Potential Use of Tannin Extracts as Additives in Semen Destined for Cryopreservation: A Review
Abstract
Simple Summary
Abstract
1. Introduction
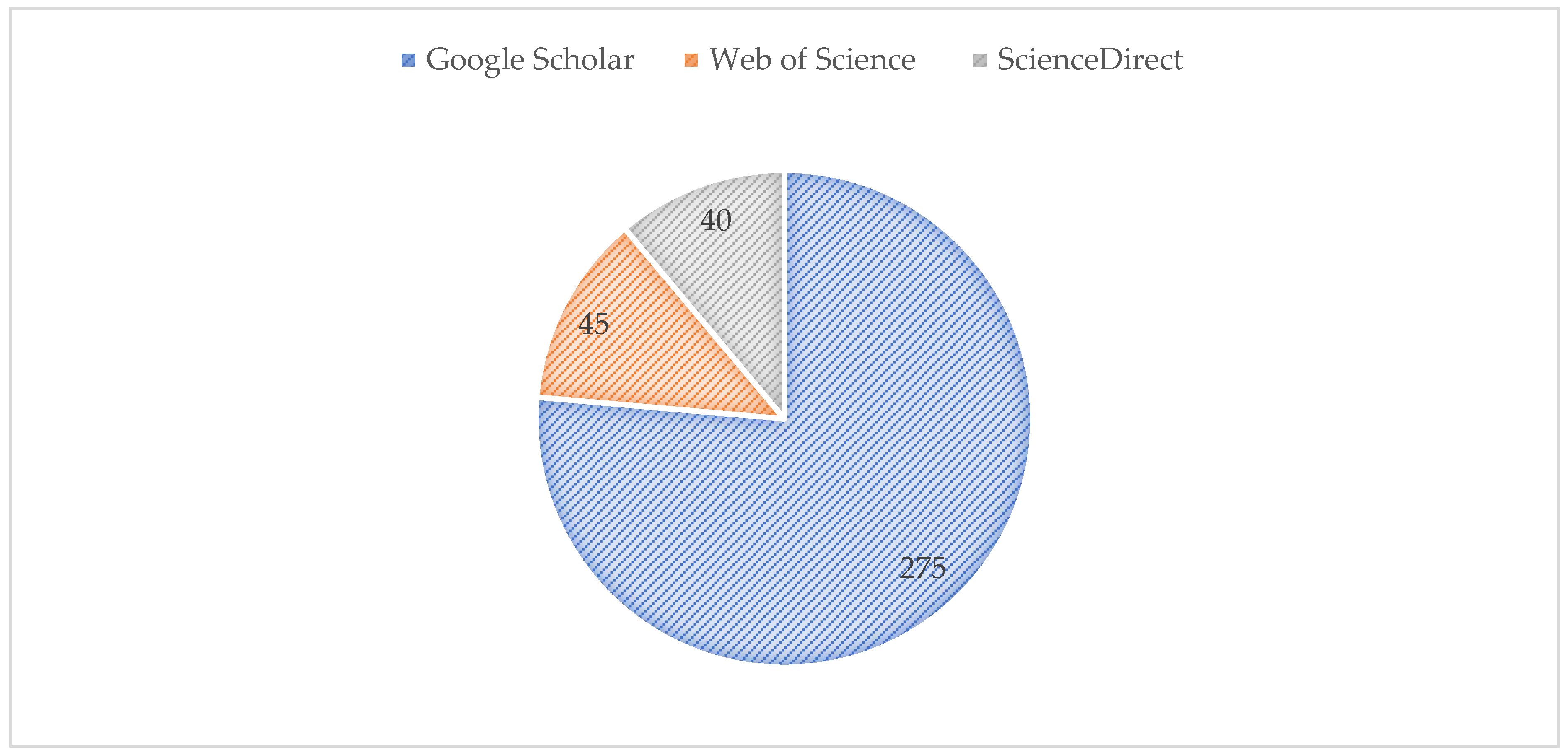
2. Methodology
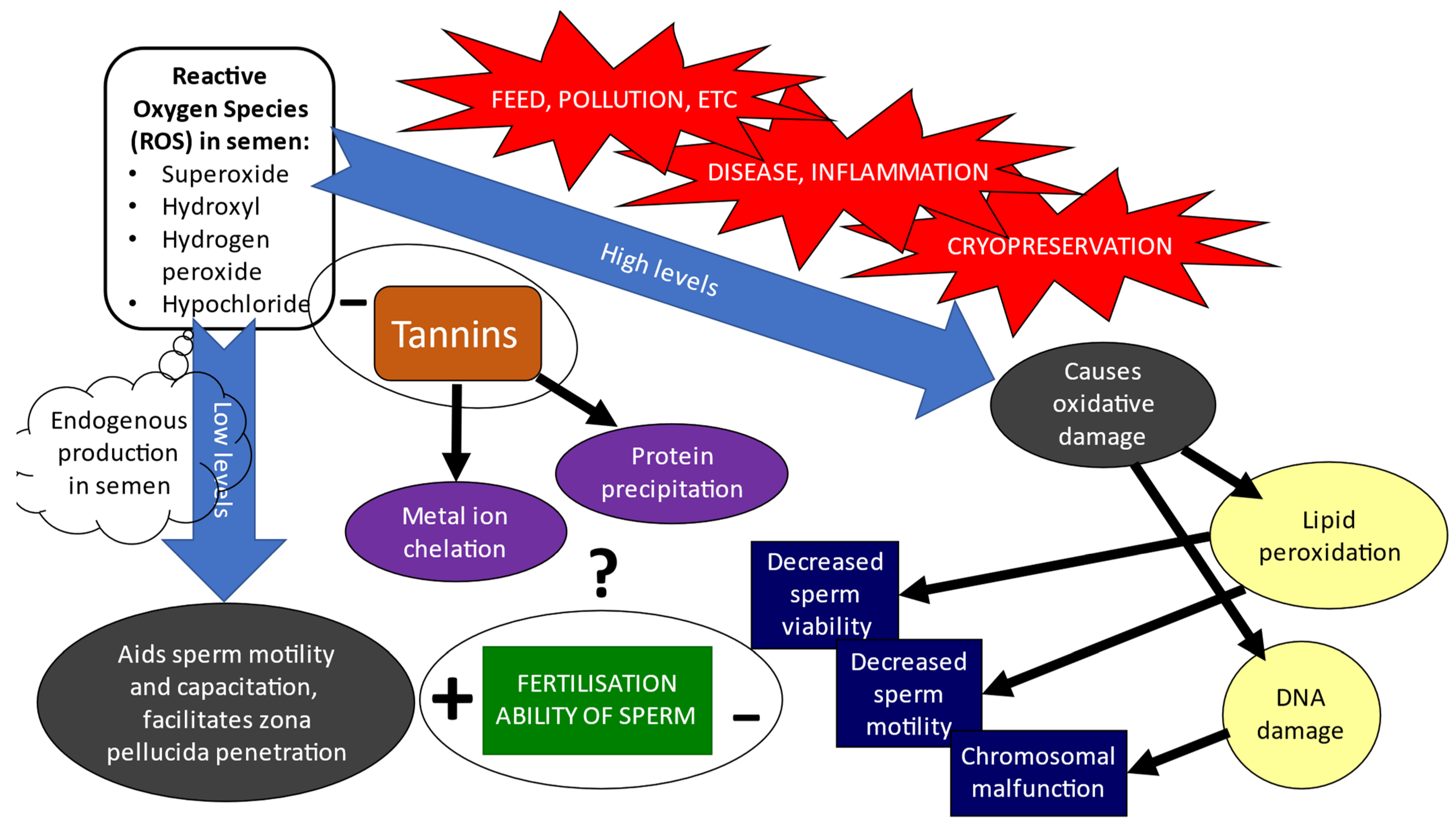
3. Effect of ROS on Cryopreserved Spermatozoa
3.1. ROS Effect on Sperm Cryopreservation and Longevity
3.2. Effects of ROS on Sperm’s Fertilising Potential
4. Tannins
4.1. Properties of Tannins
4.2. Extraction of Tannins
4.3. Medicinal Properties and Biological Functions of Tannins
4.4. Use of Tannins as Supplements to Improve Reproduction Outomces or as Semen-Protective Agents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, S.; Li, C.; Chen, L.; Zhou, X. Actions and mechanisms of reactive oxygen species and antioxidative system in semen. Mol. Cell. Toxicol. 2017, 13, 143–154. [Google Scholar] [CrossRef]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 2011, 142, 759. [Google Scholar] [CrossRef] [PubMed]
- Van Wagtendonk-De Leeuw, A.; Den Daas, J.; Kruip, T.A.; Rall, W.F. Comparison of the efficacy of conventional slow freezing and rapid cryopreservation methods for bovine embryos. Cryobiology 1995, 32, 157–167. [Google Scholar] [CrossRef][Green Version]
- El-Harairy, M.; Eid, L.N.; Zeidan, A.; Abd El-Salaam, A.; El-Kishk, M. Quality and fertility of the frozen-thawed bull semen as affected by the different cryoprotectants and glutathione levels. J. Am. Sci. 2011, 7, 791–801. [Google Scholar]
- Rodriguez-Martinez, H.; Barth, A.D. In vitro evaluation of sperm quality related to in vivo function and fertility. Soc. Reprod. Fertil. Suppl. 2007, 64, 39–54. [Google Scholar] [CrossRef]
- Taşdemir, U.; Büyükleblebici, S.; Tuncer, P.B.; Coşkun, E.; Özgürtaş, T.; Aydın, F.N.; Büyükleblebici, O.; Gürcan, I.S. Effects of various cryoprotectants on bull sperm quality, DNA integrity and oxidative stress parameters. Cryobiology 2013, 66, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Watson, P. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef]
- Sahashi, Y.; Otsuki, T.; Higaki, S.; Nagano, M.; Yamashita, Y.; Hishinuma, M. Effect of butylated hydroxytoluene on dog sperm longevity in chilling storage and cryopreservation. J. Vet. Med. Sci. 2011, 73, 1103090461. [Google Scholar] [CrossRef][Green Version]
- Naijian, H.R.; Kohram, H.; Shahneh, A.Z.; Sharafi, M.; Bucak, M.N. Effects of different concentrations of BHT on microscopic and oxidative parameters of Mahabadi goat semen following the freeze–thaw process. Cryobiology 2013, 66, 151–155. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amiri, I.; Khodadadi, I.; Fattahi, A.; Atabakhsh, M.; Tavilani, H. Influence of BHT inclusion on post-thaw attributes of human semen. Syst. Biol. Reprod. Med. 2015, 61, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, P.B.; Buyukleblebici, S.; Eken, A.; Tasdemir, U.; Durmaz, E.; Büyükleblebici, O.; Coskun, E. Comparison of Cryoprotective Effects of Lycopene and Cysteamine in Different Cryoprotectants on Bull Semen and Fertility Results. Reprod. Domest. Anim. 2014, 49, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Tomizuka, T.; Hanada, A.; Hosoda, T.; Morimoto, H. Cryoprotective action of some amide solutions on spermatozoa of domestic animals. I. Effects of formamide, acetamide and lactamide on the motility of bovine spermatozoa after pellet freezing. Jpn. J. Anim. Reprod. 1972, 18, 15–21. [Google Scholar] [CrossRef][Green Version]
