A High-Energy Diet and Spirulina Supplementation during Pre-Gestation, Gestation, and Lactation do Not Affect the Reproductive and Lactational Performance of Primiparous Sows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
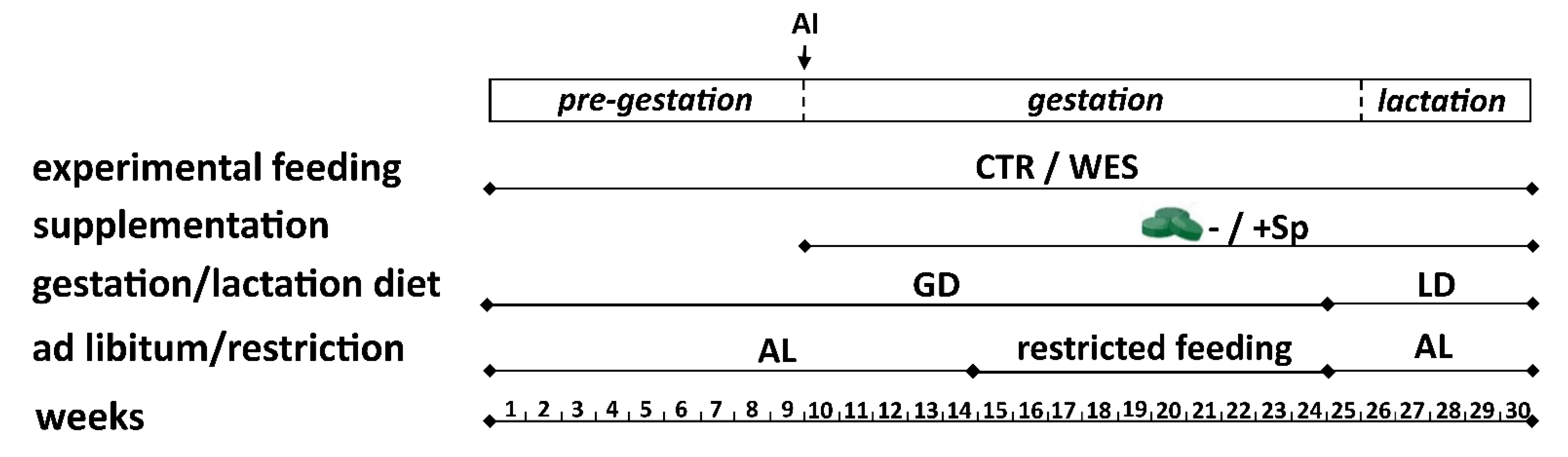
2.1. Animals, Housing, and Experimental Protocol
2.2. Experimental Diets
2.3. Reproductive and Lactational Performance
2.4. Composition of the Colostrum
2.5. Group sizes and Statistical Analyses
3. Results
3.1. Feed and Energy Intake during Gestation and Lactation
3.2. Reproductive and Lactational Performance
3.3. Composition of the Colostrum
4. Discussion
4.1. Effects of a High-Energy Diet
4.2. Effects of Spirulina Supplementation
4.3. Interaction of Maternal Spirulina Supplementation and Maternal diet
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rekiel, A.; Wiecek, J.; Batorska, M.; Kulisiewicz, J. Effect of sow prolificacy and nutrition on pre- and postnatal growth of progeny—A review. Ann. Anim. Sci. 2014, 14, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Noblet, J.; Dourmad, J.Y.; Etienne, M. Energy utilization in pregnant and lactating sows: Modeling of energy requirements. J. Anim. Sci. 1990, 68, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.Y.C.; Bilkei, G. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 2005, 88, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yang, X.; Baidoo, S.K. Relationship between body weight of primiparous sows during late gestation and subsequent reproductive efficiency over six parities. Asian-Australas. J. Anim. Sci. 2016, 29, 768–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkwood, R.N.; Baidoo, S.K.; Aherne, F.X. The influence of feeding level during lactation and gestation on the reproductive performance of second parity sows. Can. J. Anim. Sci. 1990, 70, 1119–1126. [Google Scholar] [CrossRef]
- Baidoo, S.K.; Aherne, F.X.; Kirkwood, R.N.; Foxcroft, G.R. Effect of feed intake during lactation and after weaning on sow reproductive performance. Can. J. Anim. Sci. 1992, 72, 911–917. [Google Scholar] [CrossRef]
- Baidoo, S.K.; Lythgoe, E.S.; Kirkwood, R.N.; Aherne, F.X.; Foxcroft, G.R. Effect of lactation feed intake on endocrine status and metabolite levels in sows. Can. J. Anim. Sci. 1992, 72, 799–807. [Google Scholar] [CrossRef]
- Costerman, N.G.J.; Soede, N.M.; Middlekoop, A.; Laurenssen, B.F.A.; Koopmanschap, R.E.; Zak, L.J.; Knol, E.F.; Keijer, J.; Teerds, K.J.; Kemp, B. Influence of the metabolic state during lactation on milk production in modern sows. Animal 2020, 14, 2543–2553. [Google Scholar] [CrossRef]
- Hrolfsdottir, L.; Schalkwijk, C.G.; Birgisdottir, B.E.; Gunnarsdottir, I.; Maslova, E.; Granström, C.; Strøm, M.; Olsen, S.F.; Halldorsson, T.I. Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity 2016, 24, 2133–2139. [Google Scholar] [CrossRef]
- Averette, L.A.; Odle, J.; Monaco, M.H.; Donovan, S.M. Dietary fat during pregnancy and lactation increases milk fat and insulin-like growth factor I concentrations and improves neonatal growth rates in swine. J. Nutr. 1999, 129, 2123–2129. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Yang, M.; Lee, S.; Behrendt, C.L.; Hooper, L.V.; Saghatelian, A.; Wan, Y. Maternal western diet causes inflammatory milk and TLR2/4-dependent neonatal toxicity. Genes Dev. 2012, 26, 1306–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ratel, I.T. Reproductive performance, oxidative status and blood metabolites of doe rabbits administrated with spirulina alga. Egypt Poult. Sci. 2017, 37, 1153–1172. [Google Scholar] [CrossRef] [Green Version]
