High Prevalence of Tick-Borne Zoonotic Rickettsia slovaca in Ticks from Wild Boars, Northeastern Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
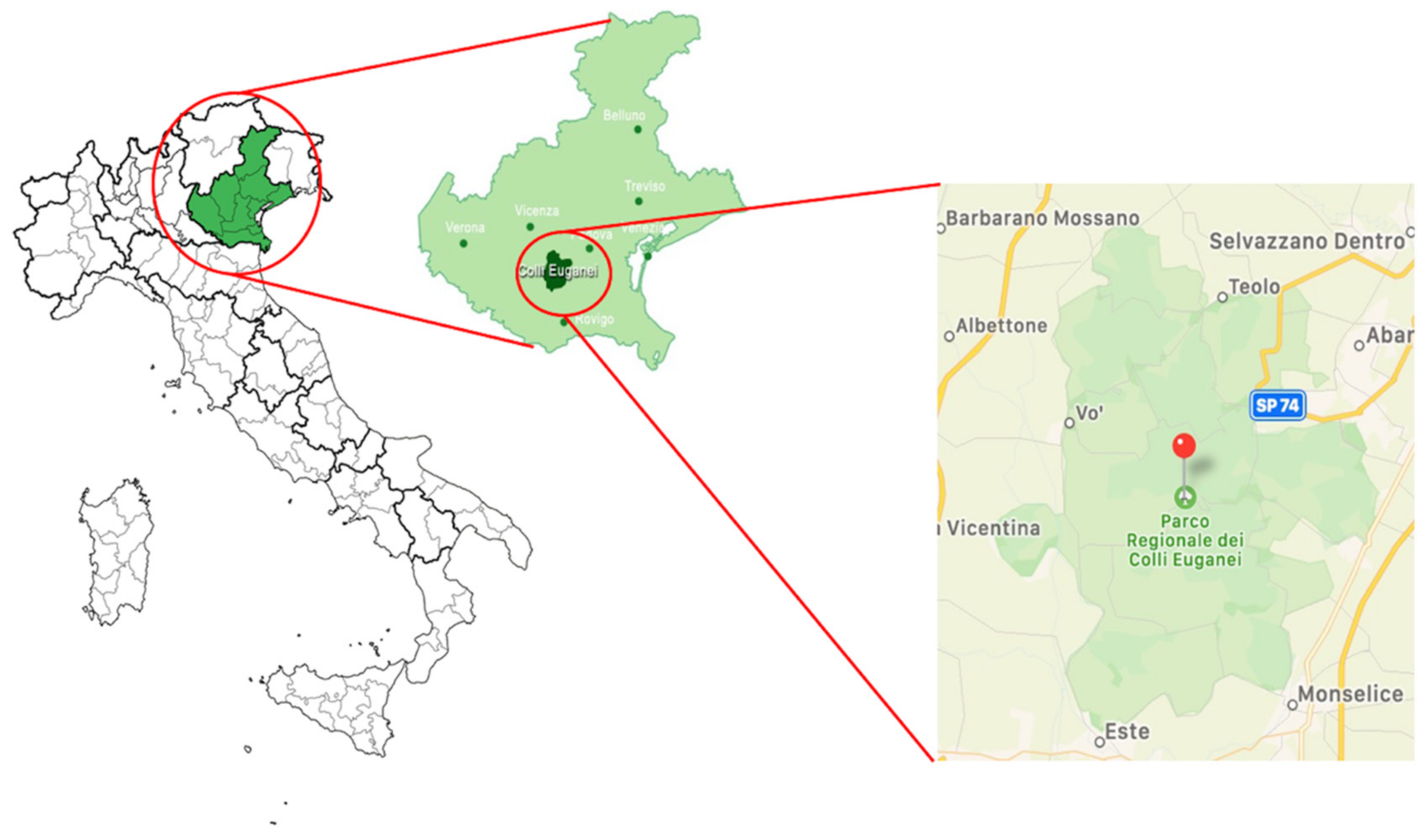
2.1. Sampling Site and Specimen Collection
2.2. Biomolecular Analysis
2.3. Stastical Analysis
3. Results
3.1. Sampled Ticks and Wild Boars
3.2. Biomolecular Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pinto, A.; De Lurdes Santos, M.; Sarmento, A. Tick-borne lymphadenopathy, an emerging disease. Ticks Tick Borne Dis. 2014, 5, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; De Sousa, R.; Santibanez, S.; Duarte, A.; Edouard, S.; Fonseca, I.P.; Marques, C.; Novakova, M.; Palomar, A.M.; Santos, M. Guidelines for the Detection of Rickettsia spp. Vector-Borne Zoonotic Dis. 2017, 17, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Parola, P.; Fournier, P.E.; Raoult, D. Spotted fever rickettsioses in southern and eastern Europe. FEMS Immunol Med. Microbiol. 2007, 49, 2–12. [Google Scholar] [CrossRef]
- Levin, M.L.; Zemtsova, G.E.; Montgomery, M.; Killmaster, L.F. Effects of homologous and heterologous immunization on the reservoir competence of domestic dogs for Rickettsia conorii (israelensis). Ticks Tick Borne Dis. 2014, 5, 33–40. [Google Scholar] [CrossRef]
- Selmi, M.; Ballardini, M.; Salvato, L.; Ricci, E. Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host-vector-pathogen interactions in a northern Mediterranean area. Exp. Appl. Acarol. 2017, 72, 79–91. [Google Scholar] [CrossRef]
- Nováková, M.; Šmajs, D. Rickettsial Endosymbionts of Ticks. In Ticks Tick-Borne Pathogens, 1st ed.; Abubakar, M., Perera, P.K., Eds.; IntechOpen: London, UK, 2019; pp. 81–94. [Google Scholar]
- Murray, G.G.R.; Weinert, L.A.; Rhule, E.L.; Welch, J.J. The Phylogeny of Rickettsia Using Different Evolutionary Signatures: How Tree-Like is Bacterial Evolution? Syst. Biol. 2016, 65, 265–279. [Google Scholar] [CrossRef]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. The relationship between spotted fever group rickettsiae and ixodid ticks. Vet. Res. 2009, 40, 34. [Google Scholar] [CrossRef]
- Parola, P.; Raoult, D. Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef]
- Tomassone, L.; Portillo, A.; Nováková, M.; De Sousa, R.; Oteo, J.A. Neglected aspects of tick-borne rickettsioses. Parasites Vectors 2018, 11, 263. [Google Scholar] [CrossRef]
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The emerging diversity of Rickettsia. Proc. R. Soc. B Boil. Sci. 2006, 273, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.P. Rickettsial infections. Ann. N. Y. Acad. Sci. 2005, 1063, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Barlozzari, G.; Romiti, F.; Zini, M.; Magliano, A.; De Liberato, C.; Corrias, F.; Capponi, G.; Galli, L.; Scarpulla, M.; Montagnani, C. Scalp eschar and neck lymphadenopathy by Rickettsia slovaca after Dermacentor marginatus tick bite case report: Multidisciplinary approach to a tick-borne disease. BMC Infect. Dis. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Allombert, C.; Supputamongkol, Y.; Caruso, G.; Brouqui, P.; Raoult, D. Aneruptive Fever Associated with Antibodies to Rickettsia helvetica in Europe and Thailand. J. Clin. Microbiol. 2004, 42, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.A.; Portillo, A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012, 3, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Guccione, C.; Colomba, C.; Tolomeo, M.; Trizzino, M.; Iaria, C.; Cascio, A. Rickettsiales in Italy. Pathogens 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. ECDC Technical report. In Epidemiological Situation of Rickettsioses in EU/EFTA Countries; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2013; pp. 1–46. [Google Scholar]
- Gomez-Barroso, D.; Vescio, M.F.; Bella, A.; Ciervo, A.; Busani, L.; Rizzo, C.; Rezza, G.; Pezzotti, P. Mediterranean spotted fever rickettsiosis in Italy, 2001–2015: Spatio-temporal distribution based on hospitalization records. Ticks Tick Borne Dis. 2019, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Mancini, F.; Caddeo, A.; Ciervo, A.; Babudieri, S.; Maida, I.; Fiori, M.L.; Rezza, G.; Mura, M.S. Rickettsia monacensis as cause of mediterranean spotted fever-like Illness, Italy. Emerg. Infect. Dis. 2012, 18, 702–704. [Google Scholar] [CrossRef]
- Tosoni, A.; Mirijello, A.; Ciervo, A.; Mancini, F.; Rezza, G.; Damiano, F.; Cauda, R.; Gasbarrini, A.; Addolorato, G. Human Rickettsia aeschlimannii infection: First case with acute hepatitis and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2630–2633. [Google Scholar]
- Rgili, G.; Attard, L.; Edouard, S.; Viale, P.; Raoult, D.; Parola, P. Rickettsia massiliae infection after a tick bite on the eyelid. Travel Med. Infect. Dis. 2018, 26, 66–68. [Google Scholar]
- Vitale, G.; Mansueto, S.; Rolain, J.M.; Raoult, D. Rickettsia massiliae human isolation. Emerg. Infect. Dis. 2006, 12, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Giglio, G.; Ramassa, E.; Nobili, F.; Rossi, L.; Tomassone, L. Dermacentor marginatus and Dermacentor reticulatus, and their infection by SFG Rickettsiae and Francisella-like endosymbionts, in mountain and periurban habitats of Northwestern Italy. Vet. Sci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Selmi, M.; Bertolotti, L.; Tomassone, L.; Mannelli, A. Rickettsia slovaca in Dermacentor marginatus and tick-borne lymphadenopathy, Tuscany, Italy. Emerg. Infect. Dis. 2008, 14, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Cinco, M.; Luzzati, R.; Mascioli, M.; Floris, R.; Brouqui, P. Serological evidence of Rickettsia infections in forestry rangers in north-eastern Italy. Clin. Microbiol. Infect. 2006, 12, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Iori, A.; Rinaldi, L.; Veneziano, V.; Genchi, C. Zecche—Mappe Parassitologiche; Rolando Ed.: Naples, Italy, 2005. [Google Scholar]
