Elevated Gamma Connectivity in Nidopallium Caudolaterale of Pigeons during Spatial Path Adjustment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
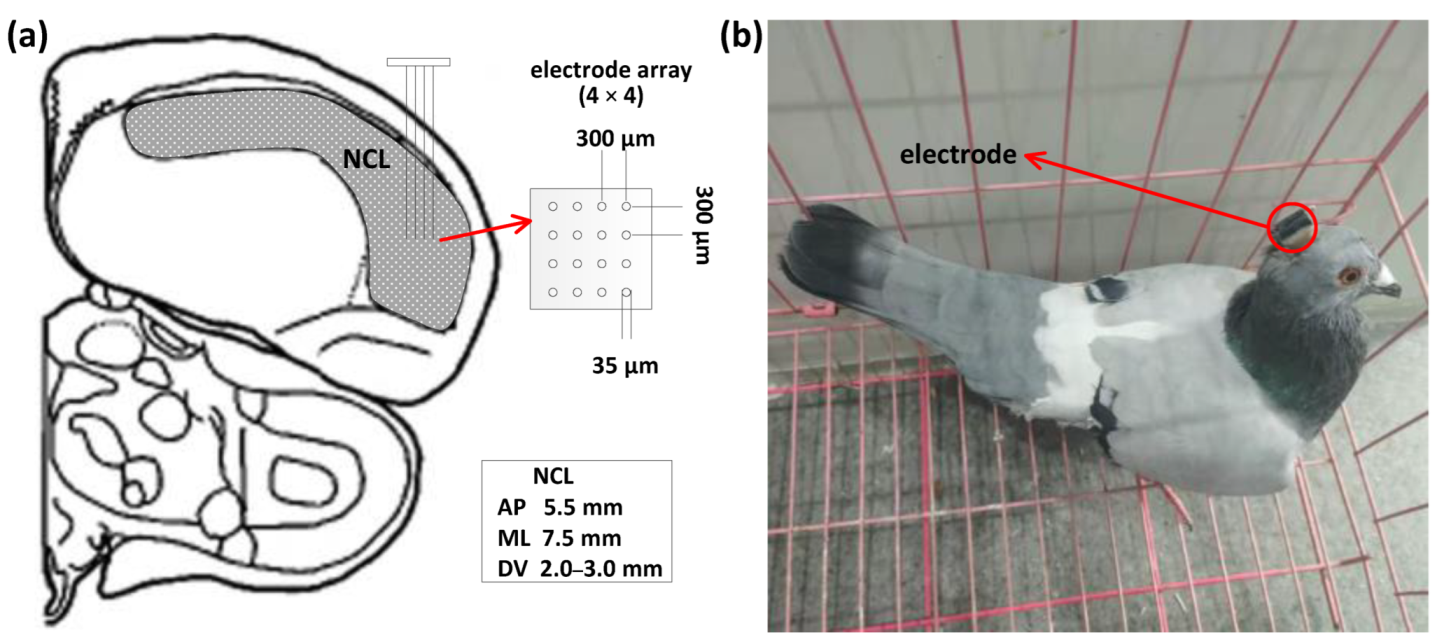
2.1. Subjects, Surgery, and Electrode Implantation
2.2. Spatial Cognitive Experiment and Apparatus
2.3. Behavioural Data and LFPs’ Recording
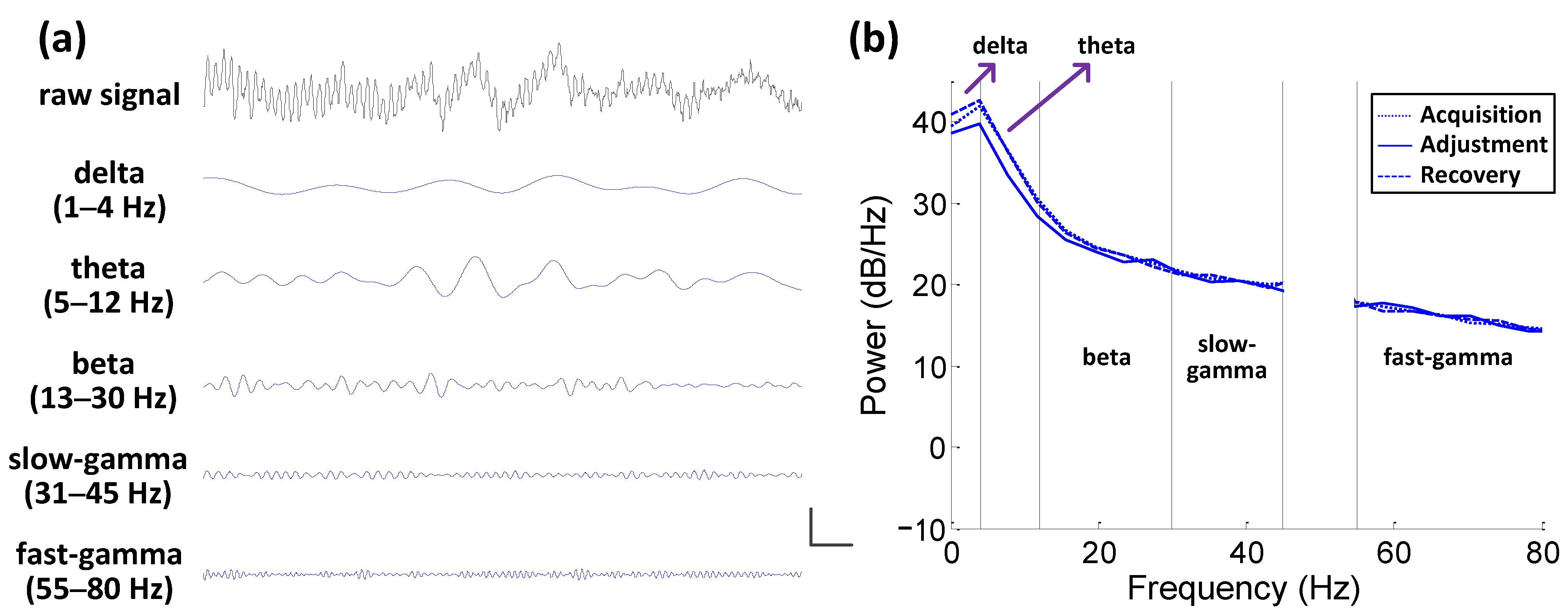
2.4. Spectral Analysis
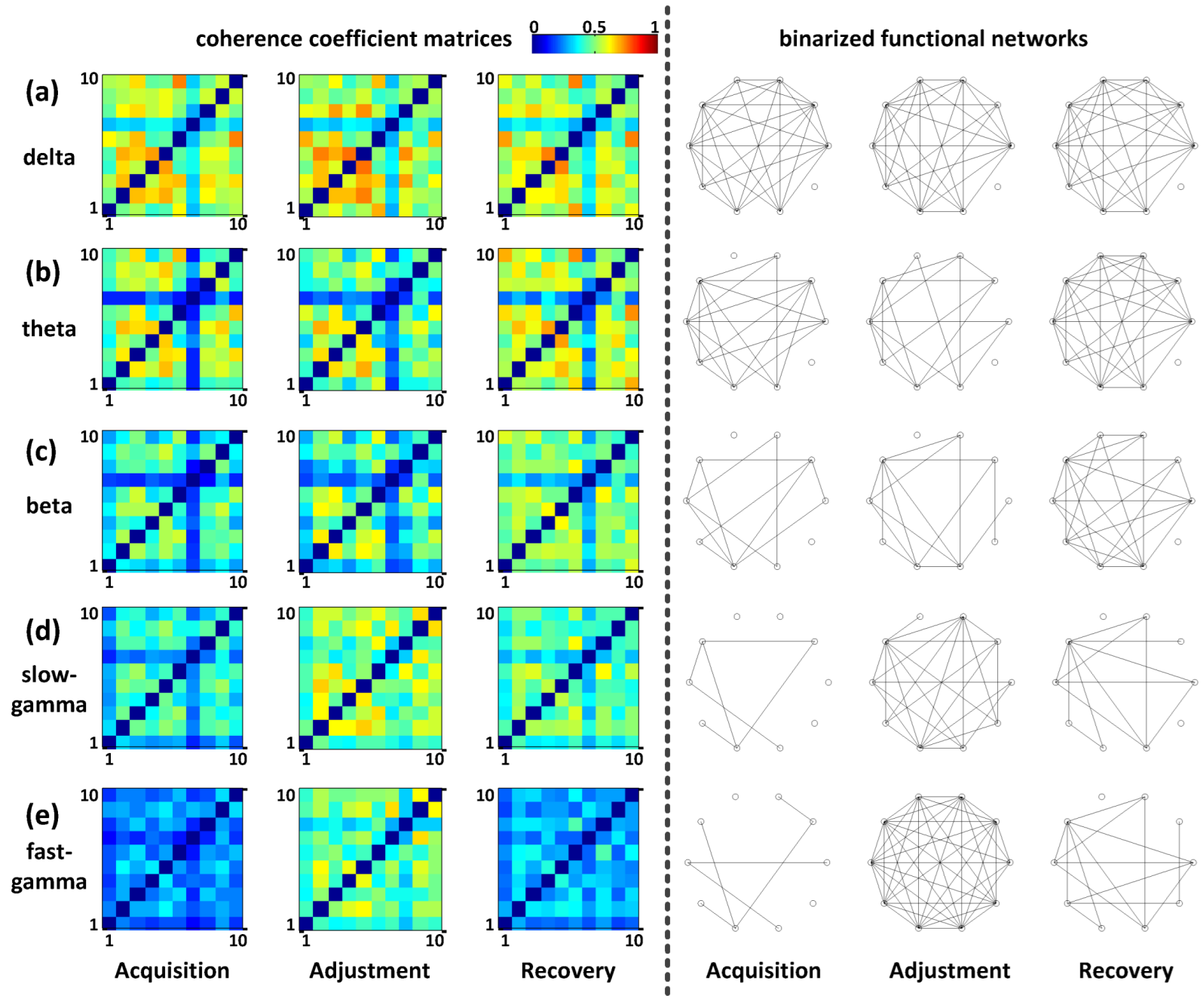
2.5. Functional Connectivity Analysis
2.6. PLS-DA Multivariate Model
2.7. Statistical Analysis
3. Results
3.1. Behavioural Results
3.2. Neural Results
3.2.1. PSD Analysis
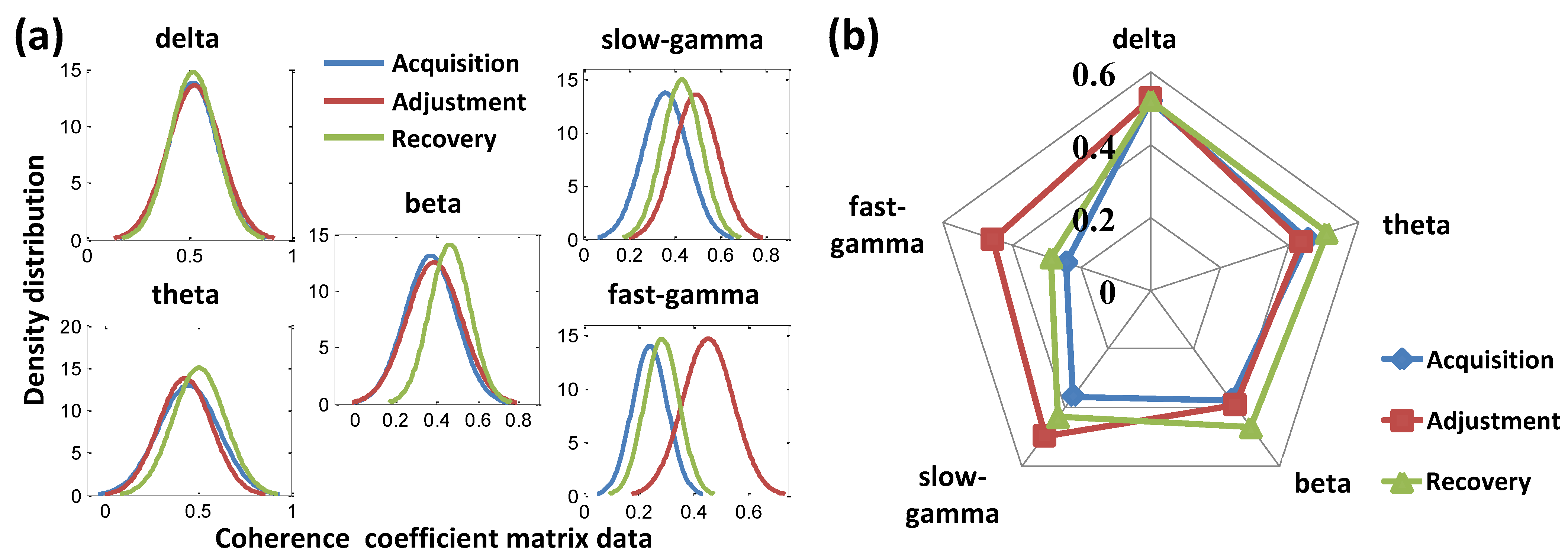
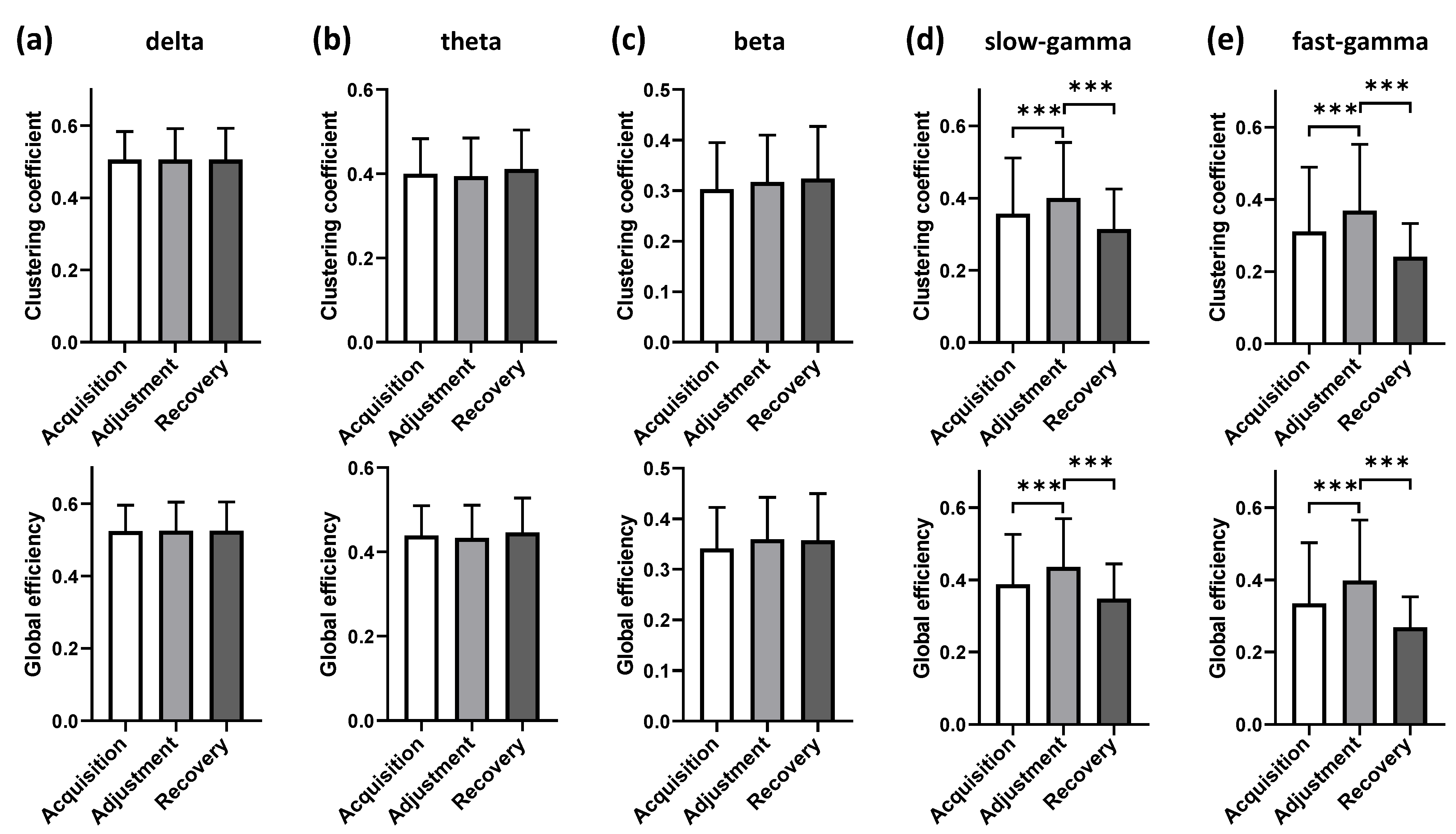
3.2.2. Functional Connectivity Analysis
3.2.3. Validation of Spatial-Associated Functional Connectivity Signatures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabadayi, C.; Bobrowicz, K.; Osvath, M. The detour paradigm in animal cognition. Anim. Cogn. 2018, 21, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolman, E.C. Cognitive maps in rats and men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poucet, B.; Thinus-Blanc, C.; Chapuis, N. Route planning in cats, in relation to the visibility of the goal. Anim. Behav. 1983, 31, 594–599. [Google Scholar] [CrossRef]
- Yu, J.Y.; Frank, L.M. Hippocampal–cortical interaction in decision making. Neurobiol. Learn. Mem. 2015, 117, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrón-Oyarzo, I.; Espinosa, N.; Aguilar-Rivera, M.; Fuenzalida, M.; Aboitiz, F.; Fuentealba, P. Coordinated prefrontal–hippocampal activity and navigation strategy-related prefrontal firing during spatial memory formation. Proc. Natl. Acad. Sci. USA 2018, 115, 7123–7128. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, M.C.; Shin, J.D.; Jadhav, S.P. Coherent coding of spatial position mediated by theta oscillations in the hippocampus and prefrontal cortex. J. Neurosci. 2019, 39, 4550–4565. [Google Scholar] [CrossRef] [Green Version]
