Long Term Dietary Supplementation with Omega-3 Fatty Acids in Charolais Beef Cattle Reared in Italian Intensive Systems: Nutritional Profile and Fatty Acids Composition of Longissimus lumborum Muscle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Carcass Traits
2.3. Physical Parameters
2.4. Chemical Parameters
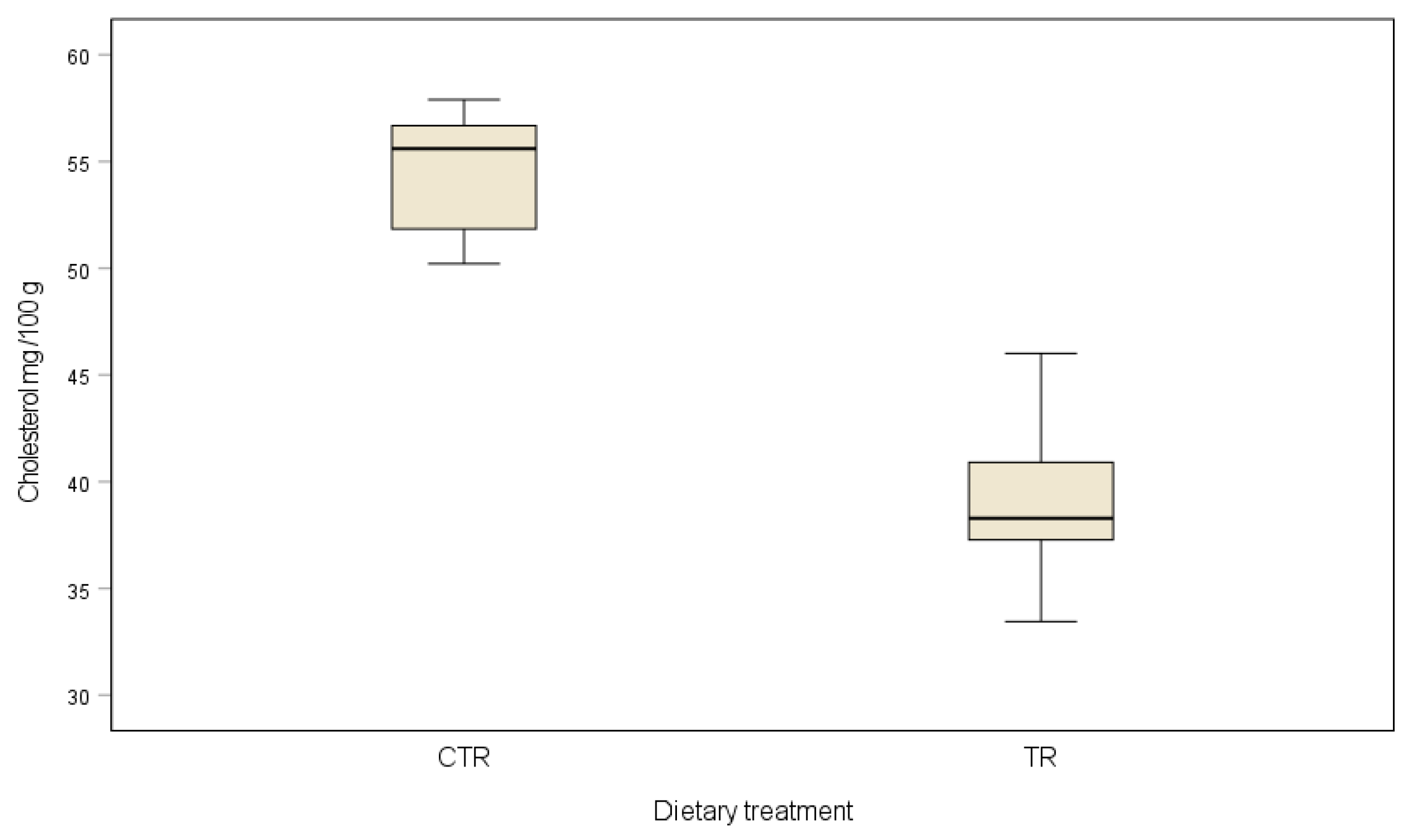
2.5. Cholesterol Content
2.6. Fatty Acid Composition
2.7. Oxidative Stability
2.8. Statistical Analyses
3. Results
3.1. Growth Performance and Carcass Characteristic
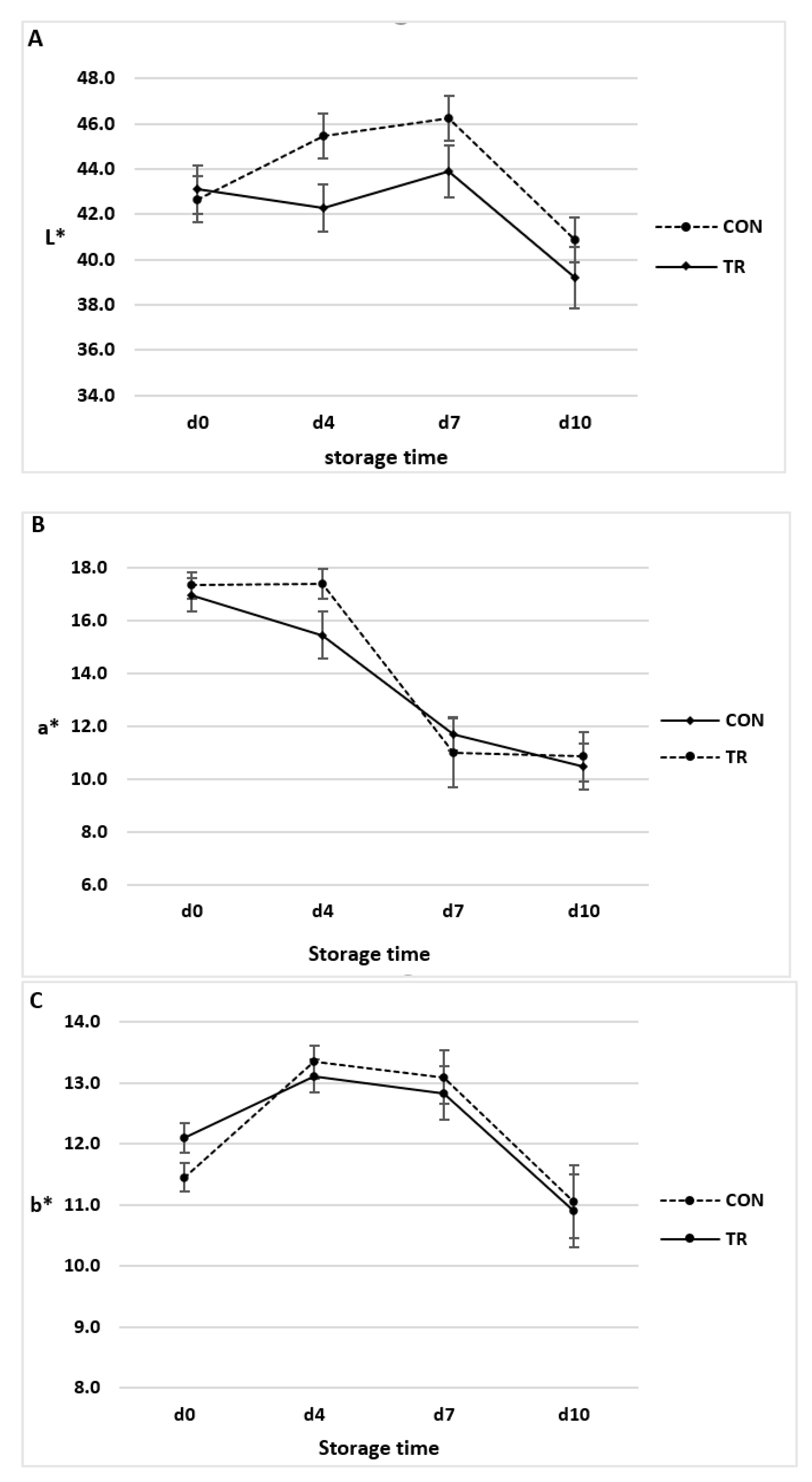
3.2. Colour Indexes
3.3. Chemical Parameters
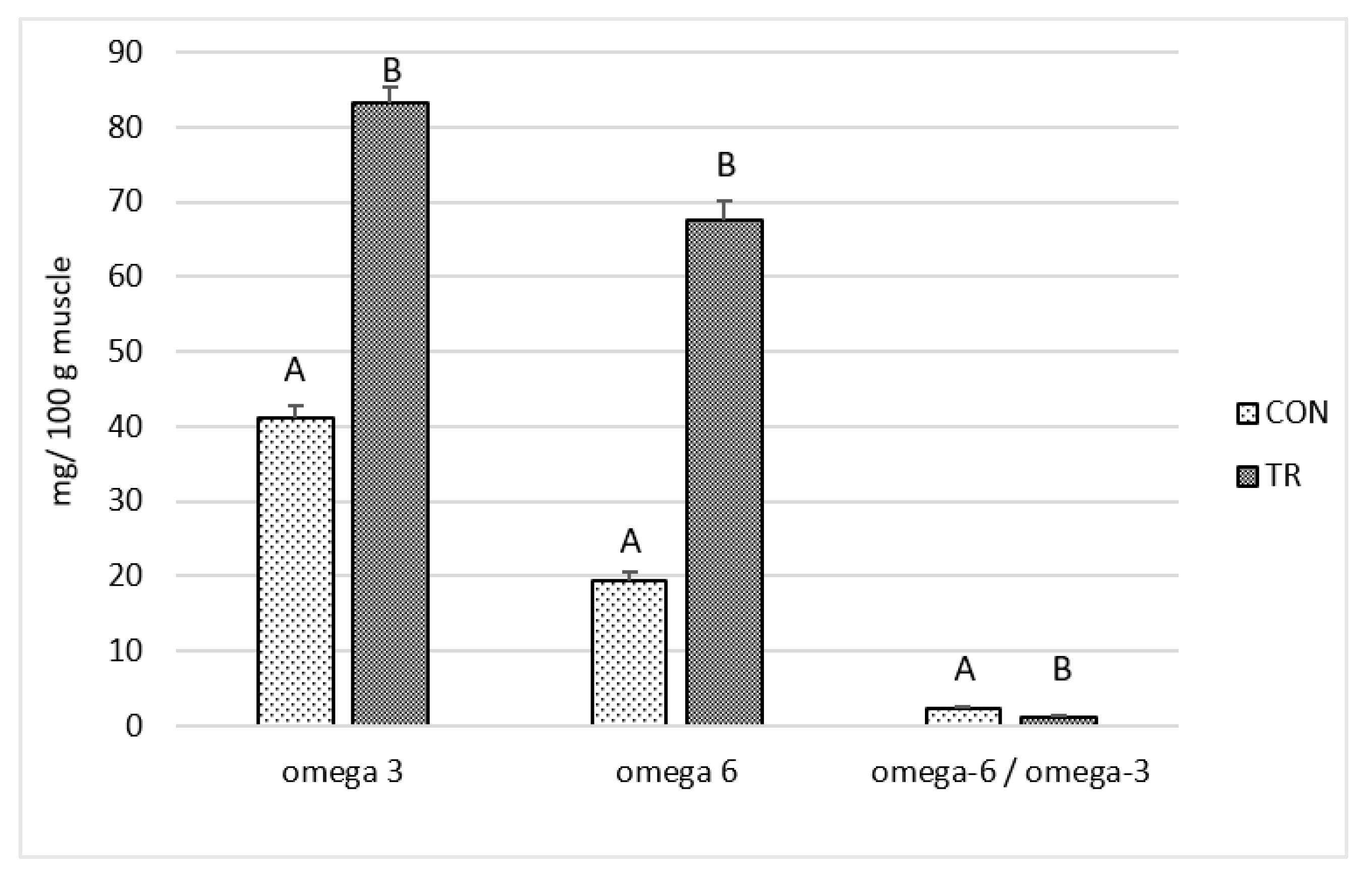
3.4. Fatty Acid Composition
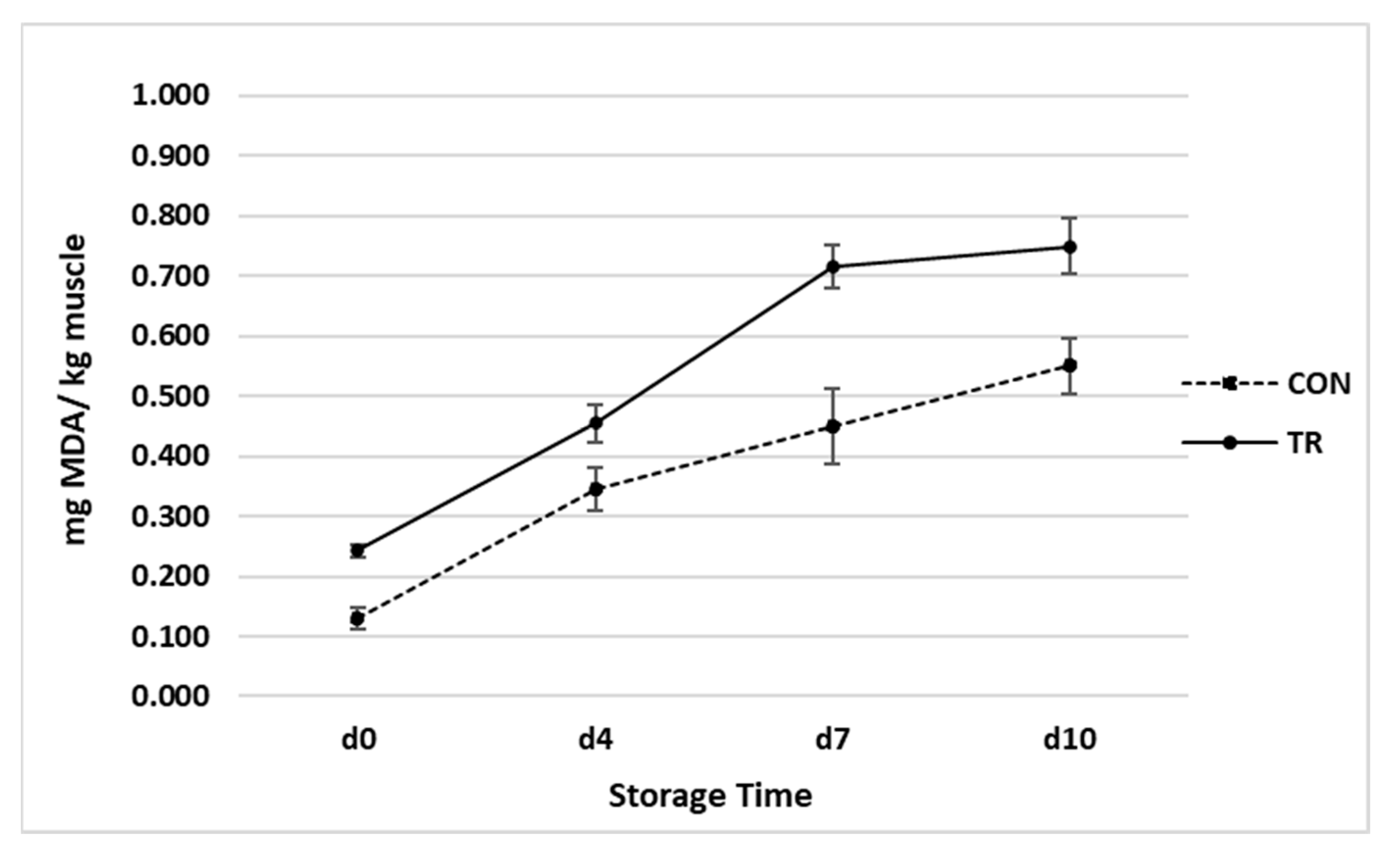
3.5. Oxidative Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabrera, M.C.; Saadoun, A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014, 98, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef] [Green Version]
- Arihara, K. Strategies for designing novel functional meat products. Meat Sci. 2006, 74, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, W.; Van Wezemael, L.; de Barcellos, M.D.; Kugler, J.O.; Hocquette, J.F.; Ueland, O.; Grunert, K.G. European beef consumers’ interest in a beef eating-quality guarantee. Insights from a qualitative study in four EU countries. Appetite 2010, 54, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Mapiye, C.; Aalhus, J.L.; Turner, T.D.; Rolland, D.C.; Basarab, J.A.; Baron, V.S.; McAllister, T.A.; Block, H.C.; Uttaro, B.; Lopez-Campos, O.; et al. Effects of feeding flaxseed or sunflower-seed in high-forage diets on beef production, quality and fatty acid composition. Meat Sci. 2013, 95, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Dannenberger, D.; Nuernberg, K.; Richardson, R.I.; MacKintosh, S.; Hocquette, J.-F.; Moloney, A.P. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2014, 97, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Realini, C.E.; Font Furnols, M.; Sañudo, C.; Montossi, F.; Oliver, M.A.; Guerrero, L. Spanish, French and British consumers’ acceptability of Uruguayan beef and consumers’ beef choice associated with country of origin, finishing diet and meat price. Meat Sci. 2013, 95, 14–21. [Google Scholar] [CrossRef]
