Does Carrying a Rider Change Motor and Sensory Laterality in Horses?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Locations and Circumstances
2.2. Animals
2.3. Experimenters and Their Tasks
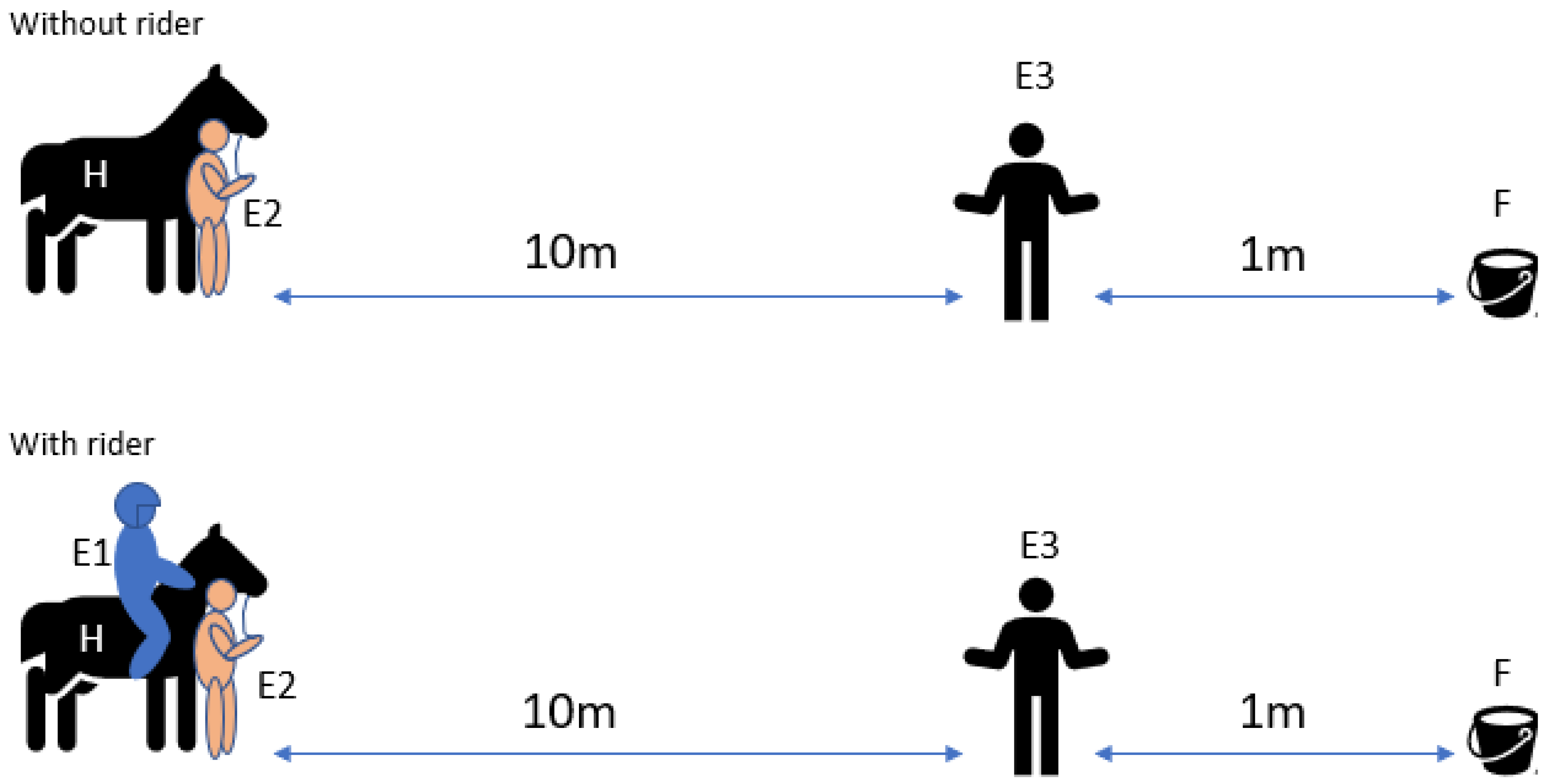
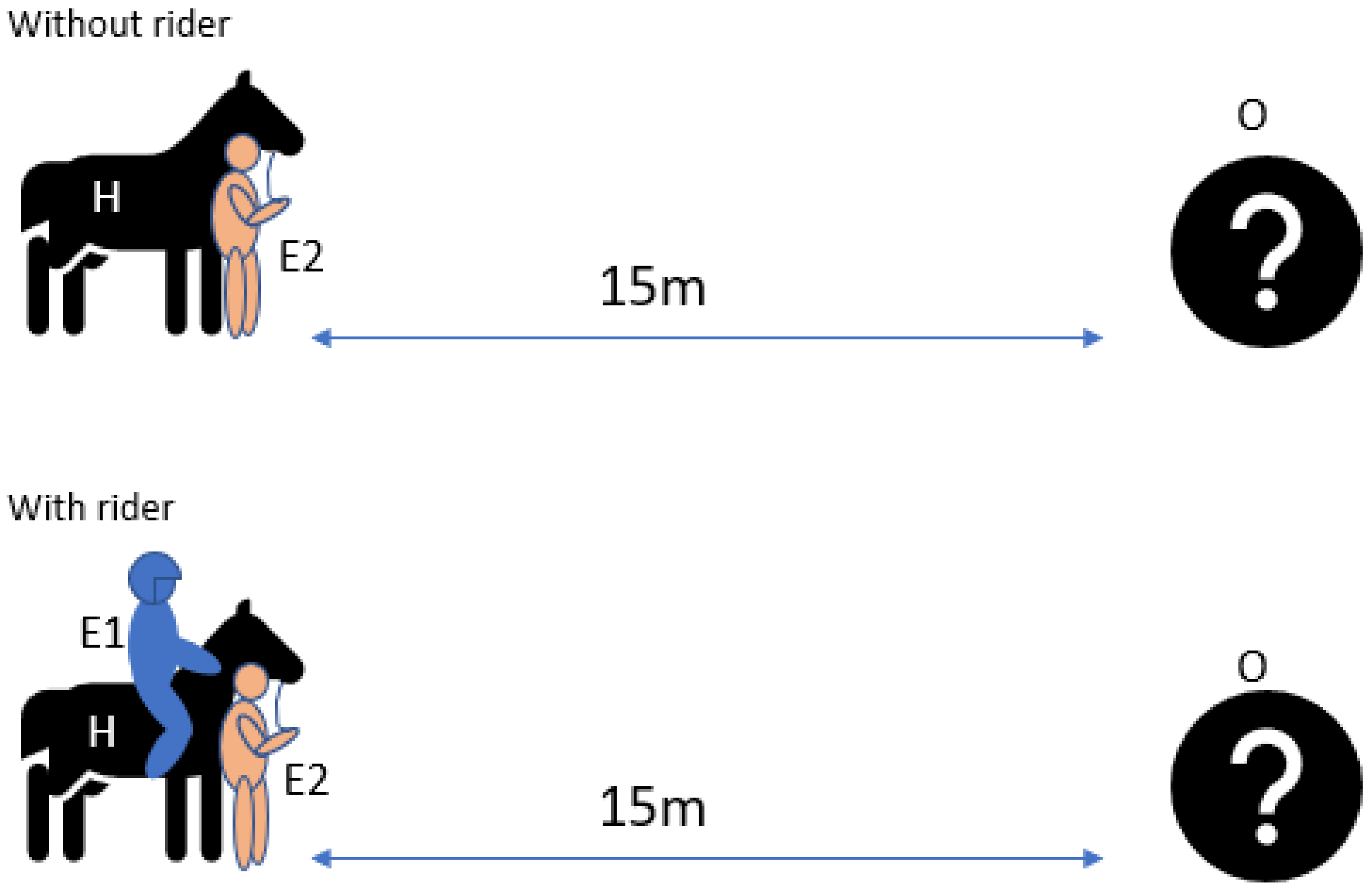
2.3.1. Experimenter 1 (E1)
2.3.2. Experimenter 2 (E2)
2.3.3. Experimenter 3 (E3)
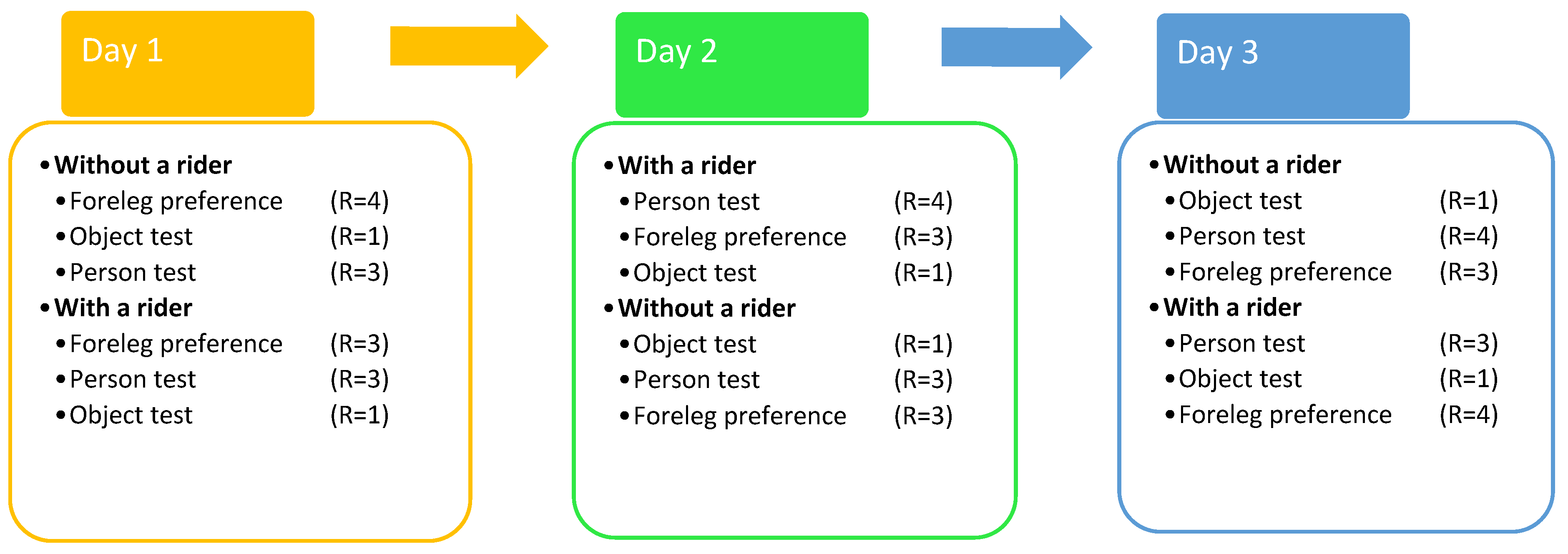
2.4. Experiments and Procedure
2.4.1. Experimental Procedure
2.4.2. Motor Laterality Foreleg Preference Test
2.4.3. Sensory Laterality Person Test
2.4.4. Sensory Laterality Object Test
2.5. Ethics Statement
2.6. Data Collection and Analysis
2.6.1. Data Preparation
2.6.2. Data Analysis
3. Results
3.1. General Results
3.2. Influence of a Rider on the Direction of Laterality
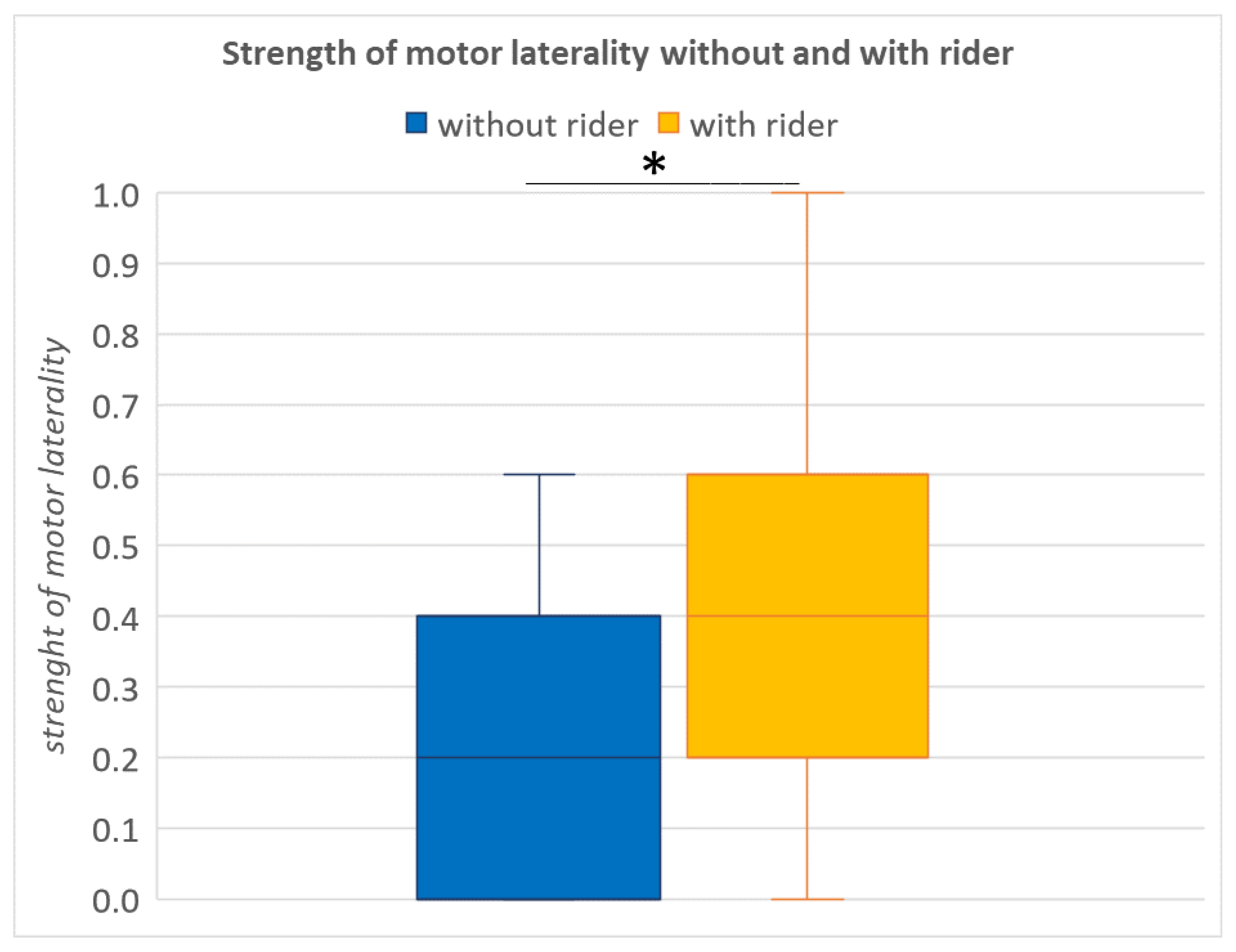
3.3. Influence of a Rider on the Strength of Laterality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Röthig, P. Beitrage Zum Studium Des Zentralnervensystems Der Wirbeltiere. 8. Über Das Zwischenhirn Der Amphibien. Arch. Mikrosk. Anat. Entwickl. 1923, 98, 616–645. [Google Scholar] [CrossRef]
- Braitenberg, V.; Kemali, M. Exceptions to Bilateral Symmetry in the Epithalamus of Lower Vertebrates. J. Comp. Neurol. 1970, 138, 137–146. [Google Scholar] [CrossRef]
- Vallortigara, G.; Bisazza, A. How Ancient Is Brain Lateralization? In Comparative Vertebrate Lateralization; Rogers, L.J., Andrew, R., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 9–69. ISBN 0-521-78161-2. [Google Scholar]
