Potential of Oral Nanoparticles Containing Cytokines as Intestinal Mucosal Immunostimulants in Pigs: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial and Culture Strains
2.2. Genetic Construct Design
2.3. Cytokine Nanoparticle Production and Purification
2.4. Immunoassays
2.5. Gene Expression Analyses
| Target Gene | Primer Name | Sequence (5′-3′) | T° Annealing (°C) | Conc (μM) | PCR Product (bp) | Reference |
|---|---|---|---|---|---|---|
| Interleukin-6 | IL6-Fw | CAAGGAGGTACTGGCAGAAA | 60 | 0.25 | 185 | |
| IL6-Rv | CAGCCTCGACATTTCCCTTAT | |||||
| β-defensin 1 | BD1-Fw | TGCCACAGGTGCCGATCT | 60 | 0.25 | 81 | |
| BD1-Rv | CTGTTAGCTGCTTAAGGAATAAAGGC | |||||
| β-defensin 2 | BD2-Fw | ACCTGCTTACGGGTCTTG | 60 | 0.25 | 168 | |
| BD2-Rv | CTCTGCTGTGGCTTCTGG | |||||
| Tumor necrosis factor-α | TNFa-Fw | ATCGGCCCCCAGAAGGAAGAG | 60 | 0.25 | 351 | [19] |
| TNFa-Rv | GATGGCAGAGAGGAGGTTGAC | |||||
| Claudin-4 | CLDN4-Fw | CGCCCTCATCGTCATCTGTATC | 60 | 0.25 | 121 | |
| CLDN4-Rv | GGCCACGATCATGGTCTTG | |||||
| Mucine-1 | Muc1-Fw | GTGCCGACGAAAGAACTG | 60 | 0.25 | 187 | |
| Muc1-Rv | TGCCAGGTTCGAGTAAGAG | |||||
| Occludin | Occludin-Fw | GCTTTGGTGGCTATGGAAGT | 60 | 0.5 | 157 | |
| Occludin-Rv | CCAGGAAGAATCCCTTTGCT | |||||
| Ribosomal protein L4 | RPL4-Fw | CAAGAGTAACTACAACCTTC | 60 | 0.5 | 122 | [18] |
| RPL4-Rv | GAACTCTACGATGAATCTTC | |||||
| Glyceraldehyde 3- phosphate dehydrogenase | GDPH-Fw | GTCGGTTGTGGATCTGACCT | 60 | 0.2 | 135 | [20] |
| GDPH-Rv | TCACAGGACACAACCTGGTC | |||||
| TATA-Box Binding Protein | TBP-Fw | AACAGTTCAGTAGTTATGAGCCAGA | 63 | 0.2 | 153 | [18] |
| TBP-Rv | AGATGTTCTCAAACGCTTCG |
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Temperature and pH IB Stability
2.8. In Vivo Assay
2.9. Statistical Analysis
3. Results
3.1. Production and Characterization of Cytokine-Based Nanoparticles
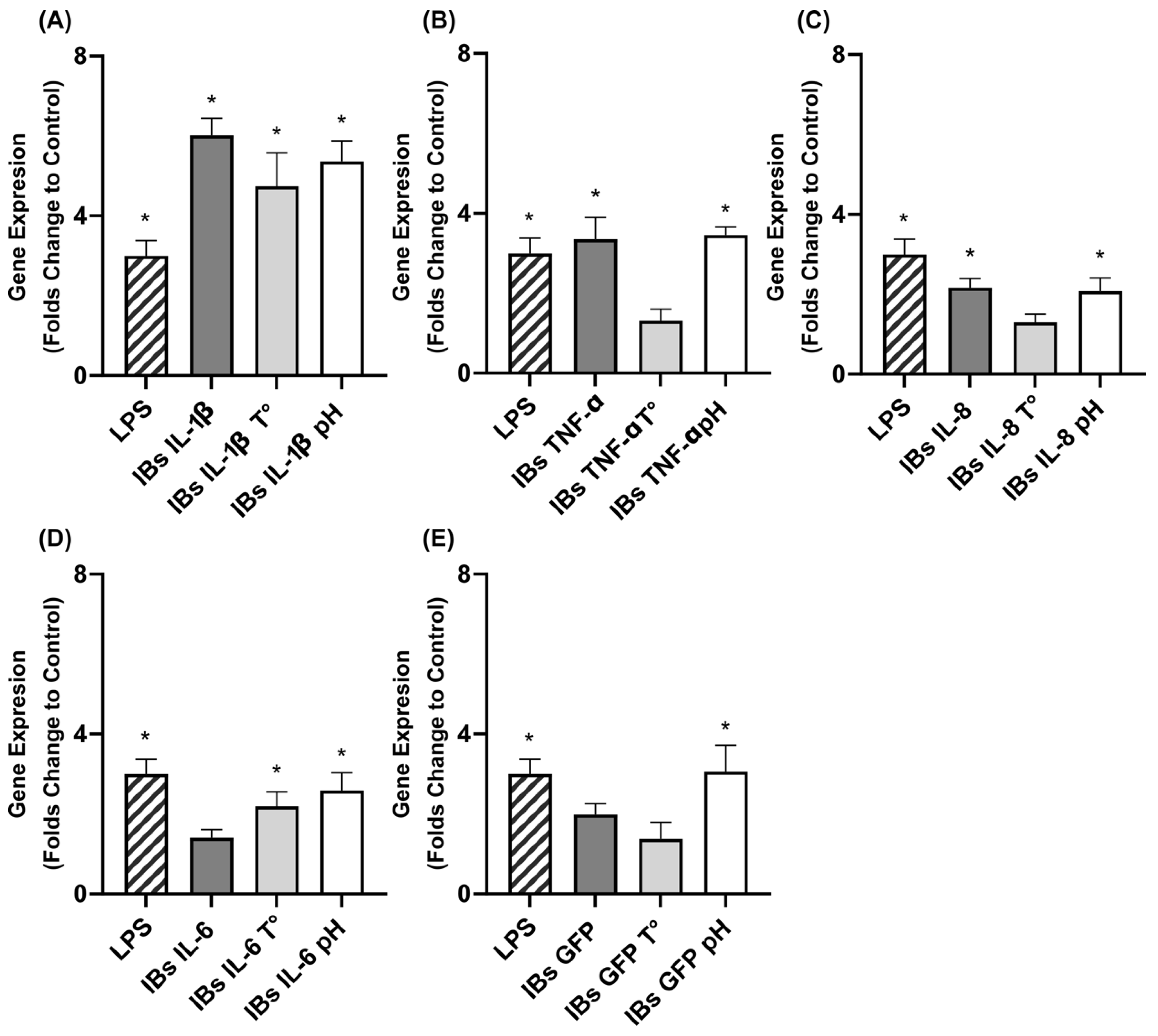
3.2. Immunostimulation of Swine Intestinal Cells and Macrophages
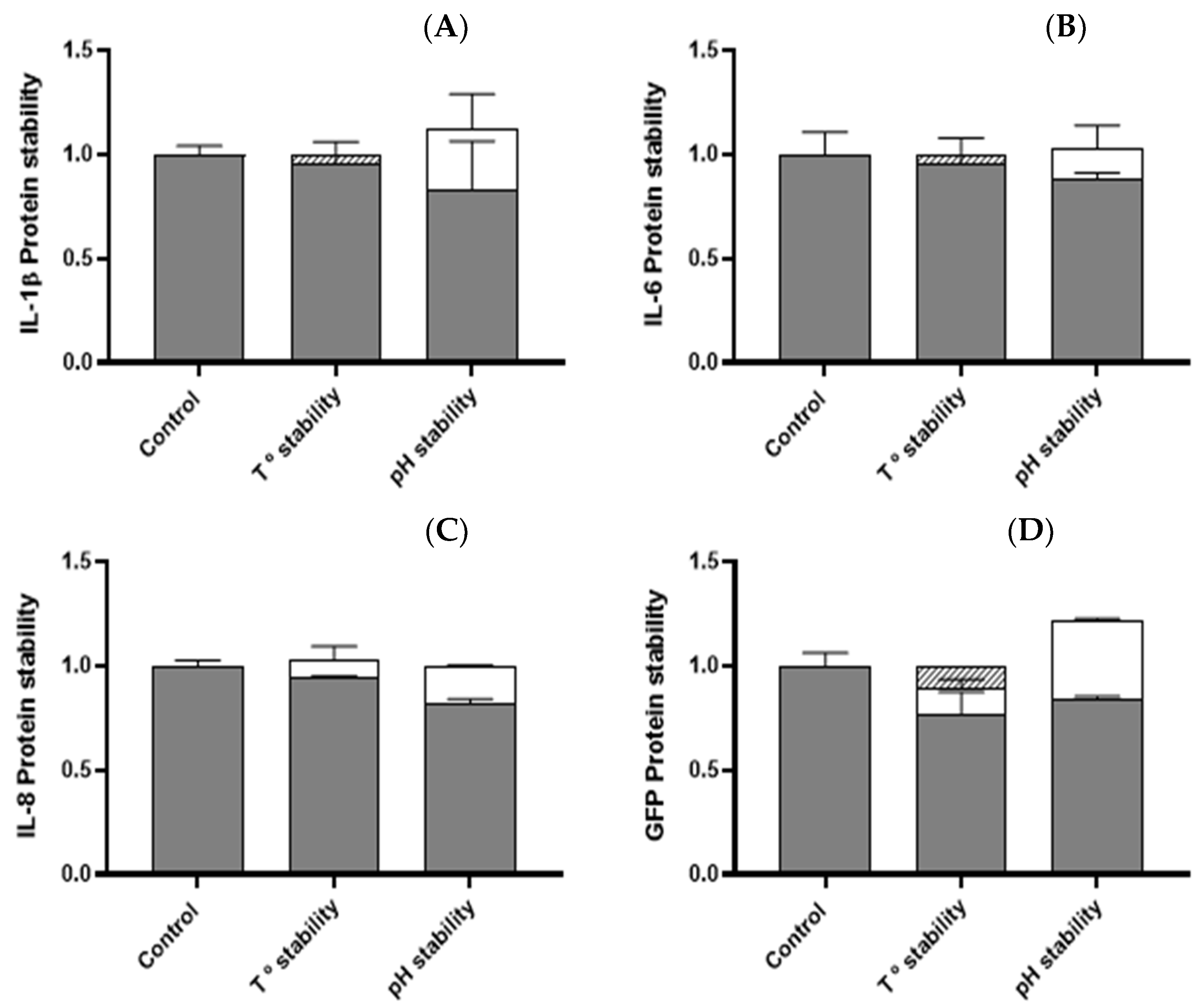
3.3. Temperature and pH Stability of Cytokine Nanoparticles