- Ros-Santaella, J.L.; Pintus, E. Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants 2021, 10, 772. [Google Scholar] [CrossRef]
- Clément, C.; Witschi, U.; Kreuzer, M. The potential influence of plant-based feed supplements on sperm quantity and quality in livestock: A review. Anim. Reprod. Sci. 2012, 132, 1–10. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar]
- Sutovsky, P. New approaches to boar semen evaluation, processing and improvement. Reprod. Domest. Anim. 2015, 50, 11–19. [Google Scholar] [CrossRef]
- Senger, P.L. Endocrinology of the male and spermatogenesis. In Pathways to Pregnancy and Parturation, 3rd ed.; Current Conceptions: Pullman, WA, USA, 2012; pp. 202–227. [Google Scholar]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm cohort-specific zinc signature acquisition and capacitation-induced zinc flux regulate sperm-oviduct and sperm-zona pellucida interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef]
- Ecroyd, H.W.; Jones, R.C.; Aitken, R.J. Endogenous redox activity in mouse spermatozoa and its role in regulating the tyrosine phosphorylation events associated with sperm capacitation. Biol. Reprod. 2003, 69, 347–354. [Google Scholar] [CrossRef][Green Version]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31. [Google Scholar] [CrossRef]
- Khojasteh, S.M.B.; Khameneh, R.J.; Houresfsnd, M.; Yaldagard, E. A review on medicinal plants used for improvement of spermatogenesis. Biol. Med. 2016, 8, 1. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Kantola, M.; Saaranen, M.; Vanha-Perttula, T. Selenium and glutathione peroxidase in seminal plasma of men and bulls. Reproduction 1988, 83, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Aurich, J.; Schönherr, U.; Hoppe, H.; Aurich, C. Effects of antioxidants on motility and membrane integrity of chilled-stored stallion semen. Theriogenology 1997, 48, 185–192. [Google Scholar] [CrossRef]
- De Lamirande, E.; Leclerc, P.; Gagnon, C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef]
- Çoyan, K.; Başpınar, N.; Bucak, M.N.; Akalın, P.P.; Ataman, M.B.; Ömür, A.D.; Güngör, Ş.; Küçükgünay, S.; Özkalp, B.; Sarıözkan, S. Influence of methionine and dithioerythritol on sperm motility, lipid peroxidation and antioxidant capacities during liquid storage of ram semen. Res. Vet. Sci. 2010, 89, 426–431. [Google Scholar] [CrossRef]
- Paulenz, H.; Söderquist, L.; Ådnøy, T.; Fossen, O.H.; Berg, K.A. Effect of milk-and TRIS-based extenders on the fertility of sheep inseminated vaginally once or twice with liquid semen. Theriogenology 2003, 60, 759–766. [Google Scholar] [CrossRef]