- Troy, D.J.; Tiwari, B.K.; Joo, S.T. Health Implications of Beef Intramuscular Fat Consumption. Korean. J. Food Sci Anim Resour. 2016, 36, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Gaeini, Z.; Mirmiran, P.; Bahadoran, Z.; Aghayan, M.; Azizi, F. The association between dietary fats and the incidence risk of cardiovascular outcomes: Tehran Lipid and Glucose Study. Nutr. Metab. (Lond.) 2021, 18, 96. [Google Scholar] [CrossRef]
- Zárate, R.; Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Covadonga, R. Significance of long chain polyunsaturated fatty acids in human health. J. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Rodrigues, L.B.O.; Lisboa, M.; Silva Pereira, M.M.; De Souza, S.O. Conjugated Linoleic Acid (CLA): A Review. Int. J. App. Sci. Technol. 2014, 4, 154–170. [Google Scholar]
- Corino, C.; Rossi, R.; Cannata, S.; Ratti, S. Effect of dietary linseed on the nutritional value and quality of pork and pork products: Systematic review and meta-analysis. Meat Sci. 2014, 98, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.B.; Fearon, A.M. Dietary sources of unsaturated fatty acids for animals and their transfer in meat, milk and eggs: A review. Livest. Sci. 2009, 126, 1–20. [Google Scholar] [CrossRef]
- Sturaro, E.; Cassandro, M.; Cozzi, G. Sustainability of cattle farms in Italy. Acta Agric. Slov. 2014, 3, 27–33. [Google Scholar]
- Scollan, N.D.; Choi, N.J.; Kurt, E.; Fisher, A.V.; Enser, M.; Wood, J.D. Manipulating of fatty acid composition of muscle and adipose tissue in beef cattle. Br. J. Nutr. 2001, 85, 115–124. [Google Scholar] [CrossRef] [Green Version]
- INRA (Institut National de la Recherche Agronomique). Alimentation des Bovins, Ovins et Caprins. Besoins des Animaux. Valeurs des Aliments; Editions Quae: Versailles, France, 2007. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Maraschiello, C.; Diaz, I.; Garcia Regueiro, J.A. Determination of cholesterol in fat and muscle of pig by HPLC and capillary gas chromatography with solvent venting injection. J. High Resolut. Chromatogr. 1996, 19, 165–168. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. Int. J. Biol. Chem. 1957, 226, 495–509. [Google Scholar]
