Loline Alkaloid Effects on Gastrointestinal Nematodes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Experiment 1: Loline Effects on Larval Migration
2.2. In Vitro Experiment 2: Establishment of T. circumcincta in Abomasal Tissues
2.3. In Vitro Experiment 3: Establishment of H. contortus in Abomasal Tissues
2.4. In Vivo Study 1: Loline Effects against T. circumcincta and T. colubriformis
2.5. In Vivo Study 2: Loline Effects against H. contortus
2.6. In Vivo Study 3: Oral Dosing Loline and Its Effects against Mixed Infection of L4 T. circumcincta, T. colubriformis, and Adult H. contortus
3. Results
3.1. In Vitro Experiments
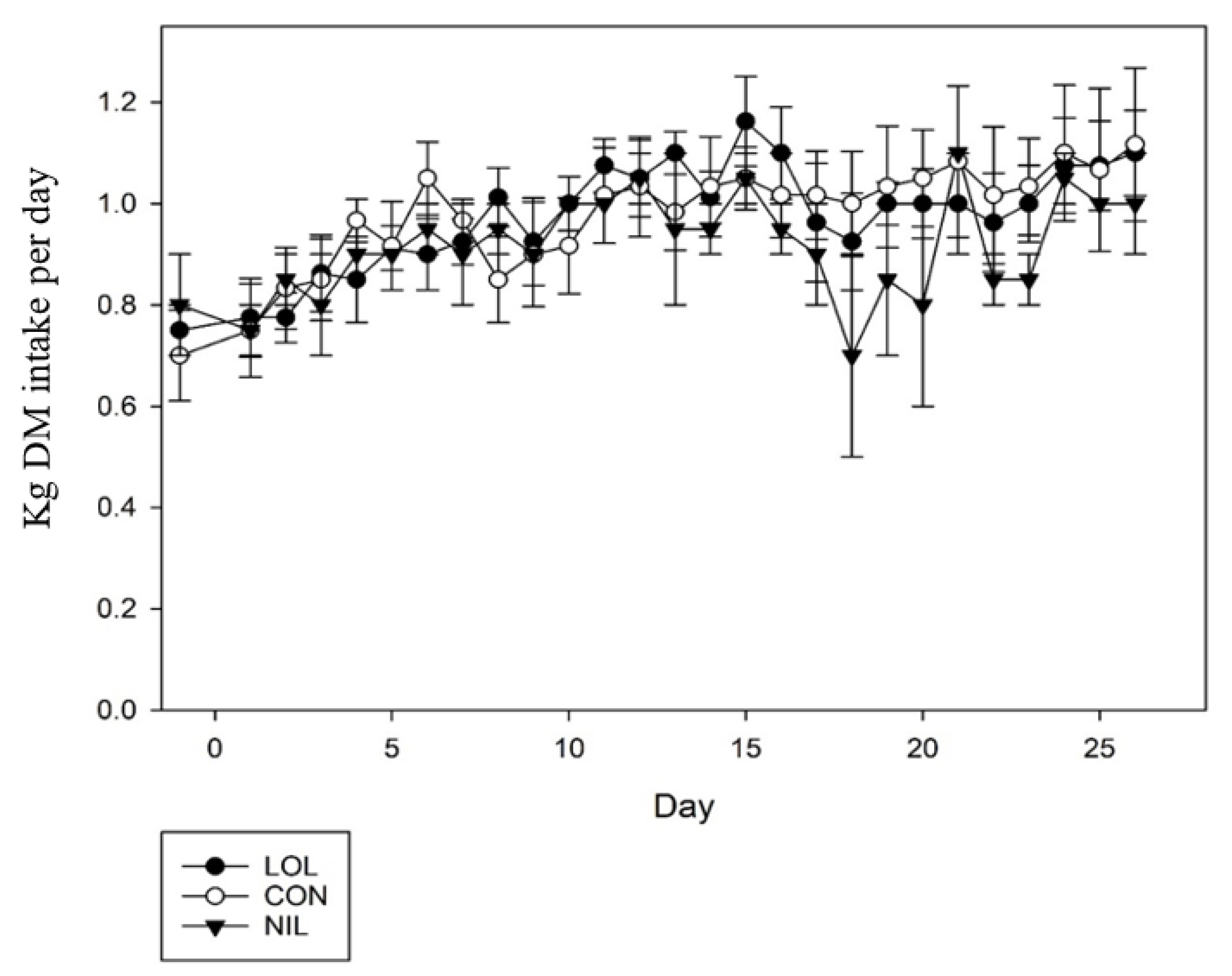
3.2. In Vivo Studies 1 and 2: Loline Effects against T. circumcincta and T. colubriformis, or H. contortus Establishment
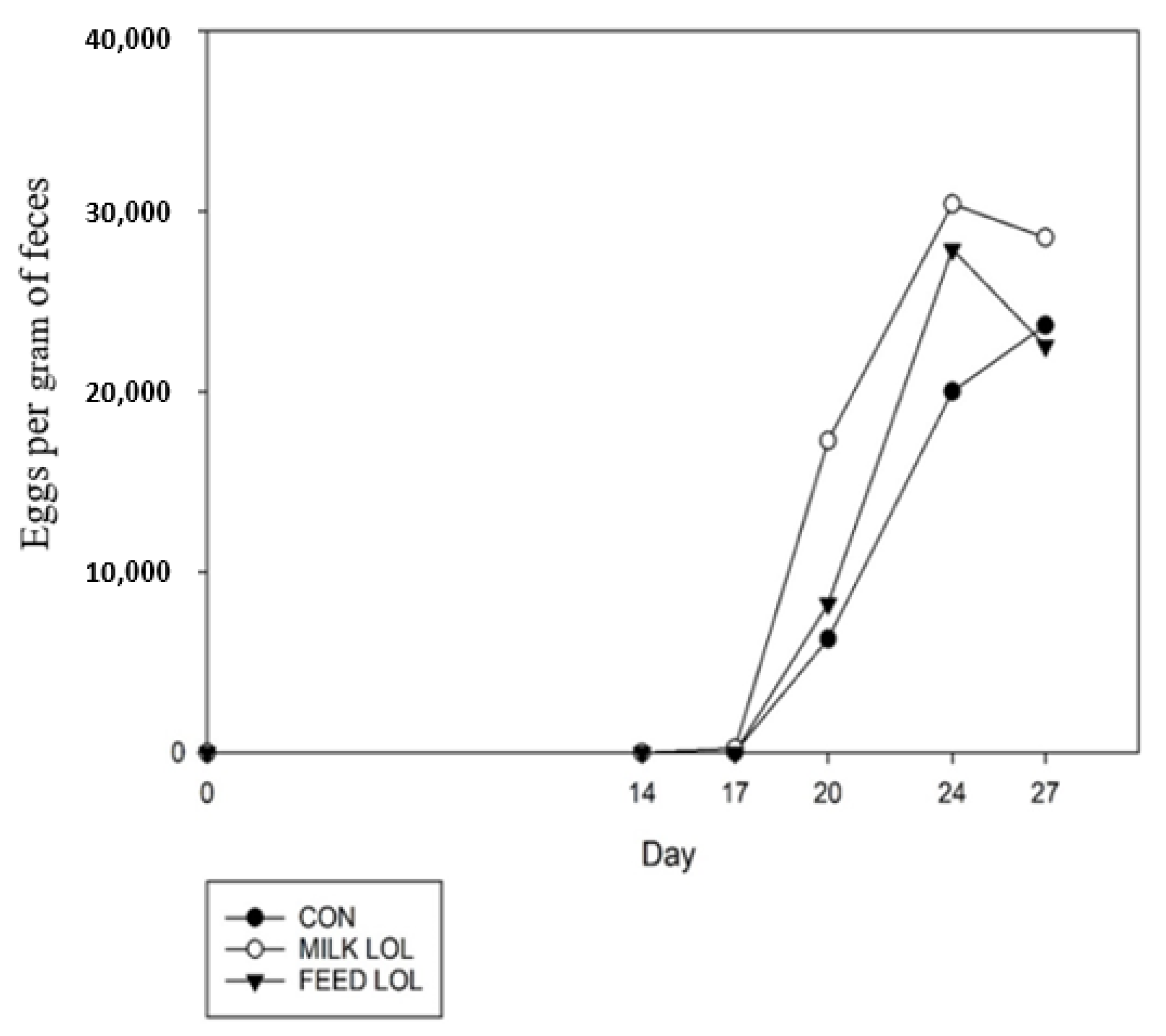
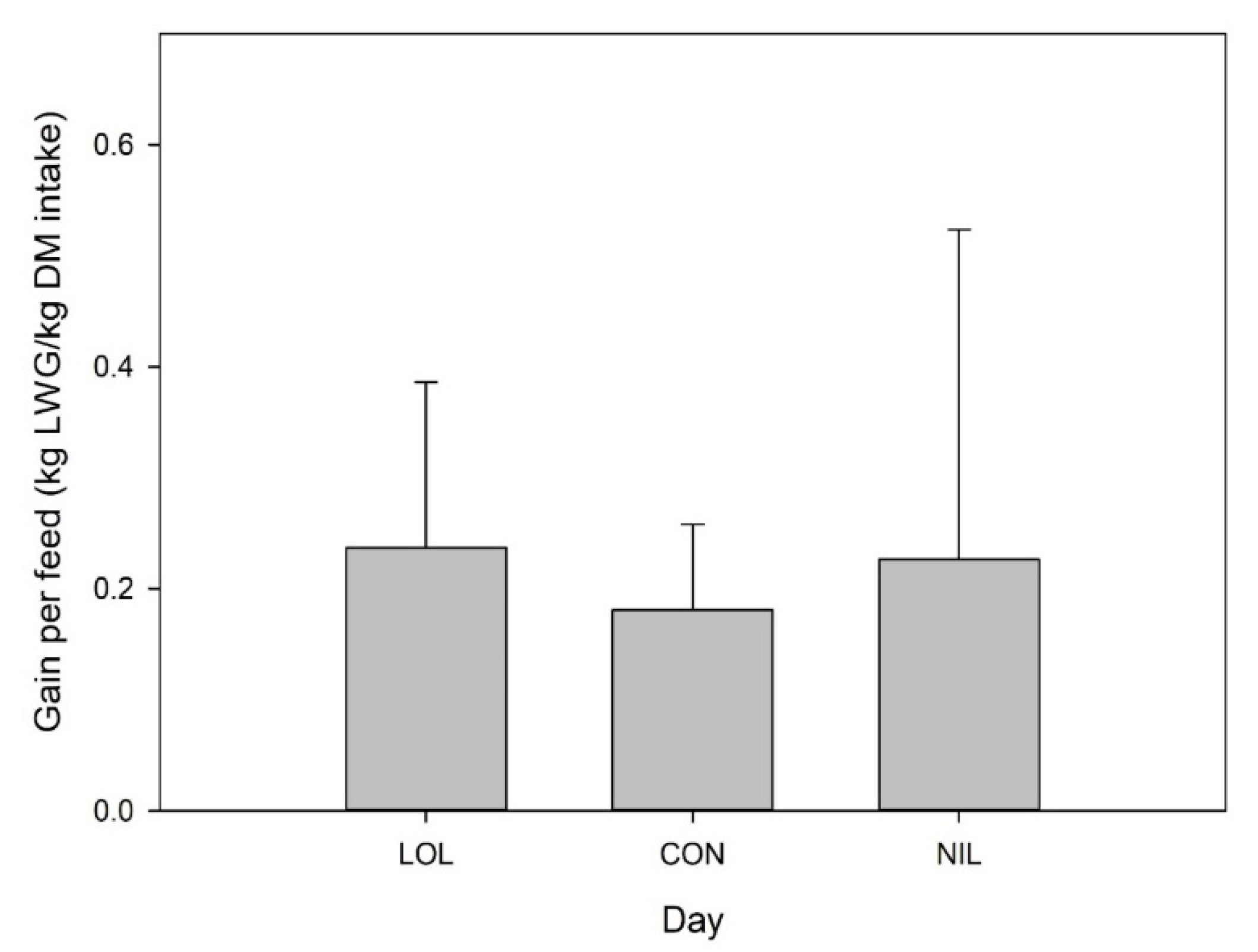
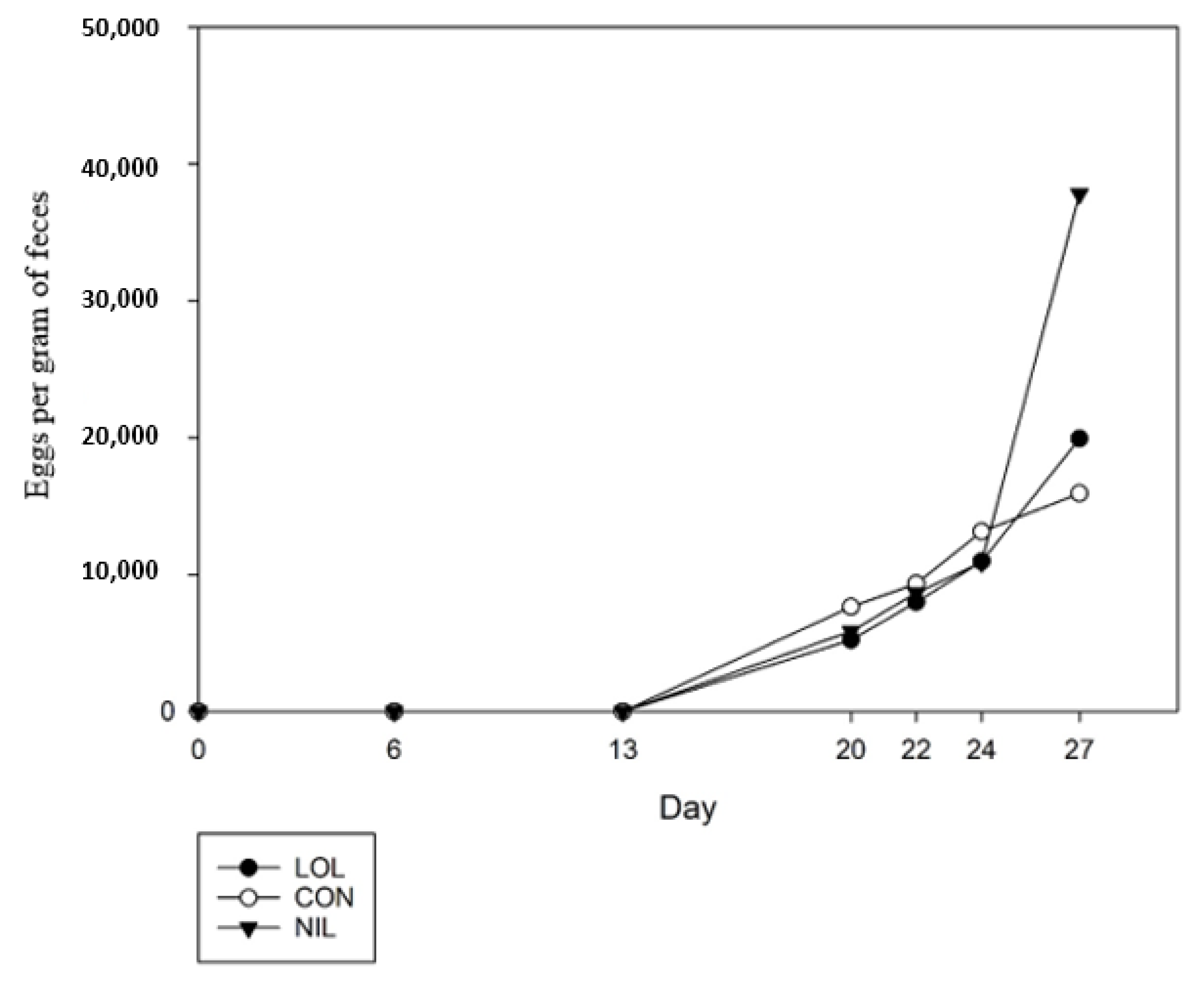
3.3. In Vivo Study 3: Oral Dosing Loline and Its Effects against Mixed Infection of L4 T. circumcincta, T. colubriformis, and Adult H. contortus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Githiori, J.B.; Athanasiadou, S.; Thamsborg, S.M. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet. Parasitol. 2006, 139, 308–320. [Google Scholar] [CrossRef]
- Waller, P.J. From discovery to development: Current industry perspectives for the development of novel methods of helminth control in livestock. Vet. Parasitol. 