- Hollinshead, F.; Evans, G.; Evans, K.; Catt, S.L.; Maxwell, W.; O’Brien, J. Birth of lambs of a pre-determined sex after in vitro production of embryos using frozen–thawed sex-sorted and re-frozen–thawed ram spermatozoa. Reproduction 2004, 127, 557–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salvador, I.; Yániz, J.; Viudes-de-Castro, M.; Gómez, E.; Silvestre, M. Effect of solid storage on caprine semen conservation at 5 C. Theriogenology 2006, 66, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, V.G.; Maldera, J.A.; Willis, W.D.; Cohen, D.J.; Goulding, E.H.; Gelman, D.M.; Rubinstein, M.; Eddy, E.M.; Cuasnicu, P.S. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev. Biol. 2008, 320, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Pini, T.; Leahy, T.; de Graaf, S.P. Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172–181. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Roca, J.; Broekhuijse, M.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.; Bolarin, A. Boar differences in artificial insemination outcomes: Can they be minimized? Reprod. Domest. Anim. 2015, 50, 48–55. [Google Scholar] [CrossRef]
- Yeste, M. Recent advances in boar sperm cryopreservation: State of the art and current perspectives. Reprod. Domest. Anim. 2015, 50, 71–79. [Google Scholar] [CrossRef]
- Abdalla, H.; Ali, M.A.E.; El-Tarabany, M.S. Fertility of commercial sexed semen and the economic analyses of its application in Holstein heifers. Adv. Anim. Vet. Sci. 2014, 2, 535–542. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2021, 106904. [Google Scholar] [CrossRef]
- Brym, P.; Wasilewska-Sakowska, K.; Mogielnicka-Brzozowska, M.; Mańkowska, A.; Paukszto, Ł.; Pareek, C.S.; Kordan, W.; Kondracki, S.; Fraser, L. Gene promoter polymorphisms in boar spermatozoa differing in freezability. Theriogenology 2021, 166, 112–123. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Tannins: Major Sources, Properties and Applications. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. [Google Scholar]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants—Hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Martinez, J.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral drug discovery: Broad-spectrum drugs from nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Yang, Y.; Lian, G.; Yu, B. Naturally occurring polyphenolic glucosidase inhibitors. Isr. J. Chem. 2015, 55, 268–284. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. ISSN 2012, 2277, 2502. [Google Scholar]
- Aisen, E.; Medina, V.; Venturino, A. Cryopreservation and post-thawed fertility of ram semen frozen in different trehalose concentrations. Theriogenology 2002, 57, 1801–1808. [Google Scholar] [CrossRef]
- Akalin, P.P.; Bucak, M.N.; Güngör, Ş.; Başpinar, N.; Coyan, K.; Dursun, Ş.; Ili, P.; Aksoy, A.; Karaşör, F.; Bilgili, A.; et al. Influence of lycopene and cysteamine on sperm and oxidative stress parameters during liquid storage of ram semen at 5 C. Small Rumin. Res. 2016, 137, 117–123. [Google Scholar] [CrossRef]