- Alvernhe, A.; Van Cauter, T.; Save, E.; Poucet, B. Different CA1 and CA3 representations of novel routes in a shortcut situation. J. Neurosci. 2008, 28, 7324–7333. [Google Scholar] [CrossRef]
- Alvernhe, A.; Save, E.; Poucet, B. Local remapping of place cell firing in the Tolman detour task. Eur. J. Neurosci. 2011, 33, 1696–1705. [Google Scholar] [CrossRef]
- Haller, M.; Case, J.; Crone, N.E.; Chang, E.F.; King-Stephens, D.; Laxer, K.D.; Weber, P.B.; Parvizi, J.; Knight, R.T.; Shestyuk, A.Y. Persistent neuronal activity in human prefrontal cortex links perception and action. Nat. Hum. Behav. 2018, 2, 80–91. [Google Scholar] [CrossRef]
- Yang, S.T.; Shi, Y.; Wang, Q.; Peng, J.Y.; Li, B.M. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol. Brain 2014, 7, 61. [Google Scholar] [CrossRef]
- Hok, V.; Save, E.; Lenck-Santini, P.P.; Poucet, B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc. Natl. Acad. Sci. USA 2005, 102, 4602–4607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, J.M.; Ma, L.; Balaguer-Ballester, E.; Durstewitz, D.; Seamans, J.K. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 5086–5091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Shang, Z.; Zhao, K.; Cheng, S.; Wan, H. The role of Hp-NCL network in goal-directed routing information encoding of bird: A review. Brain Sci. 2020, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Rinnert, P.; Kirschhock, M.E.; Nieder, A. Neuronal correlates of spatial working memory in the endbrain of crows. Curr. Biol. 2019, 29, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Kroner, S.; Güntürkün, O. Afferent and efferent connections of the caudolateral neostriatum in the pigeon (Columba livia): A retro- and anterograde pathway tracing study. J. Comp. Neurol. 1999, 407, 228–260. [Google Scholar] [CrossRef]
