Effect of β-Mannanase Supplementation on Growth Performance, Ileal Digestibility, Carcass Traits, Intestinal Morphology, and Meat Quality in Broilers Fed Low-ME Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Samples Collection and Measurements
2.2.1. Growth Performance
2.2.2. Digestibility Trial
2.2.3. Carcass Characteristics
2.2.4. Intestinal Segments and Morphology
2.2.5. Meat Quality Analysis
2.3. Statistical Analysis
3. Results
3.1. Growth Performance
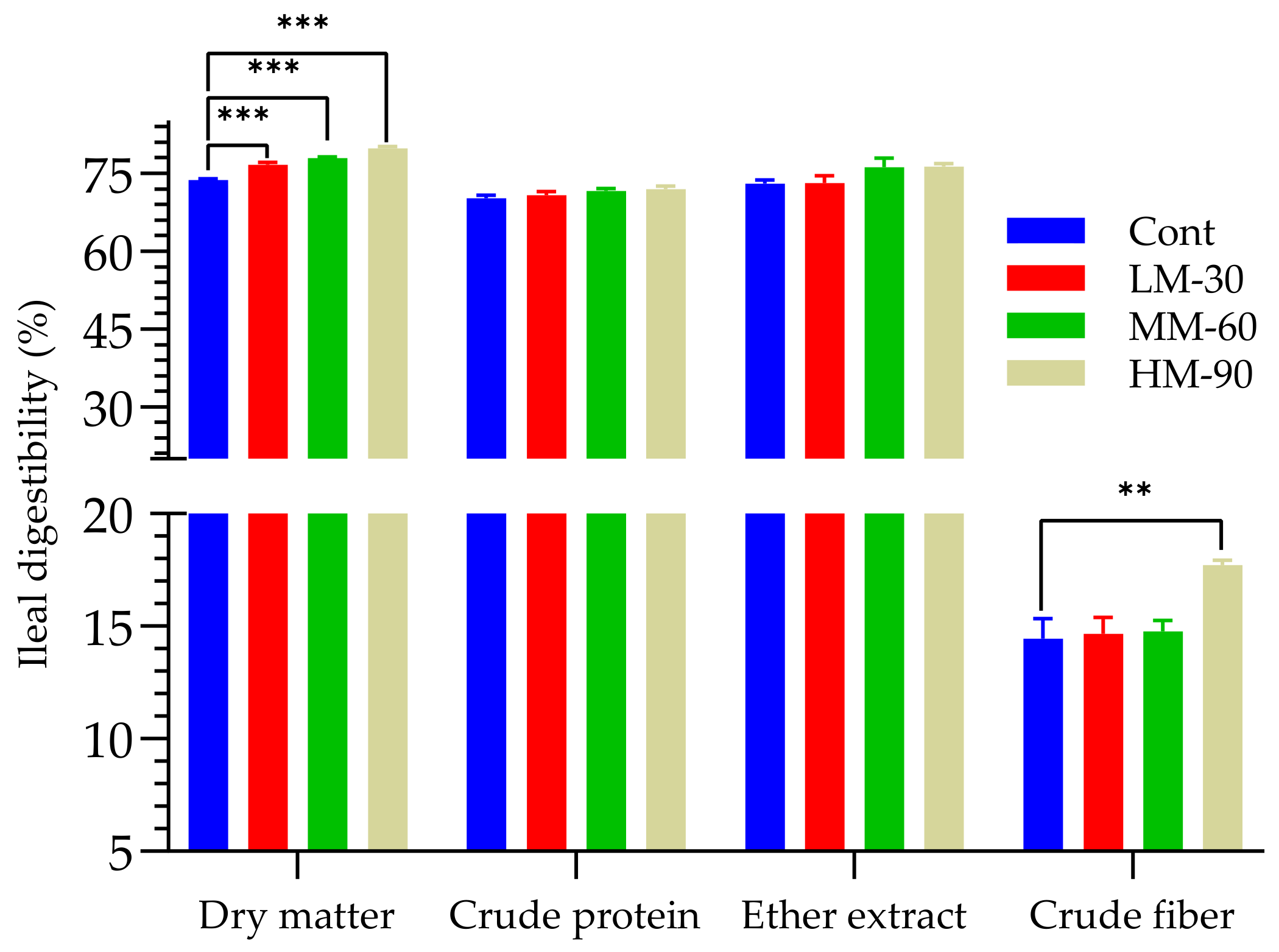
3.2. Ileal Digestibility
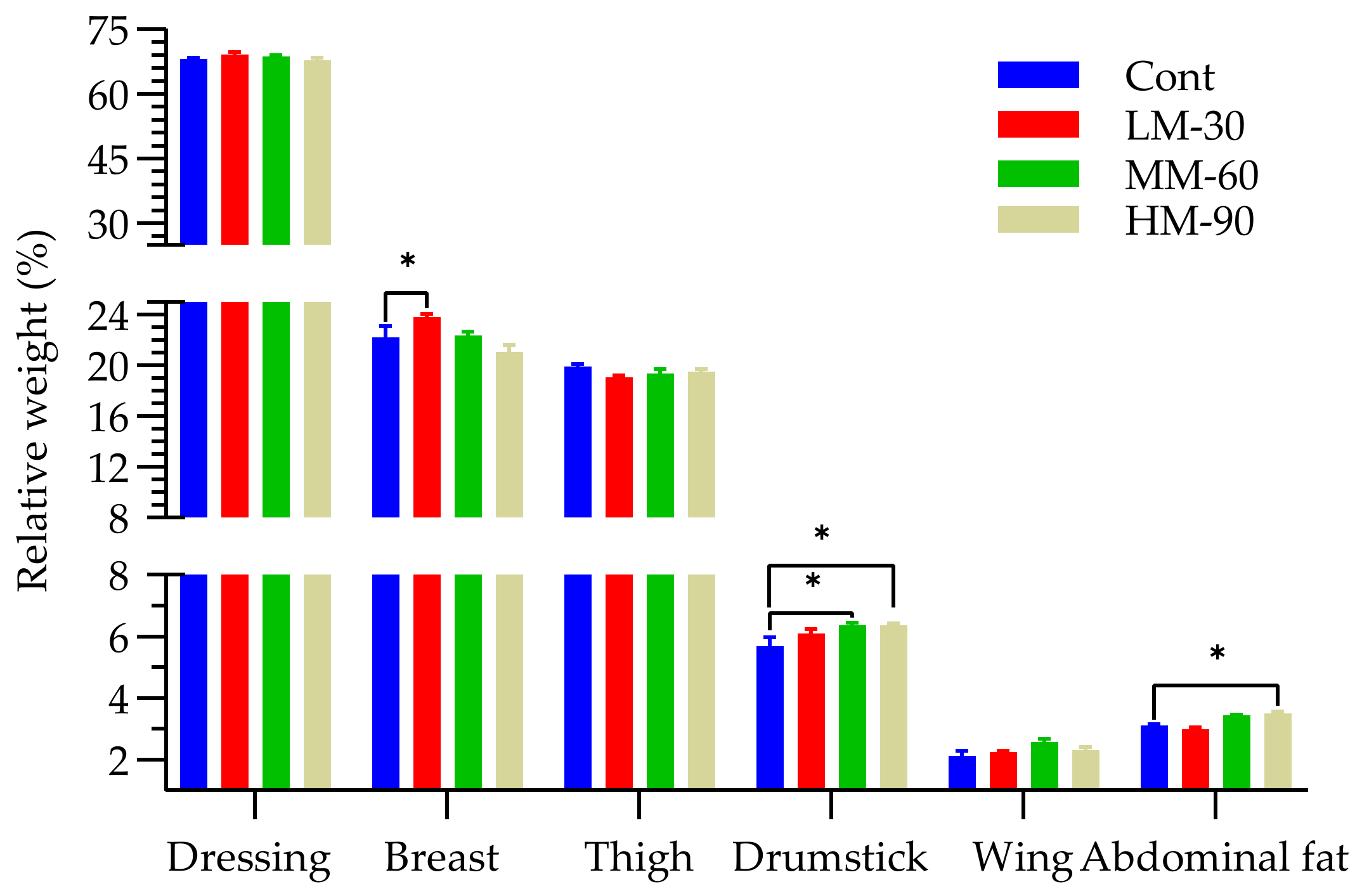
3.3. Carcass Characteristics
3.4. Internal Organ Indexes
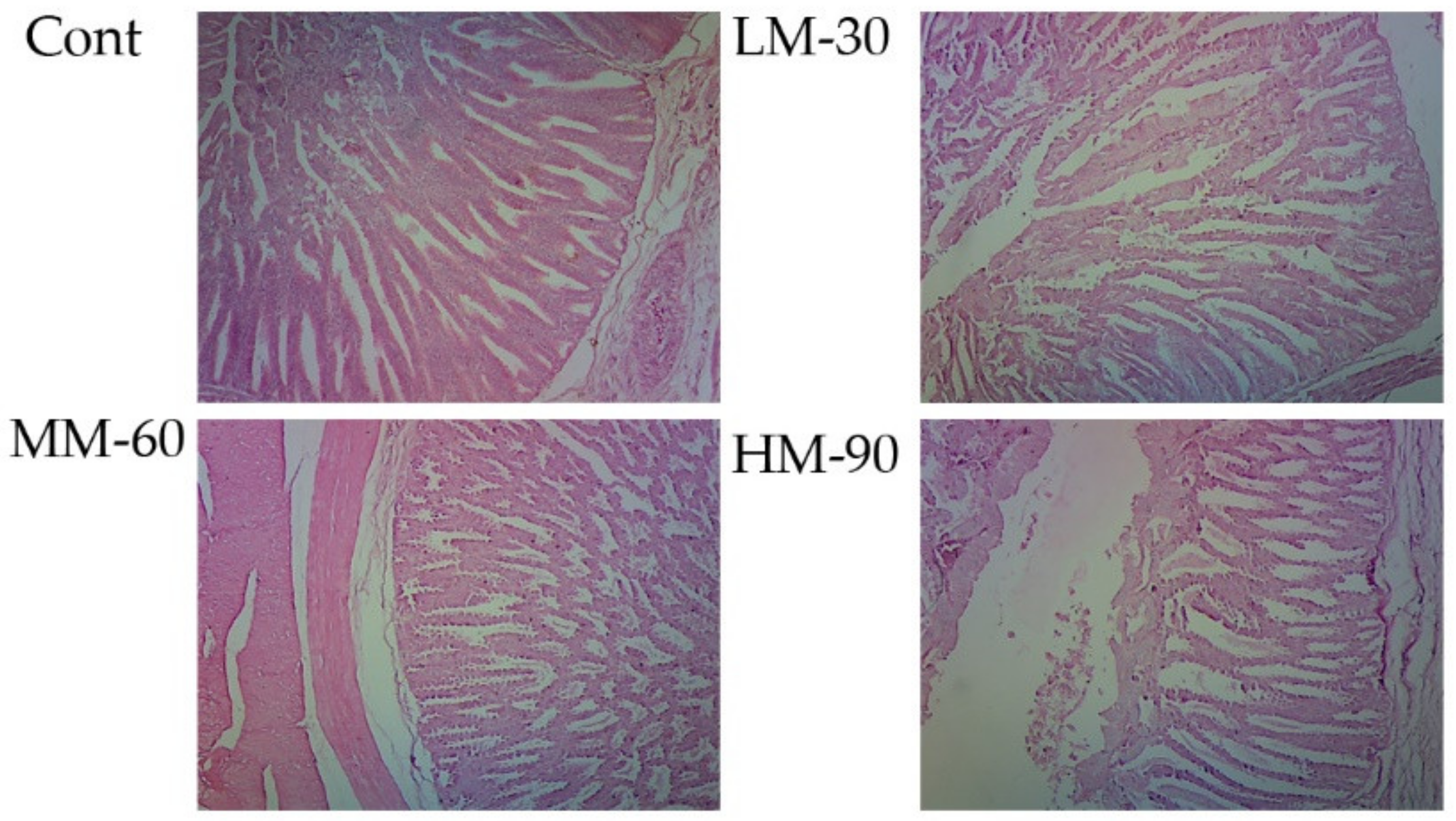
3.5. Intestinal Segments and Morphology
3.6. Meat Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhawan, S.; Kaur, J. Microbial mannanases: An overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Odetallah, N.H.; Ferket, P.R.; Grimes, J.L.; McNaughton, J.L. Effect of mannanendo- 1,4-beta-mannosidase on the growth performance of turkeys fed diets containing 44 and 48% crude protein soybean meal. Poult. Sci. 2002, 81, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Warnick, R.E. Value of enzyme supplements in rations containing certain legume seed meals or gums. Poult. Sci. 1964, 43, 1091–1097. [Google Scholar] [CrossRef]
- Lee, J.T.; Bailey, C.A.; Cartwright, A.L. β-Mannanase ameliorates viscosity associated depression of growth in broiler chickens fed guar germ and hull fractions. Poult. Sci. 2003, 82, 1925–1931. [Google Scholar] [CrossRef]