- Rogers, L.J. Laterality in Animals. Int. J. Comp. Psychol. 1989, 3, 22. [Google Scholar]
- Rogers, L.J.; Andrew, R.J. Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-521-78161-9. [Google Scholar]
- Rogers, L.J. Relevance of Brain and Behavioural Lateralization to Animal Welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- MacNeilage, P.; Rogers, L.J.; Vallortigara, G. Origins of the Left and Right Brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. Survival with an Asymmetrical Brain: Advantages and Disadvantages of Cerebral Lateralization. Behav. Brain Sci. 2005, 28, 575–633. [Google Scholar] [CrossRef]
- Rogers, L.J. A Matter of Degree: Strength of Brain Asymmetry and Behaviour. Symmetry 2017, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of Having a Lateralized Brain. Proc. Biol. Sci. 2004, 271 (Suppl. S6), S420–S422. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.S. The Domestic Horse; Cambridge University Press: Cambridge, UK, 2005; ISBN 978-0-521-89113-4. [Google Scholar]
- Saslow, C.A. Understanding the Perceptual World of Horses. Appl. Anim. Behav. Sci. 2002, 78, 209–224. [Google Scholar] [CrossRef]
- Goodwin, D. Horse Behaviour: Evolution, Domestication and Feralisation. In The Welfare of Horses; Waran, N., Ed.; Animal Welfare; Springer: Dordrecht, The Netherlands, 2002; Volume 1, pp. 1–18. ISBN 978-1-4020-6142-4. [Google Scholar]