3.4. Swine In Vivo Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, K.; Ebner, F.; Schmidt, S.; Hartmann, S.; Merle, R.; Friese, A.; Roesler, U. Influence of immune status on the airborne colonization of piglets with methicillin-resistant staphylococcus aureus (MRSA) clonal complex (CC) 398. Eur. J. Microbiol. Immunol. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J. Anim. Sci. 2012, 90, 505–514. [Google Scholar] [CrossRef]
- Krakowski, L.; Krzyżanowski, J.; Wrona, Z.; Kostro, K.; Siwicki, A.K. The influence of nonspecific immunostimulation of pregnant sows on the immunological value of colostrum. Veter-Immunol. Immunopathol. 2002, 87, 89–95. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Nijnik, A.; Philpott, D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Bricknell, I.; Dalmo, R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. 2005, 19, 457–472. [Google Scholar] [CrossRef]
- Byrne, K.A.; Loving, C.L.; McGill, J.L. Innate immunomodulation in food animals: Evidence for trained immunity? Front. Immunol. 2020, 11, 1099. [Google Scholar] [CrossRef]
- Kiczorowska, B.; Samolińska, W.; Al-Yasiry, A.R.M.; Kiczorowski, P.; Winiarska-Mieczan, A. The natural feed additives as immunostimulants in monogastric animal nutrition—A review. Ann. Anim. Sci. 2017, 17, 605–625. [Google Scholar] [CrossRef] [Green Version]
- Wilson-Welder, J.H.; Torres, M.P.; Kipper, M.J.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Vaccine adjuvants: Current challenges and future approaches. J. Pharm. Sci. 2009, 98, 1278–1316. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, D.; Seras-Franzoso, J.; Mamat, U.; Wilke, K.; Villaverde, A.; Roher, N.; Garcia-Fruitós, E. Complex Particulate Biomaterials as Immunostimulant-Delivery Platforms. PLoS ONE 2016, 11, e0164073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gifre-Renom, L.; Cano-Garrido, O.; Fàbregas, F.; Roca-Pinilla, R.; Seras-Franzoso, J.; Ferrer-Miralles, N.; Villaverde, A.; Bach, A.; Devant, M.; Arís, A.; et al. A new approach to obtain pure and active proteins from Lactococcus lactis protein aggregates. Sci. Rep. 2018, 8, 13917. [Google Scholar] [CrossRef] [PubMed]