- Duncan, C.J.; Pugh, N.; Pasco, D.S.; Ross, S.A. Isolation of a galactomannan that enhances macrophage activation from the edible fungus Morchella esculenta. J. Agric. Food Chem. 2002, 50, 5683–5685. [Google Scholar] [CrossRef]
- Jackson, M.E.; Geronian, K.; Knox, A.; McNab, J.; McCartney, E. A dose-response study with the feed enzyme β-mannanase in broilers provided with corn-soybean meal based diets in the absence of antibiotic. Poult. Sci. 2004, 83, 1992–1996. [Google Scholar] [CrossRef]
- Wu, G.; Bryant, M.M.; Voitle, R.A.; Roland, D.A. Effet of beta-mannanase in corn-soy diets on commercial leghorns in second-cycle hens. Poult. Sci. 2005, 84, 894–897. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim. Feed Sci. Technol. 2015, 208, 119–131. [Google Scholar] [CrossRef]
- Jang, A.; Liu, X.D.; Shin, M.H.; Lee, B.D.; Lee, S.K.; Lee, J.H.; Jo, C. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult. Sci. 2008, 87, 2382–2389. [Google Scholar] [CrossRef]
- Hussein, E.O.S.; Suliman, G.M.; Alowaimer, A.N.; Ahmed, S.H.; Abd El-Hack, M.E.; Taha, A.E.; Swelum, A.A. Growth, carcass characteristics, and meat quality of broilers fed a low-energy diet supplemented with a multienzyme preparation. Poult. Sci. 2020, 99, 1988–1994. [Google Scholar] [CrossRef]
- Naqvi, L.U.; Nadeem, A. Bioavailability of metabolizable energy through Kemzyme supplementation in broiler rations. Pak. Vet. J. 2004, 24, 98–100. [Google Scholar]
- Gunal, M.; Yasar, S.; Forbes, J.M. Performance and some digesta parameters of broiler chickens given low or high viscosity wheat-based diets with or without enzyme supplementation. Turk. J. Vet. Anim. Sci. 2004, 28, 323–327. [Google Scholar]
