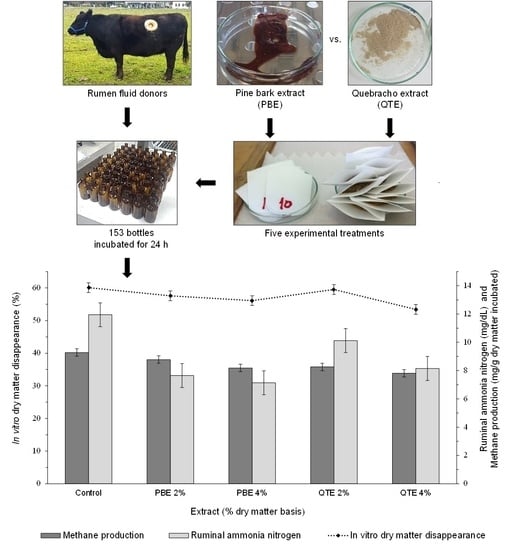
Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In Vitro Rumen Fermentation Parameters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Plant Extracts and Experimental Substrates
2.3. Animals, Rumen Inoculum and Incubation
2.4. IVDMD, Gas and CH4 Production Measurements
2.5. Determination of Culture pH, NH3-N and VFA
2.6. Substrate Analysis and Calculations
2.7. Statistical Analyses
3. Results
3.1. IVDMD, Gas and CH4 Production Kinetics
3.2. IVDMD, Gas and CH4 Outputs
3.3. In Vitro Ruminal Fermentation Parameters
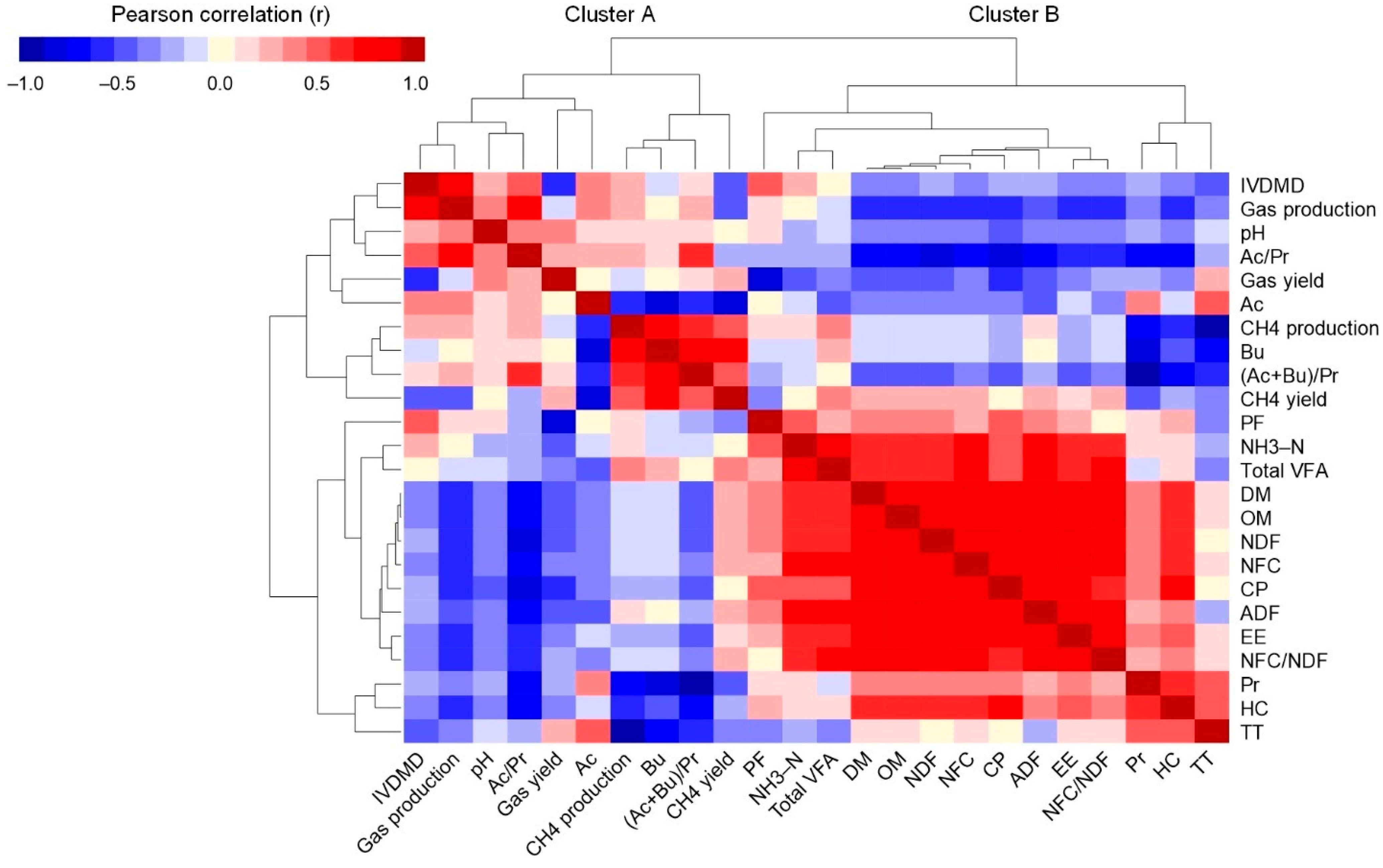
3.4. Pearson Correlation Coefficients and Hierarchical Clustering Analysis
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Livestock’s Long Shadow-Environmental Issues and Options; Food and Agriculture Organization: Rome, Italy, 2006; Volume 3, pp. 1–377. [Google Scholar]
- Bueno, I.; Brandi, R.; Franzolin, R.; Benetel, G.; Fagundes, G.; Abdalla, A.; Louvandini, H.; Muir, J. In vitro methane production and tolerance to condensed tannins in five ruminant species. Anim. Feed Sci. Technol. 2015, 205, 1–9. [Google Scholar] [CrossRef]
- Guglielmelli, A.; Calabro, S.; Primi, R.; Carone, F.; Cutrignelli, M.; Tudisco, R.; Piccolo, G.; Ronchi, B.; Danieli, P. In vitro fermentation patterns and methane production of sainfoin (Onobrychis viciifolia Scop.) hay with different condensed tannin contents. Grass Forage Sci. 2011, 66, 488–500. [Google Scholar] [CrossRef] [Green Version]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rira, M.; Morgavi, D.P.; Archimède, H.; Marie–Magdeleine, C.; Popova, M.; Bousseboua, H.; Doreau, M. Potential of tannin–rich plants for modulating ruminal microbes and ruminal fermentation in sheep. J. Anim. Sci. 2015, 93, 334–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutti, D.D.; Paula, N.F.D.; Zervoudakis, J.T.; Cabral, L.S.; Fonseca, M.A.; Macedo, B.G.; Lima, L.R. Effects of tannins and monensin on the modulation of in vitro ruminal fermentation and ammonia production of nitrogen-fertilized and non-fertilized Urochloa brizantha cv. Marandu. Grassl. Sci. 2019, 65, 101–108. [Google Scholar] [CrossRef]
- Avila, J.; Chaves, A.; Hernandez–Calva, M.; Beauchemin, K.; McGinn, S.; Wang, Y.; Harstad, O.; McAllister, T. Effects of replacing barley grain in feedlot diets with increasing levels of glycerol on in vitro fermentation and methane production. Anim. Feed Sci. Technol. 2011, 166–167, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Ishlak, A.; Günal, M.; AbuGhazaleh, A.A. The effects of cinnamaldehyde, monensin and quebracho condensed tannin on rumen fermentation, biohydrogenation and bacteria in continuous culture system. Anim. Feed Sci. Technol. 