- Manilla, G. Ixodida, Acari (Fauna d’Italia); Edizioni Calderini: Bologna, Italy, 1998. [Google Scholar]
- Socolovschi, C.; Mediannikov, O.; Sokhna, C.; Tall, A.; Diatta, G.; Bassene, H.; Trape, J.F.; Raoult, D. Rickettsia felis—Associated uneruptive fever, Senegal. Emerg. Infect. Dis. 2010, 16, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Jang, W.-J.; Kim, J.-H.; Ryu, J.-S.; Lee, S.-H.; Park, K.-H.; Paik, H.-S.; Koh, Y.-S.; Choi, M.-S.; Kim, I.-S. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg. Infect. Dis. 2005, 11, 237–244. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Lee, S.-H.; Park, K.-H.; Koh, Y.-S.; Lee, K.-H.; Baik, H.-S.; Choi, M.-S.; Kim, I.-S.; Jang, W.-J. Evaluation of PCR-based assay for diagnosis of spotted fever group rickettsiosis in human serum samples. Clin. Vaccine Immunol. 2005, 12, 759–763. [Google Scholar] [CrossRef][Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sgroi, G.; Iatta, R.; Lia, R.P.; D’Alessio, N.; Manoj, R.R.S.; Veneziano, V.; Otranto, D. Spotted fever group rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound. Emerg. Dis. 2021, 68, 2111–2120. [Google Scholar] [CrossRef]
- Selmi, M.; Martello, E.; Bertolotti, L.; Bisanzio, D.; Tomassone, L. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus ticks collected on wild boars in Tuscany, Italy. J. Med. Entomol. 2009, 46, 1490–1493. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Giannelli, A.; Latrofa, M.S.; Cascio, A.; Cazzin, S.; Ravagnan, S.; Montarsi, F.; Zanzani, S.A.; Manfredi, M.T.; et al. Ticks infesting humans in Italy and associated pathogens. Parasites Vectors 2014, 7, 328. [Google Scholar] [CrossRef] [PubMed]
- Capelli, G.; Ravagnan, S.; Montarsi, F.; Ciocchetta, S.; Cazzin, S.; Porcellato, E.; Babiker, A.M.; Cassini, R.; Salviato, A.; Cattoli, G.; et al. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: A cost-effectiveness analysis in north-eastern Italy. Parasites Vectors 2012, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Bertola, M.; Montarsi, F.; Obber, F.; Da Rold, G.; Carlin, S.; Toniolo, F.; Porcellato, E.; Falcaro, C.; Mondardini, V.; Ormelli, S.; et al. Occurrence and identification of Ixodes ricinus borne pathogens in Northeastern Italy. Pathogens 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Floris, R.; Yurtman, A.N.; Margoni, E.F.; Mignozzi, K.; Boemo, B.; Altobelli, A.; Cinco, M. Detection and identification of Rickettsia species in the Northeast of Italy. Vector-Borne Zoonotic Dis. 2008, 8, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Cammà, C.; Curini, V.; Dall’Acqua, F.; Di Sabatino, D.; Pascucci, I. Molecular survey of tick-borne pathogens in wild boars from Central Italy. In Proceedings of the 15th Medical Biodefence Conference, Munich, Germany, 26–29 April 2016. [Google Scholar]