- Mehri, M.; Adibmoradi, M.; Samie, A.; Shivazad, M. Effects of β-Mannanase on broiler performance, gut morphology and immune system. Afr. J. Biotechnol. 2010, 9, 6221–6228. [Google Scholar]
- Chegeni, A.; Torki, M.; Kamyab, A. Effects of β-mannanase-based enzyme in corn-soy and corn-soy-canola diets on broiler performance. J. Appl. Anim. Res. 2011, 39, 261–268. [Google Scholar] [CrossRef]
- Sundu, B.; Kumar, A.; Dingle, J. Response of broiler chicks fed increasing levels of copra meal and enzymes. Int. J. Poult. Sci. 2006, 5, 13–18. [Google Scholar]
- Saleh, F.; Tahir, M.; Ohtsuka, A.; Hayashi, K. A mixture of pure cellulase, hemicellulase and pectinase improves broiler performance. Brit. Poult. Sci. 2005, 46, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wu, S.; Liu, C.; Gauthier, R.; Chiou, P.W. Effects of enzyme inclusion in a maize-soybean diet on broiler performance. Anim. Feed Sci. Technol. 2007, 134, 283–294. [Google Scholar] [CrossRef]
- Azarfar, A. Effect of hemicell enzyme on the performance, growth parameter, some blood factors and ileal digestibility of broiler chickens fed corn/soybean-based diets. J. Cell Anim. Biol. 2013, 7, 85–91. [Google Scholar] [CrossRef]
- Bedford, M.R. Exogenous enzymes in monogastric nutrition-their current value and future benefits. Anim. Feed Sci. Technol. 2000, 86, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.R.; Qiao, S.Y.; Lu, W.Q.; Li, D.F. Effects of enzyme supplementation on performance, nutrient digestibility, gastrointestinal morphology, and volatile fatty acid profiles in the hindgut of broilers fed wheat-based diets. Poult. Sci. 2005, 84, 875–881. [Google Scholar] [CrossRef]