2015, 207, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Alves, S.P.; Cabo, Â.; Guerreiro, O.; Stilwell, G.; Dentinho, M.T.; Bessa, R.J. Modulation of in vitro rumen biohydrogenation by Cistus ladanifer tannins compared with other tannin sources. J. Sci. Food Agric. 2017, 97, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Egea, A.V.; Allegretti, L.I.; Lama, S.A.P.; Grilli, D.; Fucili, M.; Guevara, J.C.; Villalba, J.J. Diet mixing and condensed tannins help explain foraging preferences by Creole goats facing the physical and chemical diversity of native woody plants in the central Monte desert (Argentina). Anim. Feed Sci. Technol. 2016, 215, 47–57. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, A.R.; Álvarez del Pino, M.C. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vázquez-Armijo, J.; López-Villalobos, N.; Lee-Rangel, H.; Salem, A.; Borquez-Gastelum, J.; Domínguez-Vara, I.; Rojo-Rubio, R. In vitro gas production of foliage from three browse tree species treated with different dose levels of exogenous fibrolytic enzymes. J. Anim. Physiol. Anim. Nutr. 2016, 100, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buccioni, A.; Minieri, S.; Rapaccini, S.; Antongiovanni, M.; Mele, M. Effect of chestnut and quebracho tannins on fatty acid profile in rumen liquid- and solid-associated bacteria: An in vitro study. Animal 2011, 5, 1521–1530. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Costa, M.; Cabo, Â.; Duarte, M.F.; Jerónimo, E.; Bessa, R.J.B. Effects of extracts obtained from Cistus ladanifer L. on in vitro rumen biohydrogenation. Anim. Feed Sci. Technol. 2016, 219, 304–312. [Google Scholar] [CrossRef]
- Berg, A.; Olave, L.; Navarrete, P. Process for Obtaining Low and Medium Molecular Weight Polyphenols and Standardized Solid Fuel from Tree Wood or Bark. US Patent US 20090077871 A1, 26 March 2009. [Google Scholar]
- Vera, N.; Gutiérrez, C.; Allende, R.; Williams, P.; Fuentealba, C.; Ávila–Stagno, J. Dose–response effect of a pine bark extract on in vitro ruminal ammonia and methane formation kinetics. Acta Agric. Scand. A Anim. Sci. 2018, 68, 181–189. [Google Scholar] [CrossRef]
- Vera, N.; Gutiérrez, C.; Williams, P.; Fuentealba, C.; Allende, R.; Ávila-Stagno, J. Low concentrations of a polyphenolic extract from pine bark in high–concentrate diets decrease in vitro rumen ammonia nitrogen but not methane production. J. Appl. Anim. Res. 2021, 49, 413–422. [Google Scholar] [CrossRef]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Beef Cattle, 8th Revised ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Fedorak, P.M.; Hrudey, S.E. A simple apparatus for measuring gas production by methanogenic culture in serum bottles. Environ. Technol. Lett. 1983, 4, 425–432. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase–treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [CrossRef] [PubMed]