- Christie, W.W. Lipid Analysis, 2nd ed.; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 85–92. [Google Scholar] [CrossRef]
- Realin, C.E.; Duckett, S.K.; Brito, G.W.; Dalla Rizza, M.; De Mattos, D. Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 2004, 66, 567–577. [Google Scholar] [CrossRef]
- Razminowicz, R.H.; Kreuzer, M.; Leuenberger, H.; Scheeder, M.R.L. Efficiency of extruded linseed for the finishing of grass-fed steers to counteract a decline of omega-3 fatty acids in the beef. Livest. Sci. 2008, 114, 150–163. [Google Scholar] [CrossRef]
- Morittu, V.M.; Spina, A.A.; Iommelli, P.; Poerio, A.; Oliverio, F.V.; Britti, D.; Tudisco, R. Effect of Integration of Linseed and Vitamin E in Charolaise × Podolica Bulls’ Diet on Fatty Acids Profile, Beef Color and Lipid Stability. Agriculture 2021, 11, 1032. [Google Scholar] [CrossRef]
- Fusaro, I.; Cavallini, D.; Giammarco, M.; Manetta, A.C.; Martuscelli, M.; Mammi, L.M.E.; Lanzoni, L.; Formigoni, A.; Vignola, G. Oxidative status of Marchigiana beef enriched in n-3 fatty acids and vitamin E, treated with a blend of oregano and rosemary essential oils. Front. Vet. Sci. 2021, 8, 662079. [Google Scholar] [CrossRef] [PubMed]
- Baltrukonienė, G.; Uchockis, V.; Švirmicka, G.J. The influence of compound feed enrichment with rapeseed and linseed cake on the meat characteristics and fatty acids composition of beef bulls. Agriculture 2015, 102, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Albertí, P.; Beriain, M.J.; Ripoll, G.; Sarriés, V.; Panea, B.; Mendizabal, J.A.; Purroy, A.; Olleta, J.L.; Sañudo, C. Effect of including linseed in a concentrate fed to young bulls on intramuscular fatty acids and beef color. Meat Sci. 2014, 96, 1258–1265. [Google Scholar] [CrossRef]