- Brenes, A.; Smith, M.; Guenter, W.; Marquardt, R.R. Effect of enzyme supplementation on the performance and digestive tract size of broiler chickens fed wheat- and barley-based diets. Poult. Sci. 1993, 72, 731–1739. [Google Scholar] [CrossRef]
- Salim, H.; Kang, H.; Akter, N.; Kim, D.; Kim, J.; Kim, M. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult. Sci. 2013, 92, 2084–2090. [Google Scholar] [CrossRef]
- Mohammadigheisar, M.; Kim, H.S.; Kim, I.H. Effect of inclusion of lysolecithin or multi-enzyme in low energy diet of broiler chickens. J. Appl. Anim. Res. 2018, 46, 1198–1201. [Google Scholar] [CrossRef] [Green Version]
- Bin Baraik, B.S.S. Effect of Adding Xylanase and Phytase Enzymes to Broiler Diets on Performance and Carcass Yield and Quality. Ph.D. Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2010. [Google Scholar]
- Smith, D.; Lyon, C.; Lyon, B. The effect of age, dietary carbohydrate source, and feed withdrawal on broiler breast fillet color. Poult. Sci. 2002, 81, 1584–1588. [Google Scholar] [CrossRef]


| Treatments | Chicks: Replicates | ME kcal/kg | β-Mannanase | |
|---|---|---|---|---|
| Starter Phase | Finisher Phase | |||
| T1: Cont | n = 100; R = 5 | 3100 | 3200 | - |
| T2: LM-30 | n = 100; R = 5 | 3070 | 3170 | 200 mg/kg |
| T3: MM-60 | n = 100; R = 5 | 3040 | 3140 | 400 mg/kg |
| T4: HM-90 | n = 100; R = 5 | 3010 | 3110 | 600 mg/kg |
| Ingredients (%) | Treatments | |||
|---|---|---|---|---|
| Cont | LM-30 | MM-60 | HM-90 | |
| Corn | 55.67 | 56.32 | 56.98 | 57.59 |
| Soya bean meal | 27.79 | 27.67 | 27.56 | 27.44 |