- Shanahan, M.; Bingman, V.P.; Shimizu, T.; Wild, M.; Güntürkün, O. Large-scale network organization in the avian forebrain: A connectivity matrix and theoretical analysis. Front. Comput. Neurosci. 2013, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, B.; Güntürkün, O. Selective deficits in reversal learning after neostriatum caudolaterale lesions in pigeons: Possible behavioral equivalencies to the mammalian prefrontal system. Behav. Brain Res. 1998, 96, 125–133. [Google Scholar] [CrossRef]
- Diekamp, B.; Gagliardo, A.; Güntürkün, O. Nonspatial and subdivision-specific working memory deficits after selective lesions of the avian prefrontal cortex. J. Neurosci. 2002, 22, 9573–9580. [Google Scholar] [CrossRef] [Green Version]
- Güntürkün, O. The convergent evolution of neural substrates for cognition. Psychol. Res. 2012, 76, 212–219. [Google Scholar] [CrossRef]
- Mouritsen, H.; Heyers, D.; Güntürkün, O. The neural basis of long-distance navigation in birds. Annu. Rev. Physiol. 2016, 78, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wan, H.; Li, S.; Shang, Z.; Shi, L. The role of nidopallium caudolaterale in the goal-directed behavior of pigeons. Behav. Brain Res. 2017, 326, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, J.; Cheng, S.; Yang, L.; Yang, L.; Wang, L.; Shang, Z.; Wan, H. Enhanced hippocampus-nidopallium caudolaterale connectivity during route formation in goal-directed spatial learning of pigeons. Animals 2021, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Karten, H.; Hodos, W. A Stereotaxic Atlas of the Brain of the Pigeon (Columba Livia); Johns Hopkins Press: Baltimore, MD, USA, 1967. [Google Scholar]