2006, 139, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Ropiak, H.M.; Fryganas, C.; Desrues, O.; Mueller-Harvey, I.; Thamsborg, S.M. Assessment of the anthelmintic activity of medicinal plant extracts and purified condensed tannins against free-living and parasitic stages of Oesophagostomum dentatum. Parasites Vectors 2014, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Worku, E.; Kiros, A.; Asgedom, H.; Tadesse, B. Alternative Control Methods of Gastrointestinal Nematode Infections in Small Ruminants: Biological Method and Use of Medicinal Plant Extracts. ARC J. Anim. Vet. Sci. 2017, 3, 11–28. [Google Scholar]
- Demain, A.L. Pharmaceutically active secondary metabolites of microorganisms. Appl. Microbiol. Biotechnol. 1999, 52, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.O.; Cerqueira, A.P.M.; Branco, A.; Batatinha, M.J.M.; Botura, M.B. Anthelmintic activity of plants against gastrointestinal nematodes of goats: A review. Parasitology 2019, 146, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Ramsay, A.; Hansen, T.; Ropiak, H.M.; Mejer, H.; Nejsum, P.; Mueller-Harvey, I.; Thamsborg, S.M. Anthelmintic activity of trans-cinnamaldehyde and A- and B-type proanthocyanidins derived from cinnamon (Cinnamomum verum). Sci. Rep. 2015, 5, 14791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compant, S.; Saikkonen, K.; Mitter, B.; Campisano, A.; Mercado-Blanco, J. Editorial special issue: Soil, plants and endophytes. Plant Soil 2016, 405, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, S.; Kyriazakis, I. Plant secondary metabolites: Antiparasitic effects and their role in ruminant production systems. Proc. Nutr. Soc. 2004, 63, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef]