| Canola meal | 8.00 | 8.01 | 7.99 | 8.00 |
| Soybean oil | 5.04 | 4.48 | 3.92 | 3.36 |
| Monocalcium phosphate | 0.74 | 0.74 | 0.74 | 0.74 |
| Limestone | 1.24 | 1.24 | 1.24 | 1.24 |
| L-Lysine HCl | 0.38 | 0.38 | 0.39 | 0.39 |
| DL-Methionine | 0.27 | 0.27 | 0.27 | 0.26 |
| Threonine | 0.08 | 0.08 | 0.08 | 0.08 |
| β-mannanase | - | 0.02 | 0.04 | 0.06 |
| Sodium chloride | 0.25 | 0.25 | 0.25 | 0.25 |
| NaHCO3 | 0.23 | 0.23 | 0.23 | 0.28 |
| Phytase | 0.01 | 0.01 | 0.01 | 0.01 |
| VitaMin Premix * | 0.30 | 0.30 | 0.30 | 0.30 |
| Total | 100 | 100 | 100 | 100 |
| ME (Kcal/kg) | 3100 | 3070 | 3040 | 3010 |
| Crude protein | 21.50 | 21.50 | 21.50 | 21.50 |
| Lysine | 1.29 | 1.29 | 1.29 | 1.29 |
| Methionine | 0.51 | 0.51 | 0.51 | 0.51 |
| Calcium | 0.87 | 0.87 | 0.87 | 0.87 |
| Available P | 0.44 | 0.44 | 0.44 | 0.44 |
| Ingredients (%) | Treatments | |||
|---|---|---|---|---|
| Cont | LM-30 | MM-60 | HM-90 | |
| Corn | 62.27 | 62.95 | 63.64 | 64.32 |
| Canola meal | 6.09 | 5.99 | 5.88 | 5.78 |
| Soya bean meal | 23.56 | 23.53 | 23.50 | 23.47 |
| Soyabean oil | 5.18 | 4.61 | 4.03 | 3.46 |
| Monocalcium phosphate | 0.51 | 0.51 | 0.51 | 0.51 |
| Limestone | 1.03 | 1.03 | 1.04 | 1.04 |
| L-Lysine HCl | 0.34 | 0.34 | 0.34 | 0.35 |
| DL-Methionine | 0.23 | 0.23 | 0.23 | 0.22 |
| Threonine | 0.06 | 0.06 | 0.06 | 0.06 |
| β-mannanase | - | 0.02 | 0.04 | 0.06 |
| Sodium chloride | 0.22 | 0.22 | 0.22 | 0.22 |
| NaHCO3 | 0.20 | 0.20 | 0.20 | 0.20 |
| VitaMin Premix * | 0.30 | 0.30 | 0.30 | 0.30 |
| Phytase | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100 | 100 | 100 | 100 |
| ME (Kcal/kg) | 3200 | 3170 | 3140 | 3110 |