- Aitken, J.; Fisher, H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk. Bioessays 1994, 16, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Bennetts, L.E.; Sawyer, D.; Wiklendt, A.M.; King, B.V. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int. J. Androl. 2005, 28, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef]
- Zhang, X.-K.; He, F.; Zhang, B.; Reeves, M.J.; Liu, Y.; Zhao, X.; Duan, C.-Q. The effect of prefermentative addition of gallic acid and ellagic acid on the red wine color, copigmentation and phenolic profiles during wine aging. Food Res. Int. 2018, 106, 568–579. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A review of polyphenolics in oak woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.K.; Ghani, A.; Ghani, E. In vitro effects of Capparis spinosa L. extract on human sperm function, DNA fragmentation, and oxidative stress. J. Ethnopharmacol. 2021, 269, 113702. [Google Scholar]
- Spinaci, M.; Muccilli, V.; Bucci, D.; Cardullo, N.; Gadani, B.; Tringali, C.; Tamanini, C.; Galeati, G. Biological effects of polyphenol-rich extract and fractions from an oenological oak-derived tannin on in vitro swine sperm capacitation and fertilizing ability. Theriogenology 2018, 108, 284–290. [Google Scholar] [CrossRef]
- Seif, M.; Abd El-Aziz, T.; Sayed, M.; Wang, Z. Zingiber officinale ethanolic extract attenuates oxidative stress, steroidogenic gene expression alterations, and testicular histopathology induced by sodium arsenite in male rats. Environ. Sci. Pollut. Res. 2021, 28, 19783–19798. [Google Scholar] [CrossRef]
- Ogunro, O.B.; Yakubu, M.T. Antifertility effects of 60-day oral gavage of ethanol extract of Spondias mombin leaves in guinea pigs: A biochemical, reproductive and histological study. Asian Pac. J. Reprod. 2021, 10, 56. [Google Scholar]
- Kahalerras, L.; Otmani, I.; Abdennour, C. Wild Garlic Allium triquetrum L. Alleviates Lead Acetate-Induced Testicular Injuries in Rats. Biol. Trace Elem. Res. 2022, 200, 2205–2222. [Google Scholar] [CrossRef] [PubMed]
- Irais, C.-M.; Claudia, B.-R.; David, P.-E.; Ashutosh, S.; Rubén, G.-G.; Agustina, R.-M.; del Carmen, V.-M.M.; Mario-Alberto, R.-G.; Luis-Benjamín, S.-G. Leaf and Fruit Methanolic Extracts of Azadirachta indica Exhibit Antifertility Activity on Rats’ Sperm Quality and Testicular Histology. Curr. Pharm. Biotechnol. 2021, 22, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Lehloenya, K.; Mphaphathi, M.; Hassen, A. Effect of Acacia mearnsii Tannin Extract Supplementation on Reproductive Performance and Oxidative Status of South African Mutton Merino Rams. Animals 2021, 11, 3266. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M. Therapeutic Potential of Date Palm against Human Infertility: A Review. Metabolites 2021, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Sabry, O.M.; Adel, R.; Dmirieh, M.; El-Shazly, A.M.; Wink, M. HPLC-PDA-MS/MS characterization of bioactive secondary metabolites from Turraea fischeri bark extract and its antioxidant and hepatoprotective activities in vivo. Molecules 2017, 22, 2089. [Google Scholar] [CrossRef]
