ||||
| Genetic Var Second parity | 0.399 (0.019) | 0.863 (0.022) | ||||
| Genetic Var Third parity | 0.382 (0.019) | 0.813 (0.023) | ||||
| Permanent Var | 0.255 (0.010) | 0.308 (0.011) | ||||
| Residual Var First parity | 0.536 (0.011) | 1.450 (0.006) | ||||
| Residual Var Second parity | 0.435 (0.011) | 1.217 (0.006) | ||||
| Residual Var Third parity | 0.412 (0.012) | 1.160 (0.007) | ||||
| Heritability First parity | 0.245 (0.013) | 0.284 (0.007) | ||||
| Heritability Second parity | 0.366 (0.015) | 0.361 (0.007) | ||||
| Heritability Third parity | 0.365 (0.016) | 0.356 (0.008) | ||||
| Repeatability First parity | 0.488 (0.011) | 0.409 (0.004) | ||||
| Repeatability Second parity | 0.601 (0.010) | 0.491 (0.004) | ||||
| Repeatability Third parity | 0.607 (0.012) | 0.491 (0.005) | ||||
| Rg first and second parity | 0.767 (0.022) | 0.456 (0.015) | ||||
| Rg first and third parity | 0.538 (0.034) | 0.178 (0.021) | ||||
| Rg second and third parity | 0.854 (0.011) | 0.658(0.011) | ||||
| Principal Components Analysis Genetic variance explained % | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 |
| 86.5 | 13.4 | 0.01 | 64.8 | 25.6 | 9.6 | |
| Eigenvector 1 | 0.417 | −0.846 | 0.331 | 0.389 | −0.843 | 0.371 |
| Eigenvector 2 | 0.666 | 0.038 | −0.745 | 0.697 | 0.006 | −0.717 |
| Eigenvector 3 | 0.618 | 0.531 | 0.580 | 0.603 | 0.538 | 0.590 |
| EBV SCC Average of Each Lactation | EBV SCC on Each Test Day | ||||
|---|---|---|---|---|---|
| First Parity | Second Parity | Third Parity | First Parity | Second Parity | Third Parity |
| 1.000 | 0.870 | 0.730 | 0.757 | 0.662 | 0.485 |
| 1.000 | 0.972 | 0.554 | 0.769 | 0.697 | |
| 1.000 | 0.407 | 0.752 | 0.735 | ||
| 1.000 | 0.622 | 0.389 | |||
| 1.000 | 0.813 | ||||
| 1.000 | |||||
| Principal Component | Average for Each Parity | Test Day Average | ||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Eigenvalues | 2.719 | 0.281 | 2.232 | 0.630 | 0.138 | |
| % Explained variance | 90.6 | 9.4 | 74.4 | 21.0 | 4.6 | |
| Eigenvector ev parity 1 | 0.551 | 0.790 | 0.505 | 0.816 | −0.281 | |
| Eigenvector ev parity 2 | 0.604 | −0.156 | 0.638 | −0.134 | 0.758 | |
| Eigenvector ev parity 3 | 0.576 | −0.592 | 0.581 | −0.562 | −0.588 | |
| Ipc1 | 0.551 × EBV1 + 0.604 × EBV2 | 0.505 × EBV1 + 0.638 × EBV2 | ||||
| Ipc2 | 0.790 × EBV1 − 0.156 × EBV2 | 0.816 × EBV1 − 0.134 × EBV2 | ||||
| IpcT | Ipc1 + Ipc2 | Ipc1 + Ipc2 | ||||
| Correlation between EBVi and Ipc | Ipc1 | Ipc2 | Ipct | Ipc1 | Ipc2 | Ipct |
| First parity | 0.901 | 0.419 | 0.947 | 0.754 | 0.648 | 0.868 |
| Second parity | 0.995 | −0.123 | 0.982 | 0.953 | −0.106 | 0.915 |
| Third parity | 0.247 | −0.530 | 0.910 | 0.868 | −0.446 | 0.761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Granado, R.; Molina, A.; Ziadi, C.; Sanchez, M.; Muñoz-Mejías, E.; Demyda-Peyrás, S.; Menendez-Buxadera, A. Genetic Parameters of Somatic Cell Score in Florida Goats Using Single and Multiple Traits Models. Animals 2022, 12, 1009. https://doi.org/10.3390/ani12081009
Jimenez-Granado R, Molina A, Ziadi C, Sanchez M, Muñoz-Mejías E, Demyda-Peyrás S, Menendez-Buxadera A. Genetic Parameters of Somatic Cell Score in Florida Goats Using Single and Multiple Traits Models. Animals. 2022; 12(8):1009. https://doi.org/10.3390/ani12081009
Chicago/Turabian StyleJimenez-Granado, Rocío, Antonio Molina, Chiraz Ziadi, Manuel Sanchez, Eva Muñoz-Mejías, Sebastián Demyda-Peyrás, and Alberto Menendez-Buxadera. 2022. "Genetic Parameters of Somatic Cell Score in Florida Goats Using Single and Multiple Traits Models" Animals 12, no. 8: 1009. https://doi.org/10.3390/ani12081009
APA StyleJimenez-Granado, R., Molina, A., Ziadi, C., Sanchez, M., Muñoz-Mejías, E., Demyda-Peyrás, S., & Menendez-Buxadera, A. (2022). Genetic Parameters of Somatic Cell Score in Florida Goats Using Single and Multiple Traits Models. Animals, 12(8), 1009. https://doi.org/10.3390/ani12081009