- Bacetty, A.A.; Snook, M.E.; Glenn, A.E.; Noe, J.P.; Nagabhyru, P.; Bacon, C.W. Chemotaxis disruption in Pratylenchus scribneri by tall fescue root extracts and alkaloids. J. Chem. Ecol. 2009, 35, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Muponda, L. An investigation into the anthelmintic properties of seed extracts from endophyte containing pasture grasses. In Animal Science; Lincoln University: Lincoln, New Zealand, 2014. [Google Scholar]
- Bush, L.P.; Fannin, F.F.; Siegel, M.R.; Dahlman, D.L.; Burton, H.R. Chemistry, occurrence and biological effects of saturated pyrrolizidine alkaloids associated with endophyte-grass interactions. Agric. Ecosyst. Environ. 1993, 44, 81–102. [Google Scholar] [CrossRef]
- Gooneratne, S.R.; Patchett, B.J.; Wellby, M.; Fletcher, L.R. Excretion of loline alkaloids in urine and faeces of sheep dosed with meadow fescue (Festuca pratensis) seed containing high concentrations of loline alkaloids. N. Z. Vet. J. 2012, 60, 176–182. [Google Scholar] [CrossRef]
- Froehlich, K.A. Metabolism of loline in ruminants and their potential effects on microflora, and gastrointestinal nematodes. In Animal Science; Lincoln University: Lincoln, New Zealand, 2020; p. 120. [Google Scholar]
- Yates, S.G.; Petroski, R.J.; Powell, R.G. Analysis of loline alkaloids in endophyte-infected tall fescue by capillary gas chromatography. J. Agric. Food Chem. 1990, 38, 182–185. [Google Scholar] [CrossRef]
- Dahlman, D.L. Insecticidal activity of N-formyl loline. In Proceedings of the XVII International Grasslands Congress, Winnipeg, MB, Canada, 8–17 June 1997. [Google Scholar]