- Iamsaard, S.; Arun, S.; Burawat, J.; Yannasithinon, S.; Tongpan, S.; Bunsueb, S.; Lapyuneyong, N.; Choowong-In, P.; Tangsrisakda, N.; Chaimontri, C.; et al. Evaluation of antioxidant capacity and reproductive toxicity of aqueous extract of Thai Mucuna pruriens seeds. J. Integr. Med. 2020, 18, 265–273. [Google Scholar] [CrossRef]
- Li, W.; Yao, R.; Xie, L.; Liu, J.; Weng, X.; Yue, X.; Li, F. Dietary supplementation of grape seed tannin extract stimulated testis development, changed fatty acid profiles and increased testis antioxidant capacity in pre-puberty hu lambs. Theriogenology 2021, 172, 160–168. [Google Scholar] [CrossRef]
- Shaik, R.; Mohamad, S.; Rao, N.V. Evaluation of Anti Fertility Activities of Bark Extracts of Caesalpinia pulcherrima Linn (Caesalpiniaceae) in Rats. Indian J. Pharm. Sci. 2021, 83, 393–397. [Google Scholar]
- Wurlina, W.; Mas’ud Hariadi, E.S.; Susilowati, S.; Meles, D.K. The effect of crude guava leaf tannins on motility, viability, and intact plasma membrane of stored spermatozoa of Etawa crossbred goats. Vet. World 2020, 13, 530. [Google Scholar] [CrossRef]
- Ros-Santaella, J.L.; Pintus, E. Rooibos (Aspalathus linearis) extract enhances boar sperm velocity up to 96 h of semen storage. PLoS ONE 2017, 12, e0183682. [Google Scholar] [CrossRef]
- Galeati, G.; Bucci, D.; Nerozzi, C.; Gadani, B.; Tamanini, C.; Mislei, B.; Spinaci, M. Improvement of in vitro fertilization by a tannin rich vegetal extract addition to frozen thawed boar sperm. Anim. Reprod. 2020, 17. [Google Scholar] [CrossRef]
- Sobeh, M.; Hassan, S.A.; Hassan, M.A.; Khalil, W.A.; Abdelfattah, M.A.; Wink, M.; Yasri, A. A polyphenol-rich extract from Entada abyssinica reduces oxidative damage in cryopreserved ram semen. Front. Vet. Sci. 2020, 956, 604477. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Banana, H.J. Effect of adding n-acetylcystiene and avena sativa extract to tris extender on post-cryopreservative semen characteristics of holstein bulls. Plant Arch. 2020, 20, 1209–1216. [Google Scholar]
- Fitriyah, A.; Said, D.O.; Harianto, H. Improvement of Sperm Quality of Bali Cattle by Supplementation of Crude Tannin in the Semen. In Proceeding of the 1st International Conference on Tropical Agriculture; Springer: Cham, Switzerland, 2017. [Google Scholar]
| Plant from Which Tannins Were Extracted (References) | Compounds Identified | Extraction Method | Subject (Animal Species and Gender) | Effect on Reproduction |
|---|---|---|---|---|
| Zingiber officinale (ginger) root extract [61] | High content of total flavonoids, tannins, alkaloids, and total phenolic components | Ethanol | Rattus rattus (Rat)—male | Restored testis histopathological alterations, reduced arsenic, and improved sperm parameters. |