- Mach, N.; Devant, M.; Bach, A.; Díaz, I.; Font, M.; Oliver, M.A.; García, J.A. Increasing the amount of omega-3 fatty acid of meat from intensively fed young Holstein bulls through nutrition. J. Anim. Sci. 2006, 84, 3039–3048. [Google Scholar] [CrossRef] [Green Version]
- Conte, G.; Serra, A.; Casarosa, L.; Ciucci, F.; Cappucci, A.; Bulleri, E.; Corrales-Retana, L.; Buccioni, A.; Mele, M. Effect of linseed supplementation on total longissimus muscle lipid composition and shelf-life of beef from young Maremmana bulls. Front. Vet. Sci. 2019, 5, 326. [Google Scholar] [CrossRef]
- Olivera, D.F.; Bambicha, R.; Laporte, G.; Cárdenas, F.C.; Mestorino, N. Kinetics of colour and texture changes of beef during storage. J. Food Sci. Technol. 2013, 50, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Font-I-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Barragán-Hernández, W.; Mahecha-Ledesma, L.; Olivera-Angel, M.; Angulo-Arizala, J. Beef consumers’ perceptions and relationships with acceptation assessed by photography. Ital. J. Anim. Sci. 2021, 20, 505–513. [Google Scholar] [CrossRef]
- Bartoň, L.; Marounek, M.; Kudrna, V.; Bureš, D.; Zahradkova, M. Growth performance and fatty acid profiles of intramuscular and subcutaneous fat from Limousin and Charolais heifers fed extruded linseed. Meat Sci. 2007, 76, 517–523. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Eilander, A.; Harika, R.K.; Zock, P.L. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations? Eur. J. Lipid Sci. Technol. 2015, 117, 1370–1377. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oilseeds Fats Crops Lipids 2010, 17, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Scollan, N.; Hocquette, J.P.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Monteiro, A.C.G.; Santos-Silva, J.; Bessa, R.J.B.; Navas, D.R.; Lemos, J.P.C. Fatty acid composition of intramuscular fat of bulls and steers. Livest. Sci. 2006, 99, 13–19. [Google Scholar] [CrossRef]
- Dunne, P.G.; Rogalski, J.; Moreno, T.; Monahan, F.J.; French, P.; Moloney, A.P. Colour, composition and quality of M. longissimus dorsi and M. extensor carpi radialis of steers housed on straw or concrete slats or accommodated outdoors on wood-chips. Meat Sci. 2008, 79, 700–708. [Google Scholar] [CrossRef]