- Li, M.; Liang, Y.; Yang, L.; Wang, H.; Yang, Z.; Zhao, K.; Shang, Z.; Wan, H. Automatic bad channel detection in implantable brain-computer interfaces using multimodal features based on local field potentials and spike signals. Comput. Biol. Med. 2020, 116, 103572. [Google Scholar] [CrossRef] [PubMed]
- Unde, S.A.; Shriram, R. PSD based coherence analysis of EEG signals for stroop task. Int. J. Comput. Appl. 2014, 95, 16. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Li, M.; Yang, L.; Wang, H.; Wan, H.; Shang, Z. Functional connectivity changes in the intra-and inter-brain during the construction of the multi-brain network of pigeons. Brain Res. Bull. 2020, 161, 147–157. [Google Scholar] [CrossRef]
- Hallquist, M.N.; Hillary, F.G. Graph theory approaches to functional network organization in brain disorders: A critique for a brave new small-world. Netw. Neurosci. 2018, 3, 1–26. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Kakkos, I.; Dai, Z.; Wang, H.; Sgarbas, K.; Thakor, N.; Bezerianos, A.; Sun, Y. Functional connectivity analysis of mental fatigue reveals different network topological alterations between driving and vigilance tasks. IEEE Trans. Neural Syst. Rehabil. 2018, 26, 740–749. [Google Scholar] [CrossRef]
- Rosenberg, A.M.; Rausser, S.; Ren, J.; Mosharov, E.V.; Sturm, G.; Ogden, R.T.; Patel, P.; Kumar Soni, R.; Lacefield, C.; Tobin, D.J.; et al. Quantitative mapping of human hair greying and reversal in relation to life stress. eLife 2021, 10, e67437. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Akçay, H.G.; Kabasakal, B.; Aksu, D.; Demir, N.; Öz, M.; Erdoğan, A. Automated bird counting with deep learning for regional bird distribution mapping. Animals 2020, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Romo, R.; Brody, C.D.; Hernández, A.; Lemus, L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 1999, 399, 470–473. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, M. From cognitive to neural models of working memory. Philos. Trans. R. Soc. B 2007, 362, 761–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, F.; Wibral, M.; Mohr, H.M.; Singer, W.; Uhlhaas, P.J. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J. Neurosci. 2012, 32, 12411–12420. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Noninvasively decoding the contents of visual working memory in the human prefrontal cortex within high-gamma oscillatory patterns. J. Cogn. Neurosci. 2012, 24, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Lundqvist, M.; Bastos, A.M. Working memory 2.0. Neuron 2018, 100, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhao, K.; Wang, D.; Ping, Y.; Wan, H. Goal-directed behavior elevates gamma oscillations in nidopallium caudolaterale of pigeon. Brain Res. Bull. 2018, 137, 10–16. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Li, S.; Wan, H. Decoding pigeon behavior outcomes using functional connections among local field potentials. Comput. Intell. Neurosci. 2018, 2018, 3505371. [Google Scholar] [CrossRef] [Green Version]
- Colgin, L.L.; Denninger, T.; Fyhn, M.; Hafting, T.; Bonnevie, T.; Jensen, O.; Moser, M.B.; Moser, E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009, 462, 353–357. [Google Scholar] [CrossRef]