- Lavrenčič, A.; Mills, C.R.; Stefanon, B. Application of the Gompertz model to describe the fermentation characteristics of chemical components in forages. Anim. Sci. 1998, 66, 155–161. [Google Scholar] [CrossRef]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fibre digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, R.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Effects of disodium fumarate on in vitro rumen microbial growth, methane production and fermentation of diets differing in their forage: Concentrate ratio. Br. J. Nutr. 2005, 94, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blümmel, M.; Steingss, H.; Becker, K. The relationship between in vitro gas production, in vitro microbial biomass yield and 15N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 5th ed.; Pearson: Boston, MA, USA, 2007. [Google Scholar]
- Carreño, D.; Hervás, G.; Toral, P.G.; Belenguer, A.; Frutos, P. Ability of different types and doses of tannin extracts to modulate in vitro ruminal biohydrogenation in sheep. Anim. Feed Sci. Technol. 2015, 202, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of ruminal Quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Salem, A.Z.M.; Olafadehan, O.A.; Kholif, A.M. Sustainable anaerobic rumen methane and carbon dioxide productions from prickly pear cactus flour by organic acid salts addition. J. Clean. Prod. 2016, 139, 1362–1369. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2013, 93, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Saravanan, M.; Baruah, L.; Prasad, C.S. Effects of graded levels of tannin–containing tropical tree leaves on in vitro rumen fermentation, total protozoa and methane production. J. Appl. Microbiol. 2015, 118, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.K.; Kolte, A.P.; Baruah, L.; Saravanan, M.; Bakshi, B.; Bhatta, R. Enteric methane mitigation in sheep through leaves of selected tanniniferous tropical tree species. Livest. Sci. 2017, 200, 29–34. [Google Scholar] [CrossRef]
- Gunun, P.; Gunun, N.; Cherdthong, A.; Wanapat, M.; Polyorach, S.; Sirilaophaisan, S.; Wachirapakorn, C.; Kang, S. In vitro rumen fermentation and methane production as affected by rambutan peel powder. J. Appl. Anim. Res. 2018, 46, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Moate, P.J.; Jacobs, J.L.; Hixson, J.L.; Deighton, M.H.; Hannah, M.C.; Morris, G.L.; Ribaux, B.E.; Wales, W.J.; Williams, S.R.O. Effects of Feeding either Red or White Grape Marc on Milk Production and Methane Emissions from Early-Lactation Dairy Cows. Animals 2020, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Sommai, S.; Cherdthong, A.; Suntara, C.; So, S.; Wanapat, M.; Polyorach, S. In vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios. Fermentation 2021, 7, 109. [Google Scholar] [CrossRef]