- Gaunitz, C.; Fages, A.; Hanghøj, K.; Albrechtsen, A.; Khan, N.; Schubert, M.; Seguin-Orlando, A.; Owens, I.J.; Felkel, S.; Bignon-Lau, O.; et al. Ancient Genomes Revisit the Ancestry of Domestic and Przewalski’s Horses. Science 2018, 360, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.; Sutherland, A.; Arkins, S. Idiosyncratic Motor Laterality in the Horse. Appl. Anim. Behav. Sci. 2005, 91, 297–310. [Google Scholar] [CrossRef]
- Lucidi, P.; Bacco, G.; Sticco, M.; Mazzoleni, G.; Benvenuti, M.; Bernabò, N.; Trentini, R. Assessment of Motor Laterality in Foals and Young Horses (Equus Caballus) through an Analysis of Derailment at Trot. Physiol. Behav. 2013, 109, 8–13. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Rogers, L.J. Motor and Sensory Laterality in Thoroughbred Horses. Appl. Anim. Behav. Sci. 2005, 92, 337–352. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Thomson, P.C. Differences in Motor Laterality between Breeds of Performance Horse. Appl. Anim. Behav. Sci. 2006, 99, 183–190. [Google Scholar] [CrossRef]
- Marr, I.; Preisler, V.; Farmer, K.; Stefanski, V.; Krüger, K. Non-Invasive Stress Evaluation in Domestic Horses (Equus Caballus): Impact of Housing Conditions on Sensory Laterality and Immunoglobulin A. R. Soc. Open Sci. 2020, 7, 191994. [Google Scholar] [CrossRef] [Green Version]