- Gifre-Renom, L.; Seras-Franzoso, J.; Rafael, D.; Andrade, F.; Cano-Garrido, O.; Martinez-Trucharte, F.; Ugarte-Berzal, E.; Martens, E.; Boon, L.; Villaverde, A.; et al. The Biological Potential Hidden in Inclusion Bodies. Pharmaceutics 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olvera, A.; Ballester, M.; Nofrarias, M.; Sibila, M.; Aragon, V. Differences in phagocytosis susceptibility in Haemophilus parasuis strains. Vet. Res. 2009, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cano-Garrido, O.; Rueda, F.L.; Sànchez-García, L.; Ruiz-Ávila, L.; Bosser, R.; Villaverde, A.; García-Fruitós, E. Expanding the recombinant protein quality in Lactococcus lactis. Microb. Cell Factories 2014, 13, 167. [Google Scholar] [CrossRef] [Green Version]
- Nygard, A.-B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Dozois, C.M.; Oswald, E.; Gautier, N.; Serthelon, J.-P.; Fairbrother, J.M.; Oswald, I. A reverse transcription-polymerase chain reaction method to analyze porcine cytokine gene expression. Vet. Immunol. Immunopathol. 1997, 58, 287–300. [Google Scholar] [CrossRef]
- Liehr, M.; Mereu, A.; Pastor, J.J.; Quintela, J.C.; Staats, S.; Rimbach, G.; Ipharraguerre, I.R. Olive oil bioactives protect pigs against experimentally-induced chronic inflammation independently of alterations in gut microbiota. PLoS ONE 2017, 12, e0174239. [Google Scholar] [CrossRef] [Green Version]
- Torrealba, D.; Parra, D.; Seras-Franzoso, J.; Vallejos-Vidal, E.; Yero, D.; Gibert, I.; Villaverde, A.; Garcia-Fruitós, E.; Roher, N. Nanostructured recombinant cytokines: A highly stable alternative to short-lived prophylactics. Biomaterials 2016, 107, 102–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Garrido, O.; Sánchez-Chardi, A.; Parés, S.; Giró, I.; Tatkiewicz, W.I.; Ferrer-Miralles, N.; Ratera, I.; Natalello, A.; Cubarsi, R.; Veciana, J.; et al. Functional protein-based nanomaterial produced in microorganisms recognized as safe: A new platform for biotechnology. Acta Biomater. 2016, 43, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef] [PubMed]