- Spellman, T.; Rigotti, M.; Ahmari, S.E.; Fusi, S.; Gogos, J.A.; Gordon, J.A. Hippocampal–prefrontal input supports spatial encoding in working memory. Nature 2015, 522, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Hirel, J.; Gaussier, P.; Quoy, M.; Banquet, J.P.; Save, E.; Poucet, B. The hippocampo-cortical loop: Spatio-temporal learning and goal-oriented planning in navigation. Neural. Netw. 2013, 43, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Treves, A.; Rolls, E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus 1994, 4, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Colgin, L.L.; Moser, E.I.; Moser, M.B. Understanding memory through hippocampal remapping. Trends Neurosci. 2008, 31, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Disarrangement and Reorganization of the Hippocampal Functional Connectivity during the Spatial Path Adjustment of Pigeons; PREPRINT (Version 1); Research Square: Durham, NC, USA, 2021. [Google Scholar] [CrossRef]
- O’Reilly, R.C.; Rudy, J.W. Computational principles of learning in the neocortex and hippocampus. Hippocampus 2000, 10, 389–397. [Google Scholar] [CrossRef]
- Sherry, D.F.; Grella, S.L.; Guigueno, M.F.; White, D.J.; Marrone, D.F. Are there place cells in the avian hippocampus? Brain Behav. Evolut. 2017, 90, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Nie, J.; Yang, L.; Liu, X.; Shang, Z.; Wan, H. Hippocampus-nidopallium caudolaterale interactions exist in the goal-directed behavior of pigeon. Brain Res. Bull. 2019, 153, 257–265. [Google Scholar] [CrossRef]
- Canolty, R.T.; Soltani, M.; Dalal, S.S.; Edwards, E.; Dronkers, N.F.; Nagarajan, S.S.; Kirsch, H.E.; Barbaro, N.M.; Knight, R.T. Spatiotemporal dynamics of word processing in the human brain. Front. Neurosci. 2007, 1, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Nieder, A. Inside the corvid brain—Probing the physiology of cognition in crows. Curr. Opin. Behav. Sci. 2017, 16, 8–14. [Google Scholar] [CrossRef]