- Siniscalchi, M.; Padalino, B.; Lusito, R.; Quaranta, A. Is the Left Forelimb Preference Indicative of a Stressful Situation in Horses? Behav. Process. 2014, 107, 61–67. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Padalino, B.; Aubé, L.; Quaranta, A. Right-Nostril Use during Sniffing at Arousing Stimuli Produces Higher Cardiac Activity in Jumper Horses. Laterality 2015, 20, 483–500. [Google Scholar] [CrossRef]
- Marr, I.; Bauer, T.; Farmer, K.; Krüger, K. Gibt Die Sensorische Lateralität Im Objekttest Aufschluss Über Das Interieur, Den Aktuellen Gemütszustand, Oder Den Trainingszustand Der Pferde? In Proceedings of the Physisch und Psychisch Gesunde und Leistungsfähige Sportpferde, Freising, Germany, 6–8 May 2016; pp. 1–5. [Google Scholar]
- Marr, I.; Farmer, K.; Krüger, K. Evidence for Right-Sided Horses Being More Optimistic than Left-Sided Horses. Animals 2018, 8, 219. [Google Scholar] [CrossRef] [Green Version]
- Miklósi, Á.; Topál, J.; Csányi, V. Comparative Social Cognition: What Can Dogs Teach Us? Anim. Behav. 2004, 67, 995–1004. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb Preferences and Lateralization of Aggression, Reactivity and Vigilance in Feral Horses. Equus Caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Rehren, K.D.; Stadler, P. Untersuchung der “Schiefe” des Pferdes: Symmetrie von Bewegungsablauf und Hufbelastung; Wissenschaftliche Reihe der Klinik für Pferde; 1. Auflage; Cuvillier Verlag: Göttingen, Germany, 2018; ISBN 978-3-7369-9804-9. [Google Scholar]