- Siegel, M.R.; Latch, G.C.M.; Bush, L.P.; Fannin, F.F.; Rowan, D.D.; Tapper, B.A.; Bacon, C.W.; Johnson, M.C. Fungal endophyte-infected grasses: Alkaloid accumulation and aphid response. J. Chem. Ecol. 1990, 16, 3301–3315. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, C.T.; Knapp, F.W.; Bush, L.P.; Maul, J.E.; Van Willigen, J. Mortality of horn fly (Diptera: Muscidae) larvae in bovine dung supplemented with loline alkaloids from tall fescue. J. Med. Entomol. 1998, 35, 798–803. [Google Scholar] [CrossRef]
- Patchett, B.J.; Chapman, R.B.; Fletcher, L.R.; Gooneratne, S.R. Endophyte infected Festuca pratensis containing loline alkaloids deters feeding by Listronotus bonariensis. N. Z. Plant Prot. 2008, 61, 205–209. [Google Scholar]
- Popay, A.J.; Tapper, B.A.; Podmore, C. Endophyte infected meadow fescue and loline alkaloids affect Argentine stem weevil larvae. N. Z. Plant Prot. 2009, 62, 19–27. [Google Scholar]
- Patchett, B.J.; Chapman, R.; Fletcher, L.; Gooneratne, S. Root loline concentration in endophyte infected meadow fescue (Festuca pratensis) is increased by grass grub (Costelytra zealandica) attack. N. Z. Plant Prot. 2008, 61, 210–214. [Google Scholar]
- Shiba, T.; Sugawara, K. Fungal loline alkaloids in grass-endophyte associations confer resistance to the rice leaf bug Trigonotylus caelestialium. Neth. Entomol. Soc. 2009, 130, 55–62. [Google Scholar] [CrossRef]