| Crude protein | 19.50 | 19.50 | 19.50 | 19.50 |
| Lysine | 1.16 | 1.16 | 1.16 | 1.16 |
| Methionine | 0.47 | 0.47 | 0.47 | 0.47 |
| Calcium | 0.79 | 0.79 | 0.79 | 0.79 |
| Available P | 0.40 | 0.40 | 0.40 | 0.40 |
| Items | Treatments | SEM | |||
|---|---|---|---|---|---|
| Cont | LM-30 | MM-60 | HM-90 | ||
| Grower (2–3 weeks) | |||||
| Feed intake (g) | 986.99 | 976.71 | 1012.42 | 984.95 | 7.69 |
| Weight gain (g) | 717.75 | 729.10 | 740.00 | 726.52 | 4.58 |
| FCR | 1.38 | 1.34 | 1.37 | 1.36 | 0.01 |
| Finisher (4–5 weeks) | |||||
| Feed intake (g) | 2179.01 | 2024.68 | 2074.38 | 2025.93 | 36.22 |
| Weight gain (g) | 1272.41 | 1142.64 | 1247.75 | 1192.17 | 29.04 |
| FCR | 1.72 | 1.78 | 1.67 | 1.70 | 0.02 |
| Overall (2–5 weeks) | |||||
| Feed intake (g) | 3167.02 | 3001.39 | 3086.80 | 3010.88 | 38.50 |
| Weight gain (g) | 1990.16 | 1871.73 | 1987.74 | 1918.68 | 28.71 |
| FCR | 1.59 | 1.60 | 1.55 | 1.57 | 0.01 |
| Parameters | Treatments | SEM | |||
|---|---|---|---|---|---|
| Cont | LM-30 | MM-60 | HM-90 | ||
| Weight (g) | |||||
| Duodenum | 22.58 | 19.5 ** | 22.75 | 21.26 | 0.75 |
| Jejunum | 32.12 | 32.00 | 32.63 | 34.50 | 0.58 |
| Ileum | 24.42 | 19.20 *** | 21.25 ** | 22.62 | 1.10 |
| Ceca | 11.81 | 11.62 | 11.50 | 10.88 | 0.20 |
| Length (cm) | |||||
| Duodenum | 14.67 | 14.32 | 15.02 | 14.60 | 0.14 |
| Jejunum | 63.10 | 75.46 *** | 77.84 *** | 77.00 *** | 3.46 |
| Ileum | 64.24 | 61.68 | 58.8 ** | 58.32 *** | 1.38 |
| Ceca | 17.86 | 18.18 | 16.60 | 16.80 | 0.39 |
| Parameters | Treatments | SEM | |||
|---|---|---|---|---|---|
| Cont | LM-30 | MM-60 | HM-90 | ||