- Braciak, T.A.; Gallichan, W.S.; Graham, F.L.; Richards, C.; Ramsay, A.J.; Rosenthal, K.L.; Gauldie, J. Recombinant adenovirus vectors expressing interleukin-5 and -6 specifically enhance mucosal immunoglobulin A responses in the lung. Immunology 2000, 101, 388–396. [Google Scholar] [CrossRef]
- Huber, V.C.; Arulanandam, B.P.; Arnaboldi, P.M.; Elmore, M.K.; Sheehan, C.E.; Kallakury, B.V.; Metzger, D.W. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 2003, 3, 801–809. [Google Scholar] [CrossRef]
- Peel, J.E.; Kolly, C.; Siegenthaler, B.; Martinod, S.R. Effects of recombinant bovine interferon-γ (rBoIFN-γ) on the clinical course of severe systemic salmonellosis caused by S. typhimurium in calves. Immunobiology 1989, 4, 121. [Google Scholar]
- Peters, S.M.; Yancy, H.; Deaver, C.; Jones, Y.L.; Kenyon, E.; Chiesa, O.A.; Esparza-Trujillo, J.; Screven, R.; Lancaster, V.; Stubbs, J.T.; et al. In vivo characterization of inflammatory biomarkers in swine and the impact of flunixin meglumine administration. Vet. Immunol. Immunopathol. 2012, 148, 236–242. [Google Scholar] [CrossRef]
- Zhong, J.-F.; Wu, W.-G.; Zhang, X.-Q.; Tu, W.; Liu, Z.-X.; Fang, R.-J. Effects of dietary addition of heat-killed Mycobacterium phlei on growth performance, immune status and anti-oxidative capacity in early weaned piglets. Arch. Anim. Nutr. 2016, 70, 249–262. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.; Lee, J.J.; Bravo, D.; Maddox, C.W.; Pettigrew, J.E. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection. J. Anim. Sci. 2014, 92, 2050–2062. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Yang, H.S.; Wang, X.C.; Hu, Q.; Liu, C.X.; Wu, X.; Deng, D.; Hou, Y.Q.; Nyachoti, C.M.; Xiao, D.; et al. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef]