- Bacetty, A.A.; Snook, M.E.; Glenn, A.E.; Bacon, C.W.; Nagabhyru, P.; Schardl, C.L. Nematotoxic effects of endophyte-infected tall fescue toxins and extracts in an in vitro bioassay using the nematode Pratylenchus scribneri. In Proceedings of the Acremonium Grass Interactions International Symposium, Christchurch, New Zealand, 25–28 March 2007. [Google Scholar]
- Jackson, F.; Greer, A.W.; Huntley, J.; McAnulty, R.W.; Bartley, D.J.; Stanley, A.; Stenhouse, L.; Stankiewicz, M.; Sykes, A.R. Studies using Teladorsagia circumcincta in an in vitro direct challenge method using abomasal tissue explants. Vet. Parasitol. 2004, 124, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Herlich, H. A digestion method for post-mortem recovery of nematodes from ruminants. Proc. Helminthol. Soc. Wash. 1956, 23, 102–103. [Google Scholar]
- Bultman, T.L.; Borowicz, K.L.; Schneble, R.M.; Coudron, T.A.; Bush, L.P. Effect of a Fungal Endophyte on the Growth and Survival of Two Euplectrus parasitoids. Oikos 1997, 78, 170–176. [Google Scholar] [CrossRef]
- Patchett, B.; Gooneratne, R.; Chapman, B.; Fletcher, L. Effects of loline-producing endophyte-infected meadow fescue ecotypes on New Zealand grass grub (Costelytra zealandica). N. Z. J. Agric. Res. 2011, 54, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Roy, H.; Chakraborty, A.; Bhanja, S.; Mishra, B.S.N.S.R.; Ellaiah, P. Preliminary phytochemical investigation and anthelmintic activity of Acanthospermum hispidum DC. J. Pharm. Sci. Technol. 2010, 2, 217–221. [Google Scholar]