- Wells, A.E.D.; Blache, D. Horses Do Not Exhibit Motor Bias When Their Balance Is Challenged. Anim. Int. J. Anim. Biosci. 2008, 2, 1645–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, N.P.; Rogers, L.J. Lateralization of Agonistic and Vigilance Responses in Przewalski Horses (Equus Przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Zucca, P.; Cerri, F.; Carluccio, A.; Baciadonna, L. Space Availability Influence Laterality in Donkeys (Equus Asinus). Behav. Process. 2011, 88, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.; Esch, L.; Farmer, K.; Marr, I. Basic Needs in Horses?—A Literature Review. Animals 2021, 11, 1798. [Google Scholar] [CrossRef]
- Larose, C.; Richard-Yris, M.; Hausberger, M.; Rogers, L.J. Laterality of Horses Associated with Emotionality in Novel Situations. Laterality 2006, 11, 355–367. [Google Scholar] [CrossRef]
- Klimke, R.; Klimke, R. Basic Training of the Young Horse; J.A. Allen & Company: London, UK, 1985; ISBN 978-0-85131-408-2. [Google Scholar]
- Podhajsky, A. The Complete Training of Horse and Rider; Harrap: London, UK, 1977; ISBN 978-0-245-59040-5. [Google Scholar]
- Dorrance, B.; Desmond, L. True Horsemanship through Feel; Diamond Lu Productions: Novato, CA, USA, 1999; ISBN 978-1-892578-00-6. [Google Scholar]
- Farmer, K.; Krüger, K.; Byrne, R.W.; Marr, I. Sensory Laterality in Affiliative Interactions in Domestic Horses and Ponies (Equus Caballus). Anim. Cogn. 2018, 21, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Farmer, K.; Krüger, K.; Byrne, R.W. Visual Laterality in the Domestic Horse (Equus Caballus) Interacting with Humans. Anim. Cogn. 2010, 13, 229–238. [Google Scholar] [CrossRef]