- Shan, T.; Wang, Y.; Liu, J.; Xu, Z. Effect of dietary lactoferrin on the immune functions and serum iron level of weanling piglets1. J. Anim. Sci. 2007, 85, 2140–2146. [Google Scholar] [CrossRef] [PubMed]

| Cytokine | Yield (mg/L) a | Recombinant Protein Content (%) |
|---|---|---|
| Interleukin-1β | 0.51 ± 0.20 | 16.19 ± 0.06 |
| Interleukin-6 | 1.19 ± 0.12 | 34.40 ± 0.04 |
| Interleukin-8 | 25.69 ± 3.49 | 23.39 ± 6.82 |
| Tumor necrosis factor-α | n.d | n.d |
| GFP | 1.67 ± 0.15 | 11.01 ± 1.39 |
| Tissue | IB Treatment (10 μg/mL) | IL-6 Secretion (ng/mL) | TNF-α Secretion (ng/mL) |
|---|---|---|---|
| Alveolar macrophages | IL-1β | 8.868 ± 0.182 * | n.d |
| IL-6 | n.d | n.d | |
| IL-8 | 0.031 ± 0 | 4.622 ± 0.109 * | |
| TNF-α | 0.381 ± 0 * | 1.206 ± 0 * | |
| GFP | 2.235 ± 0.016 * | 0.796 ± 0.091 * | |
| LPS | 0.067 ± 0.010 * | 5.277 ± 0.062 * | |
| PBS | 0.036 ± 0.018 | 2.214 ± 0.061 | |
| Intestinal Epithelial cells (IPEC-J2) | IL-1β | 21.125 ± 4.598 * | n.d |
| IL-6 | n.d | n.d | |
| IL-8 | n.d | n.d | |
| TNF-α | 0.300 ± 0.006 | 2.646 ± 0.055 | |
| GFP | 7.006 ± 0.403 * | n.d | |
| LPS | 0.018 ± 0.004 | n.d | |
| PBS | 0.015 ± 0.003 | n.d |
| Day-27 | Day-34 | SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Cytokine | Control | Treatment | Control | Treatment | T | D | TxD | |
| IL-8 | 0.066 | 0.097 | 0.089 | 0.251 | 0.080 | 0.116 | 0.476 | 0.112 |
| IL-6 | 0.435 | 0.620 | 0.511 | 1.324 | 0.286 | 0.136 | 0.105 | 0.328 |
| TNF-α | 1.188 | 0.522 | 0.570 | 54.106 | 16.370 | 0.122 | 0.120 | 0.076 |
| IL-10 | 0.350 | 0.680 | 0.300 | 3.202 | 1.569 | 0.854 | 0.697 | 0.900 |
| Day-27 | Day-34 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | Gene | Control | Treatment | Control | Treatment | T | D | TxD | |
| Ileum | TNF-α | 2.8 × 10−9 | 0.265 | 0.244 | 0.423 | 0.527 | 0.679 | 0.708 | 0.936 |
| (A) | IL6 | 1.009 | 0.953 | 1.020 | 1.045 | 0.144 | 0.917 | 0.724 | 0.784 |
| BD1 | 1.416 | 1.393 | 3.175 | 2.898 | 1.279 | 0.679 | 0.708 | 0.936 | |
| BD2 | 1.092 | 0.873 | 1.594 | 1.165 | 0.251 | 0.215 | 0.133 | 0.680 | |