- Pilarczyk, R.; Wójcik, J. Fatty acids profile and health lipid indices in the longissimus lumborum muscle of different beef cattle breeds reared under intensive production systems. Acta Sci. Pol. Zootech. 2015, 14, 109–126. [Google Scholar]
- Enser, M.; Hallett, K.; Hewitt, B.; Fursey, G.A.J.; Wood, J.D. Fatty acid content and composition of English beef, lamb and pork at retail. Meat Sci. 1996, 42, 443–456. [Google Scholar] [CrossRef]
- Nuernberg, K.; Dannenberger, D.; Nuernberg, G.; Ender, K.; Voigt, J.; Scollan, N.D.; Wood, J.D.; Nute, G.R.; Richardson, R.I. Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livest. Prod. Sci. 2005, 94, 137–147. [Google Scholar] [CrossRef]
- Warren, H.E.; Scollan, N.D.; Enser, M.; Hughes, S.I.; Richardson, R.I.; Wood, J.D. Effects of breed and a concentrate or grass silage diet on beef quality in cattle of 3 ages. I, Animal performance, carcass quality and muscle fatty acid composition. Meat Sci. 2008, 78, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, C.; Cabaraux, J.F.; Dufrasne, I.; Clinquart, A.; Hocquette, J.F.; Istasse, L.; Hornick, J.L. Performance, slaughter characteristics and meat quality of young bulls from Belgian Blue, Limousin and Aberdeen Angus breeds fattened with a sugar-beet pulp or a cereal-based diet. Anim. Sci. 2006, 82, 125–132. [Google Scholar] [CrossRef]
- Williams, J.W.; Plassman, B.L.; Burke, J.; Benjamin, S. Preventing Alzheimer’s disease and cognitive decline. Evid. Rep. Technol. Assess. 2010, 1, 727. [Google Scholar]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. Br. Med. J. 2015, 350, h1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noci, F.; French, P.; Monahan, F.J.; Moloney, A.P. The fatty acid composition of muscle fat and subcutaneous adipose tissue of grazing heifers supplemented with plant oil-enriched concentrates. J. Anim. Sci. 2007, 85, 1062–1073. [Google Scholar] [CrossRef]
- Ritzenthaler, K.L.; McGuire, M.K.; Falen, R.; Schultz, T.D.; Dasgupta, N.; McGuire, M.A. Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology. J. Nutr. 2001, 131, 1548–1554. [Google Scholar] [CrossRef] [Green Version]