- Meij, J.; Rattenborg, N.C.; Beckers, G.J.L. Divergent neuronal activity patterns in the avian hippocampus and nidopallium. Eur. J. Neurosci. 2020, 52, 3124–3139. [Google Scholar] [CrossRef] [Green Version]
- Nikulin, V.V.; Brismar, T. Phase synchronization between alpha and beta oscillations in the human electroencephalogram. Neuroscience 2006, 137, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Hyafil, A.; Giraud, A.; Fontolan, L.; Gutkin, B. Neural cross-frequency coupling: Connecting architectures, mechanisms, and functions. Trends Neurosci. 2015, 38, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Bahramisharif, A.; van Gerven, M.A.J.; Jensen, O. Distinct directional couplings between slow and fast gamma power to the phase of theta oscillations in the rat hippocampus. Eur. J. Neurosci. 2020, 51, 2070–2081. [Google Scholar] [CrossRef]
- Schroeder, C.E.; Lakatos, P.; Kajikawa, Y.; Partan, S.; Puce, A. Neuronal oscillations and visual amplification of speech. Trends. Cogn. Sci. 2008, 12, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Fan, J.; Lin, L.; Shang, Z.; Wan, H. Elevated Gamma Connectivity in Nidopallium Caudolaterale of Pigeons during Spatial Path Adjustment. Animals 2022, 12, 1019. https://doi.org/10.3390/ani12081019
Li M, Fan J, Lin L, Shang Z, Wan H. Elevated Gamma Connectivity in Nidopallium Caudolaterale of Pigeons during Spatial Path Adjustment. Animals. 2022; 12(8):1019. https://doi.org/10.3390/ani12081019
Chicago/Turabian StyleLi, Mengmeng, Jiantao Fan, Lubo Lin, Zhigang Shang, and Hong Wan. 2022. "Elevated Gamma Connectivity in Nidopallium Caudolaterale of Pigeons during Spatial Path Adjustment" Animals 12, no. 8: 1019. https://doi.org/10.3390/ani12081019