- Dubois, O.; Allanic, C.; Charvet, C.L.; Guégnard, F.; Février, H.; Théry-Koné, I.; Cortet, J.; Koch, C.; Bouvier, F.; Fassier, T.; et al. Lupin (Lupinus spp.) seeds exert anthelmintic activity associated with their alkaloid content. Sci. Rep. 2019, 9, 9070. [Google Scholar] [CrossRef]
- Jain, P.; Singh, S.; Singh, S.K.; Verma, S.K.; Kharya, M.D.; Solanki, S. Anthelmintic potential of herbal drugs. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 412–427. [Google Scholar]
- Dannhardt, G.; Steindl, L. Alkaloids of Lolium temulentum: Isolation, identification and pharmacological activity. Planta Med. 1985, 51, 212–214. [Google Scholar] [CrossRef]
- Strickland, J.R.; Cross, D.L.; Jenkins, T.C.; Petroski, R.J.; Powell, R.G. The effect of alkaloids and seed extracts of endophyte-infected tall fescue on prolactin secretion in an in vitro rat pituitary perfusion system. J. Anim. Sci. 1992, 70, 2779–2786. [Google Scholar] [CrossRef]
- Larson, B.T.; Harmon, D.L.; Piper, E.L.; Griffis, L.M.; Bush, L.P. Alkaloid binding and activation of D2 dopamine receptors in cell culture. J. Anim. Sci. 1999, 77, 942–947. [Google Scholar] [CrossRef]
- Lifschitz, A.; Lanusse, C.; Alvarez, L. Host pharmacokinetics and drug accumulation of anthelmintics within target helminth parasites of ruminants. N. Z. Vet. J. 2017, 65, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.I.; Mottier, M.L.; Lanusse, C.E. Drug transfer into target helminth parasites. Trends Parasitol. 2007, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug. Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, W.; Remane, D.; Wissenbach, D.K.; Peters, F.T. Development and validation of an ultrahigh performance liquid chromatography-high resolution tandem mass spectrometry assay for nine toxic alkaloids from endophyte-infected pasture grasses in horse serum. J. Chromatogr. A 2018, 1560, 35–44. [Google Scholar] [CrossRef]