- De la Torre, A.; Debiton, E.; Juaneda, P.; Durand, D.; Chardigny, J.M.; Barthomeuf, C.; Bauchart, D.; Gruffat, D. Beef conjugated linoleic acid isomers reduce human cancer cell growth even associated with other beef fatty acids. Br. J. Nutr. 2006, 95, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Nuernberg, K.; Nuernberg, G.; Zhao, R.; Scollan, N.; Ender, K.; Dannenberger, D. Vaccenic acid and cis-9,trans-11 CLA in the rumen and different tissues of pasture and concentrate-fed beef cattle. Lipids 2007, 42, 1093–1103. [Google Scholar] [CrossRef]
- French, P.; O’Riordan, E.G.; Monahan, F.J.; Caffrey, P.J.; Moloney, A.P. Fatty acid composition of intramuscular triacylglycerols of steers fed autumn grass and concentrates. Livest. Prod. Sci. 2003, 81, 307–317. [Google Scholar] [CrossRef]
- Maddock, T.D.; Bauer, M.L.; Koch, K.B.; Anderson, V.L.; Maddock, R.J.; Barcelo-Coblijn, G.; Murphy, E.J.; Lardy, G.P. Effect of processing flax in beef feedlot diets on performance, carcass characteristics, and trained sensory panel ratings. J. Anim. Sci. 2006, 84, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaBrune, H.J.; Reinhardt, C.D.; Dikeman, M.E.; Drouillard, J.S. Effects of grain processing and dietary lipid source on performance, carcass characteristics, plasma fatty acids, and sensory properties of steaks from finishing cattle. J. Anim. Sci. 2008, 86, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Musella, M.; Cannata, S.; Rossi, R.; Mourot, J.; Baldini, P.; Corino, C. Omega-3 PUFA from extruded linseed influences fatty acid composition and sensory characteristics of dry-cured ham from heavy pigs. J. Anim. Sci. 2009, 87, 3578–3588. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.; Dugan, M.E.R.; Aldai, N.; Basarab, J.A.; Baron, V.S.; McAllister, T.A.; Aalhus, J.L. Beef quality attributes as affected by increasing the intramuscular levels of vitamin E and omega-3 fatty acids. Meat Sci. 2012, 90, 764–769. [Google Scholar] [CrossRef]