| Spondias mombin leaf extract [62] | Leaves contain saponins, alkaloids, flavonoids, tannins, steroids, phenolics, phlobatannins, cardiac glycosides, cardenolides, and dienolides with saponins | Ethanol | Cavia porcellus (Guinea pig)—male | Induced infertility in males via endocrine dysregulation, anti-spermatogenic activity, testicular dysfunction, and antioxidative stress. |
| Allium triquetrum (wild garlic) bulb and leaf extract [63] | Tannins (leaves have higher concentration) | Water | Rattus norvegicus (Wistar rat)—male | Used in the treatment of reproductive toxicity of lead acetate by reducing lead testicular injury by boosting sperm characteristics and ameliorating oxidative sperm markers. |
| Azadirachta indica leaf and fruit extracts [64] | Not reported | Methanol | Rattus norvegicus (Long Evans rat)—male | At 200 µg/mL, increased percentage of morphological defects. (Cellular detachment in the seminiferous epithelium with sperm death without decrease in number of sperm). |
| Acacia mearnsii (Black Wattle) bark extract [65] | Condensed tannins average MW 1250 (500 to 3000), non-tannin polyphenols, salts, sugars, and organic acids. Total tannins (65.5%), tannic acid, and condensed tannin (30.5%) as leucocyanidin | Water | Ovis aries (Sheep: mutton merino)—male | Increase in testicular length, semen volume, semen concentration, and reduction in sperm with morphological defects. |
| Phoenix dactylifera (Date palm) fruit extract [66] | Review study | Homo sapiens (Man)—male and female | It has a potent effect on male hormones, seminal vesicle parameters, and sperm motility and viability. | |
| Turraea fischeri bark extract [67] | 20 compounds including several isomers of flavonolignan cinchonain-I and dominant bis-dihydrophenoxyl propanoid-substituted catechins hexsoides | Methanol | Rattus norvegicus (Wistar rat)—male | Enhanced reduction in the elevated levels of aspartate aminotransferase (AST), malondialdehyde (MDA), and increased glutathione (GSH) content in the liver. |
| Mucuna pruriens (Thai (T-MP)) seed extract [68] | Not reported | Water | Rattus rattus (Rat)—male and female | Exhibit antioxidation capacity, phytoestrogenic effect on females, and increased testicular and sperm markers of male fertility. |
| Vitis vinifera (Grape) seed tannin extract (GPE) [69] | GPE has a 95% purity coefficient (56.5% condensed tannins) | Not reported | Ovis aries (Hu lambs) | Improved the seminiferous tubules’ development, diameter, and increase in Sertoli cells. Also increase in superoxide dismutase (SOD). |
| Caesalpinia pulcherrima bark extracts [70] | Alkaloids, flavonoids, steroids, and triterpenes | Water and ethanol | Rattus rattus (Rat)—female | Reduced ovarian size and increased uterine weight. |
| Plants from Which Tannins Were Extracted (References) | Compounds Identified | Extraction Methods | Subject (Animal Species and Gender) | Effect on Sperm |
|---|---|---|---|---|
| Psidium guajava (Crude guava) leaf tannin extract [71] | 2.41% of tannin, 20.80% of phenols per 17.825 g of extract | Methanol, ethyl acetate, and acetone | Capra aegagrus hircus (Etawa crossbred goat) | At 3%, increase in sperm motility, viability, and maintained intact plasma membrane integrity. |