- Hennessy, D.R. Pharmacokinetic disposition of benzimidazole drugs in the ruminant gastrointestinal tract. Parasitol. Today 1993, 9, 329–333. [Google Scholar] [CrossRef]
- Ali, D.N.; Hennessy, D.R. The effect of feed intake on the rate of flow of digesta and the disposition and activity of oxfendazole in sheep. Int. J. Parasitol. 1993, 23, 477–484. [Google Scholar] [CrossRef]
- Sánchez, S.F.; Alvarez, L.I.; Lanusse, C.E. Fasting-induced changes to the pharmacokinetic behaviour of albendazole and its metabolites in calves. J. Vet. Pharmacol. Ther. 1997, 20, 38–47. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef]
- Cakmak, M.; Mayer, P.; Trauner, D. An efficient synthesis of loline alkaloids. Nat. Chem. 2011, 3, 543–545. [Google Scholar] [CrossRef]
- Joost, R.E. Acremonium in fescue and ryegrass: Boon or bane? A review. J. Anim. Sci. 1995, 73, 881–888. [Google Scholar] [CrossRef]
- Easton, H. Endophyte in New Zealand ryegrass pastures, an overview. Ag Res. Grassl. Res. Pract. 1999, 7, 1–9. [Google Scholar] [CrossRef]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective Alkaloids of Grass-Fungal Endophyte Symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, S.G.; Fenster, J.C.; Bartelt, R.J. Assay of tall fescue seed extracts, fractions, and alkaloids using the large milkweed bug. J. Agric. Food Chem. 1989, 37, 354–357. [Google Scholar] [CrossRef]
 .