- Unnawong, N.; Cherdthong, A.; So, S. Crude saponin extract from Sesbania grandiflora (L.) Pers pod meal could modulate ruminal fermentation, and protein utilization, as well as mitigate methane production. Trop. Anim. Health Prod. 2021, 53, 1–9. [Google Scholar] [CrossRef]
- Khiaosa–Ard, R.; Bryner, S.F.; Scheeder, M.R.; Wettstein, H.R.; Leiber, F.; Kreuzer, M.; Soliva, C.R. Evidence for the inhibition of the terminal step of ruminal α–linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 2009, 92, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Firkins, J.L.; Allen, M.S.; Oldick, B.S.; St-Pierre, N.R. Modeling ruminal digestibility of carbohydrates and microbial protein flow to the duodenum. J. Dairy Sci. 1998, 81, 3350–3369. [Google Scholar] [CrossRef]
- Patra, A.K.; Kamar, D.N.; Agarwal, N. Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim. Feed Sci. Technol. 2006, 128, 276–291. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatta, R.; Baruah, L.; Saravanan, M.; Suresh, K.P.; Sampath, K.T. Effect of medicinal and aromatic plants on rumen fermentation, protozoa population and methanogenesis in vitro. J. Anim. Physiol. Anim. Nutr. 2013, 97, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.G.; Berchiell, T.T.; Pedreira, M.S.; Primavesi, O.; Frighetto, R.; Lima, M.A. Effect of tannin levels in sorghum silage and concentrate supplementation on apparent digestibility and methane emission in beef cattle. Anim. Feed Sci. Technol. 2007, 135, 236–248. [Google Scholar] [CrossRef]
- Wischer, G.; Boguhn, J.; Steingaß, H.; Schollenberger, M.; Rodehutscord, M. Effects of different tannin–rich extracts and rapeseed tannin monomers on methane formation and microbial protein synthesis in vitro. Animal 2013, 7, 1796–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, C.; Bodas, R.; Morán, L.; Mateo, J.; Andrés, S.; Giráldez, F.J. Effect of hop (Humulus lupulus L.) inclusion in the diet for fattening lambs on animal performance, ruminal characteristics and meat quality. Food Res. Int. 2018, 108, 42–47. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Stochmal, A.; Sadowinska, A.; Salem, A.Z.M.; Kowalczyk, D.; Zbonik, P.; Szumacher-Strabel, M. Effects of Two Sources of Tannins (Quercus L. and Vaccinium Vitis Idaea L.) on Rumen Microbial Fermentation: An in vitro Study. Ital. J. Anim. Sci. 2014, 13, 3133. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho–chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef] [Green Version]
- Broderick, G.A.; Grabber, J.H.; Muck, R.E.; Hymes-Fecht, U.C. Replacing alfalfa silage with tannin-containing birdsfoot trefoil silage in total mixed rations for lactating dairy cows. J. Dairy Sci. 2017, 100, 3548–3562. [Google Scholar] [CrossRef] [Green Version]