| Aspalathus linearis (Rooibos) extracts [72] | Major flavonoids, flavols, and low tannins | Water | Sus scrofa domesticus (Pig) | Enhanced the sperm velocity, protected acrosome integrity, and preserved membrane integrity during 96 h of storage. |
| Mixture of chestnut and Quebracho wood (60/40) tannin-rich vegetal extract [73] | 94.2% tannin content | Filter Freiberg-hide powder method | Sus scrofa domesticus (Pig) | Increased penetration rate with oocytes inseminated with thawed sperm pretreated with vegetal extract, and at 5 µg/mL, it exerts total efficiency on fertilisation. |
| Entada abyssinica (Splinter bean) bark extract [74] | 28 compounds including tannins and gallic acid derivatives | Methanol | Ovis aries (Sheep) | Increased post-thaw progressive sperm motility, plasma membrane integrity, % of intact sperm increased with decrease in apoptotic/necrotic sperm. |
| Quercus robur (Toasted oak wood) (Tan activ®) [56,60] | Monogalloyl glucose (332.2), Glucose esterified by hexahydroxydiphenic acid (482.2), Gallic acid (170.1), Ellagitannins, castalin (632.4), Vescalsgin (934.6), Grandinin or its isomer roburin E (1066.7) | Ethanol | Sus scrofa domesticus (Pig) | Stimulated the sperm capacitation and oocyte fertilisation rate in a swine in vitro fertilisation trial. |
| Capparis spinosa leaf extract [59] | Flavones and flavanols, total flavonoids, total phenolic content, tannins, and the total carbohydrates | Water and ethanol | Homo sapiens (Man) | Increased progressive, total in vitro motility, viability, and maintained sperm DNA integrity. |
| Avena sativa (Oats) seed extract [75] | Phenols—93.2 mg/g, Flavonoids—67 mg/g, Saponins—5.9%, Glycosides—17.6%, Terpenoids—4.6%, Rutin—179 ppm, Kaemperol—513 ppm, Quercetin 409 ppm, Gallic acid—348 ppm | Water | Bos taurus (Bovine: Holstein) | Improved sperm individual motility, viability, plasma membrane integrity, and acrosome integrity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liman, M.S.; Hassen, A.; McGaw, L.J.; Sutovsky, P.; Holm, D.E. Potential Use of Tannin Extracts as Additives in Semen Destined for Cryopreservation: A Review. Animals 2022, 12, 1130. https://doi.org/10.3390/ani12091130
Liman MS, Hassen A, McGaw LJ, Sutovsky P, Holm DE. Potential Use of Tannin Extracts as Additives in Semen Destined for Cryopreservation: A Review. Animals. 2022; 12(9):1130. https://doi.org/10.3390/ani12091130
Chicago/Turabian StyleLiman, Mohammed S., Abubeker Hassen, Lyndy J. McGaw, Peter Sutovsky, and Dietmar E. Holm. 2022. "Potential Use of Tannin Extracts as Additives in Semen Destined for Cryopreservation: A Review" Animals 12, no. 9: 1130. https://doi.org/10.3390/ani12091130
APA StyleLiman, M. S., Hassen, A., McGaw, L. J., Sutovsky, P., & Holm, D. E. (2022). Potential Use of Tannin Extracts as Additives in Semen Destined for Cryopreservation: A Review. Animals, 12(9), 1130. https://doi.org/10.3390/ani12091130