- Gabbard, C. Foot Laterality in Children, Adolescents, and Adults. Laterality Asymmetries Body Brain Cogn. 1996, 1, 199–206. [Google Scholar] [CrossRef]
- Meij, H.S.; Meij, J.C.P. Functional Asymmetry in the Motor System of the Horse. S. Afr. J. Sci. 1980, 76, 552. [Google Scholar]
- König, V.; Borstel, U.; Visser, E.K.; Hall, C. Indicators of Stress in Equitation. Appl. Anim. Behav. Sci. 2017, 190, 43–56. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, S.; Marr, I.; Farmer, K.; Graf, K.; Stefanski, V.; Krueger, K. Does Carrying a Rider Change Motor and Sensory Laterality in Horses? Animals 2022, 12, 992. https://doi.org/10.3390/ani12080992
Schwarz S, Marr I, Farmer K, Graf K, Stefanski V, Krueger K. Does Carrying a Rider Change Motor and Sensory Laterality in Horses? Animals. 2022; 12(8):992. https://doi.org/10.3390/ani12080992
Chicago/Turabian StyleSchwarz, Sophie, Isabell Marr, Kate Farmer, Katja Graf, Volker Stefanski, and Konstanze Krueger. 2022. "Does Carrying a Rider Change Motor and Sensory Laterality in Horses?" Animals 12, no. 8: 992. https://doi.org/10.3390/ani12080992
APA StyleSchwarz, S., Marr, I., Farmer, K., Graf, K., Stefanski, V., & Krueger, K. (2022). Does Carrying a Rider Change Motor and Sensory Laterality in Horses? Animals, 12(8), 992. https://doi.org/10.3390/ani12080992