- Hatami, A.; Alipour, D.; Hozhabri, F.; Tabatabaei, M. Effect of different levels of pomegranate marc with or without polyethylene glycol on performance, nutrients digestibility and protozoal population in growing lambs. Anim. Feed Sci. Technol. 2018, 235, 15–22. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkoum, S.; Yuangklang, C.; Paengkoum, P. Flavonoids and their aromatic derivatives in Piper betle powder promote in vitro methane mitigation in a variety of diets. Ciênc. Agrotec. 2020, 44, e012420. [Google Scholar] [CrossRef]
- Gresner, N.; Wichern, A.; Lumpp, L.; Hoedemaker, M.; Höltershinken, M. Effects of grass silages with two levels of free amino acids on degradation of amino acids and fixation of nitrogen in bacterial protein in bovine ruminal fluid using the rumen simulation technique (Rusitec). Anim. Feed Sci. Technol. 2015, 202, 1–11. [Google Scholar] [CrossRef]

| Item | Treatments | ||||
|---|---|---|---|---|---|
| CTL | PBE2 | PBE4 | QTE2 | QTE4 | |
| Ingredients (g/kg dry matter) | |||||
| Mixed hay of ryegrass and clover | 600 | 588 | 576 | 588 | 576 |
| Corn grain | 250 | 245 | 240 | 245 | 240 |
| Soybean meal | 150 | 147 | 144 | 147 | 144 |
| Pine bark extract | – | 20 | 40 | – | – |
| Quebracho extract | – | – | – | 20 | 40 |
| Chemical composition (% dry matter unless otherwise noted) | |||||
| Dry matter (% fresh basis) | 90.00 | 79.00 | 69.80 | 89.70 | 89.70 |
| Organic matter | 92.80 | 92.30 | 92.20 | 92.30 | 92.60 |
| Crude protein | 20.40 | 20.60 | 21.00 | 21.10 | 20.50 |
| Ether extract | 1.91 | 1.72 | 1.89 | 1.79 | 1.91 |
| Non-fibrous carbohydrates | 40.50 | 38.90 | 38.00 | 39.30 | 40.50 |
| Neutral detergent fiber | 30.00 | 31.10 | 31.30 | 30.10 | 29.70 |
| NFC/NDF | 1.35 | 1.25 | 1.21 | 1.31 | 1.36 |
| Acid detergent fiber | 22.00 | 21.40 | 21.60 | 20.80 | 20.40 |
| Hemicellulose | 8.00 | 9.70 | 9.70 | 9.30 | 9.30 |
| Item | Treatments | SEM | p-Value | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTL | PBE2 | PBE4 | QTE2 | QTE4 | 1 | 2 | 3 | |||
| IVDMD | ||||||||||
| B (%) | 56.30 AB | 57.00 AB | 58.70 A | 54.30 AB | 50.30 B | 1.57 | 0.040 | 0.643 | 0.009 | 0.816 |
| C (h−1) | 1.47 AB | 1.32 BC | 1.20 C | 1.50 AB | 1.52 A | 0.05 | 0.004 | 0.497 | <0.001 | 0.789 |
| A | 0.44 AB | 0.38 AB | 0.34 B | 0.46 A | 0.48 A | 0.02 | 0.013 | 0.665 | <0.001 | 0.913 |
| TMDR (h) | 8.39 A | 7.60 AB | 6.26 B | 8.17 A | 7.93 A | 0.42 | 0.017 | 0.130 | 0.040 | 0.146 |
| MDR (% h−1) | 1.48 AB | 3.24 A | 4.09 A | 1.01 B | 0.88 B | 0.66 | 0.014 | 0.559 | <0.001 | 0.832 |
| Gas production | ||||||||||
| b (mL g−1 incubated DM) | 125.00 | 127.90 | 129.10 | 122.60 | 120.10 | 4.51 | 0.512 | 0.883 | 0.068 | 0.822 |
| c (h−1) | 0.19 A | 0.17 AB | 0.13 B | 0.17 AB | 0.16 B | 0.01 | 0.008 | 0.009 | 0.989 | 0.004 |
| Lag (h) | 1.78 AB | 1.79 AB | 2.45 A | 1.45 BC | 1.01 C | 0.16 | 0.008 | 0.326 | 0.024 | 0.589 |
| t1/2 (h) | 4.71 B | 4.96 B | 5.94 A | 4.65 B | 4.36 B | 0.13 | <0.001 | 0.543 | 0.004 | 0.560 |