| Muc1 | 1.139 | 0.677 | 1.064 | 0.970 | 0.173 | 0.129 | 0.539 | 0.304 | |
| CLDN4 | 1.023 | 0.832 | 1.219 | 0.998 | 0.105 | 0.068 | 0.104 | 0.890 | |
| Jejunum | TNF-α | 1.030 | 1.153 | 0.948 | 0.807 | 0.113 | 0.938 | 0.077 | 0.261 |
| (B) | IL6 | 1.039 | 0.964 | 0.877 | 0.783 | 0.144 | 0.566 | 0.251 | 0.950 |
| BD1 | 1.707 | 1.761 | 1.990 | 5.290 | 2.130 | 0.917 | 0.362 | 0.321 | |
| BD2 | 1.118 | 1.555 | 1.550 | 3.406 | 0.922 | 0.225 | 0.155 | 0.497 | |
| Muc1 | 1.005 | 1.361 | 1.232 | 1.470 | 0.187 | 0.131 | 0.382 | 0.756 | |
| CLDN4 | 1.031 | 0.870 | 0.935 | 0.956 | 0.105 | 0.515 | 0.959 | 0.398 | |
| Occludin | 1.017 | 1.001 | 1.116 | 1.085 | 0.106 | 0.827 | 0.401 | 0.944 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cano, A.; Bach, A.; López-Serrano, S.; Aragon, V.; Blanch, M.; Pastor, J.J.; Tedó, G.; Morais, S.; Garcia-Fruitós, E.; Arís, A. Potential of Oral Nanoparticles Containing Cytokines as Intestinal Mucosal Immunostimulants in Pigs: A Pilot Study. Animals 2022, 12, 1075. https://doi.org/10.3390/ani12091075
López-Cano A, Bach A, López-Serrano S, Aragon V, Blanch M, Pastor JJ, Tedó G, Morais S, Garcia-Fruitós E, Arís A. Potential of Oral Nanoparticles Containing Cytokines as Intestinal Mucosal Immunostimulants in Pigs: A Pilot Study. Animals. 2022; 12(9):1075. https://doi.org/10.3390/ani12091075
Chicago/Turabian StyleLópez-Cano, Adrià, Alex Bach, Sergi López-Serrano, Virginia Aragon, Marta Blanch, Jose J. Pastor, Gemma Tedó, Sofia Morais, Elena Garcia-Fruitós, and Anna Arís. 2022. "Potential of Oral Nanoparticles Containing Cytokines as Intestinal Mucosal Immunostimulants in Pigs: A Pilot Study" Animals 12, no. 9: 1075. https://doi.org/10.3390/ani12091075
APA StyleLópez-Cano, A., Bach, A., López-Serrano, S., Aragon, V., Blanch, M., Pastor, J. J., Tedó, G., Morais, S., Garcia-Fruitós, E., & Arís, A. (2022). Potential of Oral Nanoparticles Containing Cytokines as Intestinal Mucosal Immunostimulants in Pigs: A Pilot Study. Animals, 12(9), 1075. https://doi.org/10.3390/ani12091075