- Baba, Y.; Kallas, Z.; Costa-Font, M.; Gil, J.M.; Realini, C.E. Impact of hedonic evaluation on consumers’ preferences for beef attributes including its enrichment with n-3 and CLA fatty acids. Meat Sci. 2016, 11, 9–17. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Int. Food Res. J. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- McKenna, D.R.; Mies, P.D.; Baird, B.E.; Pfeiffer, K.D.; Ellebracht, J.W.; Savell, J.W. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 2005, 70, 665–682. [Google Scholar] [CrossRef]
| Total Mixed Ration | Phase 1 | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|---|
| CON | TR | CON | TR | CON | TR | |
| Ingredient. % D.M. | ||||||
| Corn silage | 19.15 | 19.15 | 23.72 | 23.72 | 19.73 | 19.73 |
| High moisture corn | 10.11 | 10.11 | 15.96 | 15.96 | 21.27 | 21.27 |
| Beet pulp | 22.91 | 22.91 | 9.84 | 9.84 | 10.64 | 10.64 |
| Wheat straw | 18.33 | 18.33 | 11.80 | 11.80 | 9.82 | 9.82 |
| Distillers | 9.40 | 9.40 | 6.78 | 6.78 | 8.09 | 8.09 |
| Soybean meal | 6.11 | 5.64 | 11.37 | 10.93 | 8.73 | 8.36 |
| Corn meal | 5.05 | 5.05 | 12.24 | 12.24 | 14.82 | 14.82 |
| Wheat middlings | 5.17 | 5.17 | 4.81 | 4.81 | 4.00 | 4.00 |
| Premix 1 | 2.82 | 2.82 | 2.62 | 2.62 | 2.18 | 2.18 |
| Calcium soap | 0.94 | - | 0.87 | - | 0.73 | - |
| Lipid supplement | - | 1.41 | - | 1.31 | - | 1.09 |
| Nutrient levels % DM | ||||||
| Energy, UFC/kg | 0.89 | 0.89 | 0.96 | 0.96 | 0.98 | 0.98 |
| CP | 13.84 | 13.98 | 14.3 | 14.2 | 13.6 | 13.7 |
| NDF | 41.9 | 42.8 | 33.6 | 34.4 | 31.6 | 32.3 |
| Starch | 20.1 | 20.2 | 29.4 | 30.9 | 32.9 | 33.4 |
| EE | 2.99 | 3.07 | 3.4 | 3.4 | 3.5 | 3.4 |
| Ca | 0.96 | 0.95 | 0.80 | 0.80 | 0.65 | 0.67 |
| P | 0.30 | 0.28 | 0.26 | 0.26 | 0.25 | 0.25 |
| Item 1 | CON | TR | p-Value |
|---|---|---|---|
| Moisture % | 73.78 ± 0.19 | 73.48 ± 0.26 | 0.940 |
| Crude protein, % 2 | 22.78 ± 0.26 | 23.17 ± 0.19 | 0.186 |
| Crude fat, % 2 | 1.83 ± 0.12 | 2.17 ± 0.19 | 0.254 |
| Ash, % 2 | 1.17 ± 0.03 a | 1.09 ± 0.01 b | 0.031 |
| Fatty Acid Composition | CON | TR | p-Value |
|---|---|---|---|
| C14:0 | 74.02 ± 0.97 | 75.74 ± 2.53 | 0.160 |
| C16:0 | 633.84 ± 5.42 | 663.07 ± 8.68 | <0.05 |