.
 .
.

| In Vitro Studies | Parasite(s) Studied |
|---|---|
| Study 1: Larval migration | L3 T. colubriformis |
| Study 2: Abomasal establishment | L3 T. circumcincta |
| Study 3: Abomasal establishment | L3 H. contortus |
| In Vivo Lamb Establishment Studies | Parasite(s) Studied |
| Study 1 (n = 8) | L4 and adult T. circumcincta and T. colubriformis |
| Study 2 (n = 7) | L4 and adult H. contortus |
| Study 3 (n = 16) | L4 T. circumcincta and T. colubriformis, and adult H. contortus |
| Concentration, ppm | ||||
|---|---|---|---|---|
| Sample | 16,000 | 8000 | 4000 | 2000 |
| LOL, % LM | 87.2 ± 9.0 | 85.5 ± 6.1 | 88.8 ± 6.8 | 91.2 ± 5.8 |
| NIL, % LM | 59.7 ± 20.6 | 85.0 ± 3.5 | 81.2 | 93.2 |
| Average % Establishment | ||
|---|---|---|
| LOL | CON | |
| Teladorsagia circumcincta | 30.8 ± 2.4 a | 74.8 ± 2.3 b |
| Haemonchus contortus | 56.5 ± 4.4 | 50.5 ± 8.3 |
| Average Worm Count | ||||
|---|---|---|---|---|
| LOL, L4 Dose | LOL, Adult Dose | CON | ||
| Milk Fed | Feed Fed | |||
| 1Teladorsagia circumcincta | 2985 | 3765 | 3890 | |
| 1Trichostrongylus colubriformis | 1137 (589–1687) a | 8050 (7810–8290) b | 4473 (2933–4180) b | |
| 2Haemonchus contortus | 18,750 (16,875–20,625) | 19,250 (12,750–25,750) | 29,125 (16,875–49,875) | |
| Average Worm Counts | |||
|---|---|---|---|
| LOL | CON | NIL | |
| Teladorsagia circumcincta | 6900 (2700–10,000) | 6951.7 (2960–9810) | 6940 (6280–7600) |
| Trichostrongylus colubriformis | 2415 (100–4450) | 2720 (490–5590) | 4775 (3190–5590) |
| Haemonchus contortus | 14,630 (6650–29,090) | 7938 (440–11,590) | 29,750 (23,810–35,690) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froehlich, K.A.; McAnulty, R.; Greer, A. Loline Alkaloid Effects on Gastrointestinal Nematodes. Animals 2022, 12, 996. https://doi.org/10.3390/ani12080996
Froehlich KA, McAnulty R, Greer A. Loline Alkaloid Effects on Gastrointestinal Nematodes. Animals. 2022; 12(8):996. https://doi.org/10.3390/ani12080996
Chicago/Turabian StyleFroehlich, Kelly Ann, Robin McAnulty, and Andy Greer. 2022. "Loline Alkaloid Effects on Gastrointestinal Nematodes" Animals 12, no. 8: 996. https://doi.org/10.3390/ani12080996