| AR (mL g−1 incubated DM h−1) | 11.40 | 10.80 | 10.50 | 11.30 | 11.00 | 0.31 | 0.331 | 0.183 | 0.125 | 0.300 |
| CH4 production | ||||||||||
| b (mg g−1 incubated DM) | 9.37 A | 9.16 AB | 8.57 AB | 8.18 AB | 7.67 B | 0.42 | 0.039 | 0.073 | 0.019 | 0.079 |
| c (h−1) | 0.15 | 0.14 | 0.13 | 0.15 | 0.15 | 0.01 | 0.496 | 0.358 | 0.088 | 0.578 |
| Lag (h) | 2.37 AB | 2.54 AB | 3.21 A | 2.06 B | 1.75 B | 0.18 | 0.014 | 0.967 | 0.008 | 0.926 |
| t1/2 (h) | 6.08 AB | 6.20 AB | 7.06 A | 5.54 B | 5.25 B | 0.27 | 0.007 | 0.889 | 0.002 | 0.794 |
| AR (mg g−1 incubated DM h−1) | 0.74 A | 0.67 AB | 0.59 B | 0.72 A | 0.67 AB | 0.02 | 0.014 | 0.115 | 0.067 | 0.044 |
| Item | Treatments | SEM | p-Value | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTL | PBE2 | PBE4 | QTE2 | QTE4 | 1 | 2 | 3 | |||
| 6 h | ||||||||||
| IVDMD (%) | 42.50 A | 39.60 AB | 38.00 B | 41.20 AB | 39.30 AB | 0.92 | 0.023 | 0.013 | 0.091 | 0.043 |
| Gas (mL) | 18.00 A | 14.90 B | 11.10 B | 18.00 A | 17.90 A | 0.84 | <0.001 | 0.439 | <0.001 | 0.065 |
| g−1 incubated DM | 38.60 A | 34.70 AB | 31.40 B | 38.00 A | 37.20 AB | 1.37 | 0.011 | 0.196 | 0.005 | 0.091 |
| g−1 degraded DM | 99.50 A | 91.00 AB | 80.20 B | 94.40 AB | 95.50 AB | 4.65 | 0.027 | 0.276 | 0.026 | 0.114 |
| CH4 (mg) | 1.05 A | 0.82 B | 0.59 B | 1.04 A | 1.03 A | 0.05 | <0.001 | 0.254 | <0.001 | 0.038 |
| mL−1 gas | 0.059 A | 0.055 AB | 0.052 B | 0.059 A | 0.057 AB | 0.001 | 0.013 | 0.218 | 0.004 | 0.087 |
| g−1 incubated DM | 2.31 A | 2.00 AB | 1.63 B | 2.20 A | 2.14 AB | 0.15 | 0.019 | 0.189 | 0.013 | 0.089 |
| g−1 degraded DM | 5.52 A | 5.00 AB | 4.07 B | 5.92 A | 6.02 A | 0.35 | 0.023 | 0.537 | 0.011 | 0.232 |
| 12 h | ||||||||||
| IVDMD (%) | 51.40 A | 46.80 AB | 45.10 B | 48.40 AB | 46.50 B | 1.30 | 0.004 | 0.001 | 0.144 | 0.083 |
| Gas (mL) | 38.50 A | 32.20 B | 29.60 B | 34.60 AB | 32.90 B | 1.26 | 0.003 | 0.003 | 0.035 | 0.100 |
| g−1 incubated DM | 82.50 A | 77.90 AB | 71.20 B | 76.50 AB | 70.80 B | 2.21 | 0.002 | <0.001 | 0.574 | 0.003 |
| g−1 degraded DM | 160.70 | 159.70 | 153.80 | 147.20 | 148.40 | 7.20 | 0.279 | 0.060 | 0.109 | 0.744 |
| CH4 (mg) | 2.45 A | 1.95 B | 1.54 C | 2.24 AB | 2.13 AB | 0.07 | <0.001 | 0.002 | <0.001 | 0.006 |
| mL−1 gas | 0.064 A | 0.060 AB | 0.053 B | 0.065 A | 0.065 A | 0.002 | 0.001 | 0.788 | 0.001 | 0.073 |
| g−1 incubated DM | 5.25 A | 4.70 AB | 4.18 B | 4.78 AB | 4.59 AB | 0.16 | 0.007 | 0.004 | 0.157 | 0.047 |
| g−1 degraded DM | 10.23 | 9.65 | 9.32 | 9.89 | 9.84 | 0.42 | 0.655 | 0.404 | 0.377 | 0.643 |
| 24 h | ||||||||||
| IVDMD (%) | 60.10 A | 57.60 AB | 56.10 AB | 59.50 AB | 53.40 B | 1.49 | 0.041 | 0.131 | 0.809 | 0.021 |
| Gas (mL) | 56.20 | 49.20 | 46.20 | 54.00 | 51.20 | 3.26 | 0.130 | 0.203 | 0.154 | 0.303 |
| g−1 incubated DM | 120.70 | 116.20 | 117.20 | 114.00 | 112.10 | 5.83 | 0.743 | 0.219 | 0.456 | 0.475 |