- Ortuño, A.; Quesada, M.; López-Claessens, S.; Castellà, J.; Sanfeliu, I.; Antón, E.; Segura-Porta, F. The role of wild boar (Sus scrofa) in the eco-epidemiology of, R. slovaca in northeastern Spain. Vector-Borne Zoonotic Dis. 2007, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Martello, E.; Selmi, M.; Ragagli, C.; Ambrogi, C.; Stella, M.C.; Mannelli, A.; Tomassone, L. Rickettsia slovaca in immature Dermacentor marginatus and tissues from Apodemus spp. in the northern Apennines, Italy. Ticks Tick Borne Dis. 2013, 4, 518–521. [Google Scholar] [CrossRef]
| Year | Tick Sampling | Tick Species | Tick Stage | rt-PCR gltA Positive/Total | Sequencing (Positive/Total) |
|---|---|---|---|---|---|
| 2010 (n * = 233) | Dragging (n = 51) | Ixodes ricinus (I. ricinus) (n = 47) | Adult (n = 15) | 4/15 | Rickettsia monacensis (R. monacensis) (4/4) |
| Nymphs (n = 14) (n = 6 s; n = 8 p) | 1 s/14 | R. monacensis (1/3) | |||
| 2 p/14 | Rickettsia helvetica (R. helvetica) (2/3) | ||||
| Larvae (n = 18) (n = 5 s; n = 13 p) | 2 p/18 | R. monacensis (2/2) | |||
| Dermacentor marginatus (D. marginatus) (n = 3) | Adult (n = 3) | 0/3 | - | ||
| Rhipicephalus sanguineus (R. sanguineus) (n = 1) | Larvae (n = 1) | 0/1 | - | ||
| Wild boar (n = 182) | I. ricinus (n = 9) | Adult (n = 9) | 1/9 | R. monacensis (1/1) | |
| D. marginatus (n = 169) | Adult (n = 169) | 38/169 | Rickettsia slovaca (R. slovaca) (34/38) | ||
| Rickettsia spp. (4/38) | |||||
| R. sanguineus (n = 4) | Adult (n = 4) | 0/4 | - | ||
| 2018 (n = 21) | Wild boar (n = 21) | I. ricinus (n = 2) | Adult (n = 2) | 2/2 | R. slovaca (2/2) |
| D. marginatus (n = 18) | Adult (n = 18) | 13/18 | R. slovaca (11/13) | ||
| R. helvetica (2/13) | |||||
| Hyalomma marginatum (n = 1) | Adult (n = 1) | 0/1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, L.; Menandro, M.L.; Cassini, R.; Mondin, A.; Pasotto, D.; Grillini, M.; Rocca, G.; Drigo, M. High Prevalence of Tick-Borne Zoonotic Rickettsia slovaca in Ticks from Wild Boars, Northeastern Italy. Animals 2022, 12, 967. https://doi.org/10.3390/ani12080967
Grassi L, Menandro ML, Cassini R, Mondin A, Pasotto D, Grillini M, Rocca G, Drigo M. High Prevalence of Tick-Borne Zoonotic Rickettsia slovaca in Ticks from Wild Boars, Northeastern Italy. Animals. 2022; 12(8):967. https://doi.org/10.3390/ani12080967
Chicago/Turabian StyleGrassi, Laura, Maria Luisa Menandro, Rudi Cassini, Alessandra Mondin, Daniela Pasotto, Marika Grillini, Giuseppe Rocca, and Michele Drigo. 2022. "High Prevalence of Tick-Borne Zoonotic Rickettsia slovaca in Ticks from Wild Boars, Northeastern Italy" Animals 12, no. 8: 967. https://doi.org/10.3390/ani12080967
APA StyleGrassi, L., Menandro, M. L., Cassini, R., Mondin, A., Pasotto, D., Grillini, M., Rocca, G., & Drigo, M. (2022). High Prevalence of Tick-Borne Zoonotic Rickettsia slovaca in Ticks from Wild Boars, Northeastern Italy. Animals, 12(8), 967. https://doi.org/10.3390/ani12080967