| C16:1 | 7.20 ± 0.41 | 14.18 ± 0.60 | <0.001 |
| C17:0 | 20.04 ± 0.96 | 18.38 ± 1.27 | 0.103 |
| C18:0 | 323.54 ± 3.00 | 318.07 ± 6.12 | 0.142 |
| C18:1 trans 11 | 22.66 ± 1.16 | 47.44 ± 0.83 | <0.001 |
| C18:1 cis 9 | 596.57 ± 4.73 | 760.12 ± 6.95 | <0.001 |
| C18:2 n6 | 34.36 ± 1.16 | 59.76 ± 2.21 | <0.001 |
| C18:2 cis 9, trans 11 | 5.76 ± 0.76 | 24.97 ± 0.25 | <0.001 |
| C18:3 n3 | 14.02 ± 1.16 | 54.93 ± 1.49 | <0.001 |
| C20:0 | 69.39 ± 2.24 | 54.94 ± 1.49 | <0.05 |
| C20:4 n6 | 6.81 ± 0.74 | 23.48 ± 0.13 | <0.001 |
| C20:5 n3 | 5.12 ± 0.38 | 12.40 ± 1.30 | <0.001 |
| C22:0 | 0.57 ± 0.08 | 0.76 ± 0.12 | 0.125 |
| C22:5 n3 | 3.59 ± 0.21 | 6.34 ± 0.56 | <0.001 |
| C22:6 n3 | 0.35 ± 0.04 | 0.56 ± 0.09 | 0.476 |
| SFA 1 | 1120.87 ± 6.20 | 1128.93 ± 7.17 | 0.201 |
| MUFA 2 | 626.43 ± 4.85 | 821.74 ± 6.37 | <0.001 |
| PUFA 3 | 70.01 ± 1.88 | 182.43 ± 3.69 | <0.001 |
| PUFA/SFA | 0.06 ± 0.002 | 0.16 ± 0.004 | <0.001 |
| AI 4 | 1.36 ± 0.021 | 0.96 ± 0.010 | <0.001 |
| TI 5 | 2.54 ± 0.046 | 1.53 ± 0.011 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corino, C.; Vizzarri, F.; Ratti, S.; Pellizzer, M.; Rossi, R. Long Term Dietary Supplementation with Omega-3 Fatty Acids in Charolais Beef Cattle Reared in Italian Intensive Systems: Nutritional Profile and Fatty Acids Composition of Longissimus lumborum Muscle. Animals 2022, 12, 1123. https://doi.org/10.3390/ani12091123
Corino C, Vizzarri F, Ratti S, Pellizzer M, Rossi R. Long Term Dietary Supplementation with Omega-3 Fatty Acids in Charolais Beef Cattle Reared in Italian Intensive Systems: Nutritional Profile and Fatty Acids Composition of Longissimus lumborum Muscle. Animals. 2022; 12(9):1123. https://doi.org/10.3390/ani12091123
Chicago/Turabian StyleCorino, Carlo, Francesco Vizzarri, Sabrina Ratti, Mirco Pellizzer, and Raffaella Rossi. 2022. "Long Term Dietary Supplementation with Omega-3 Fatty Acids in Charolais Beef Cattle Reared in Italian Intensive Systems: Nutritional Profile and Fatty Acids Composition of Longissimus lumborum Muscle" Animals 12, no. 9: 1123. https://doi.org/10.3390/ani12091123