| g−1 degraded DM | 192.50 | 199.00 | 205.00 | 194.30 | 205.80 | 6.84 | 0.369 | 0.180 | 0.958 | 0.117 |
| CH4 (mg) | 4.35 A | 3.63 B | 3.04 C | 3.88 AB | 3.65 B | 0.13 | <0.001 | <0.001 | 0.006 | 0.005 |
| mL−1 gas | 0.077 A | 0.073 AB | 0.066 B | 0.072 AB | 0.070 AB | 0.002 | 0.038 | 0.044 | 0.616 | 0.011 |
| g−1 incubated DM | 9.28 A | 8.78 AB | 8.19 B | 8.26 B | 7.81 B | 0.26 | 0.005 | 0.004 | 0.072 | 0.002 |
| g−1 degraded DM | 15.40 | 15.20 | 13.80 | 14.10 | 14.60 | 0.65 | 0.199 | 0.095 | 0.927 | 0.199 |
| Item | Treatments | SEM | p-Value | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTL | PBE2 | PBE4 | QTE2 | QTE4 | 1 | 2 | 3 | |||
| pH | 6.48 AB | 6.52 AB | 6.54 A | 6.47 AB | 6.44 B | 0.02 | 0.031 | 0.534 | 0.828 | 0.003 |
| NH3-N (mg dL−1) | 11.95 A | 7.65 B | 7.14 B | 10.12 AB | 8.15 B | 0.85 | 0.006 | 0.002 | 0.063 | 0.006 |
| PF (mg degraded DM mL−1 gas) | 5.08 | 5.04 | 4.89 | 5.27 | 4.88 | 0.10 | 0.095 | 0.641 | 0.374 | 0.057 |
| Total volatile fatty acids (mM) | 520.90 A | 431.30 BC | 383.30 C | 475.50 AB | 454.90 B | 15.78 | <0.001 | 0.002 | 0.004 | 0.003 |
| Acetate (%) | 38.50 C | 40.90 BC | 46.20 A | 40.80 BC | 42.20 AB | 1.09 | 0.003 | 0.025 | 0.175 | 0.007 |
| Propionate (%) | 30.20 B | 31.50 AB | 31.80 AB | 37.20 AB | 38.80 A | 1.89 | 0.016 | 0.125 | 0.002 | 0.316 |
| Butyrate (%) | 38.60 A | 37.80 A | 25.50 B | 23.30 B | 20.50 B | 2.16 | 0.001 | 0.062 | 0.041 | 0.013 |
| Ac/Pr | 1.20 AB | 1.20 AB | 1.38 A | 1.10 B | 1.14 B | 0.05 | 0.017 | 0.967 | 0.009 | 0.309 |
| (Ac + Bu)/Pr | 2.71 A | 2.20 AB | 2.15 AB | 1.70 B | 1.69 B | 0.19 | 0.003 | 0.010 | 0.001 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera, N.; Gutiérrez-Gómez, C.; Williams, P.; Allende, R.; Fuentealba, C.; Ávila-Stagno, J. Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In Vitro Rumen Fermentation Parameters. Animals 2022, 12, 1080. https://doi.org/10.3390/ani12091080
Vera N, Gutiérrez-Gómez C, Williams P, Allende R, Fuentealba C, Ávila-Stagno J. Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In Vitro Rumen Fermentation Parameters. Animals. 2022; 12(9):1080. https://doi.org/10.3390/ani12091080
Chicago/Turabian StyleVera, Nelson, Constanza Gutiérrez-Gómez, Pamela Williams, Rodrigo Allende, Cecilia Fuentealba, and Jorge Ávila-Stagno. 2022. "Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In Vitro Rumen Fermentation Parameters" Animals 12, no. 9: 1080. https://doi.org/10.3390/ani12091080
APA StyleVera, N., Gutiérrez-Gómez, C., Williams, P., Allende, R., Fuentealba, C., & Ávila-Stagno, J. (2022). Comparing the Effects of a Pine (Pinus radiata D. Don) Bark Extract with a Quebracho (Schinopsis balansae Engl.) Extract on Methane Production and In Vitro Rumen Fermentation Parameters. Animals, 12(9), 1080. https://doi.org/10.3390/ani12091080