| Breast meat | |||||
| Shear force (g) | 1026.01 | 973.05 | 874.88 | 1012.13 | 34.08 |
| Cook loss (%) | 26.40 | 27.17 | 25.56 | 26.86 | 0.35 |
| WHC (%) | 44.80 | 46.27 | 45.96 | 44.81 | 0.39 |
| pH at 24 h | 5.89 * | 6.16 | 6.15 | 6.17 | 0.07 |
| L | 51.60 | 50.36 | 49.43 | 45.89 * | 1.22 |
| a | 10.10 | 7.95 | 7.77 | 7.88 | 0.54 |
| b | 13.44 | 14.00 | 11.48 | 11.36 | 0.69 |
| Thigh meat | |||||
| Cook loss (%) | 32.02 | 33.28 | 33.51 | 32.26 | 0.39 |
| WHC (%) | 42.01 | 44.08 | 44.15 | 41.06 | 0.79 |
| pH at 24 h | 6.05 | 6.56 *** | 6.42 ** | 6.48 *** | 0.11 |
| L | 53.43 | 52.71 | 56.87 | 52.78 | 0.99 |
| a | 6.69 | 7.86 | 7.95 | 9.44 | 0.55 |
| b | 7.70 | 12.25 | 7.72 | 8.01 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqoob, M.U.; Yousaf, M.; Khan, M.I.; Wang, M. Effect of β-Mannanase Supplementation on Growth Performance, Ileal Digestibility, Carcass Traits, Intestinal Morphology, and Meat Quality in Broilers Fed Low-ME Diets. Animals 2022, 12, 1126. https://doi.org/10.3390/ani12091126
Yaqoob MU, Yousaf M, Khan MI, Wang M. Effect of β-Mannanase Supplementation on Growth Performance, Ileal Digestibility, Carcass Traits, Intestinal Morphology, and Meat Quality in Broilers Fed Low-ME Diets. Animals. 2022; 12(9):1126. https://doi.org/10.3390/ani12091126
Chicago/Turabian StyleYaqoob, Muhammad Umar, Muhammad Yousaf, Muhammad Issa Khan, and Minqi Wang. 2022. "Effect of β-Mannanase Supplementation on Growth Performance, Ileal Digestibility, Carcass Traits, Intestinal Morphology, and Meat Quality in Broilers Fed Low-ME Diets" Animals 12, no. 9: 1126. https://doi.org/10.3390/ani12091126