- Gaafar, H.M.A.; Riad, W.A.; Elsadany, A.Y.; El-Reidy, K.F.A.; Abu El-Hamd, M.A. Effects of spirulina (Arthrospira platensis) on productive and reproductive performance of Friesian cows. Egypt. J. Agric. Res. 2017, 95, 893–910. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, L.; Miron, A.; Klimova, B.; Wan, D.; Kuca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lugarà, R.; Realini, L.; Kreuzer, M.; Giller, K. Effects of maternal high-energy diet and spirulina supplementation in pregnant and lactating sows on performance, quality of carcass and meat, and its fatty acid profile in male and female offspring. Meat Sci. 2022, 187, 108769. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Adewole, D.; Liu, S.; Xi, P.; Yang, C.; Yin, Y. Tryptophan supplementation increases reproduction performance, milk yield, and milk composition in lactating sows and growth performance of their piglets. J. Agric. Food Chem. 2019, 19, 5096–5104. [Google Scholar] [CrossRef]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten. Book of Methods; VDLUFA-Verlag: Darmstadt, Germany, 2010. [Google Scholar]
- Dumas, J.B.A. Procédés de l’analyse organique. Ann. Chim. Phys. 1831, T47, 198–213. (In French) [Google Scholar]
- Ineichen, S.; Kuenzler, A.D.; Kreuzer, M.; Marquardt, S.; Beat, R. Digestibility, nitrogen utilization and milk fatty acid profile of dairy cows fed hay from species rich mountainous grasslands with elevated herbal and phenolic contents. Anim. Feed Sci. Technol. 2019, 247, 210–221. [Google Scholar] [CrossRef]
- Suter, B.; Grob, K.; Pacciarelli, B. Determination of fat content and fatty acid composition though 1-min transesterification in the food sample: Principles. Z. Lebensm. Unters. Forsch. A 1997, 204, 252–258. [Google Scholar] [CrossRef]
- Collomb, M.; Bühler, T. Analyse de la composition en acides gras de la graisse de lait. Mitt. Lebensm. Hyg. 2000, 91, 306–332. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 1 September 2019).
- Lugarà, R.; Renner, S.; Wolf, E.; Liesegang, A.; Bruckmaier, R.; Giller, K. Crossbred Sows Fed a Western Diet during Pre-Gestation, Gestation, Lactation and Post-Lactation Develop Signs of Lean Metabolic Syndrome That Are Partially Attenuated by Spirulina Supplementation; ETH Zurich: Zurich, Switzerland, 2022; manuscript in preparation. [Google Scholar]
- Coffrey, M.T.; Diggs, B.G.; Handlin, D.L.; Knabe, D.A.; Maxwell, C.V.; Noland, P.R.; Prince, T.J.; Gromwell, G.L. Effects of dietary energy during gestation and lactation on reproductive performance of sows: A cooperative study. S-145 Committee on Nutritional Systems for Swine to increase reproductive efficiency. J. Anim. Sci. 1994, 72, 4–9. [Google Scholar] [CrossRef]
- Fang, L.H.; Jin, Y.H.; Jeong, J.H.; Hong, J.S.; Chung, W.L.; Kim, Y.Y. Effects of dietary energy and protein levels on reproductive performance in gestating sows and growth of their progeny. J. Anim. Sci. Technol. 2019, 61, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Vidal, E.; Lomelí, H.; Castro-Obregon, S.; Cuervo, R.; Escalante-Alcalde, D.; Covarrubias, L. Reactive oxygen species participate in the control of mouse embryonic cell death. Exp. Cell Res. 1998, 238, 136–147. [Google Scholar] [CrossRef]
- Hempstock, J.; Jauniaux, E.; Greenwold, N.; Burton, G.J. The contribution of placental oxidative stress to early pregnancy failure. Hum. Pathol. 2003, 34, 1265–1275. [Google Scholar] [CrossRef]
- McNamara, L.B.; Giblin, L.; Markham, T.; Stickland, N.C.; Berry, D.P.; O’Reilly, J.J.; Lawlor, P.G. Nutritional intervention during gestation alters growth, body composition and gene expression patterns in skeletal muscle of pig offspring. Animal 2011, 5, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Cerisuelo, A.; Baucells, M.D.; Gasa, J.; Coma, J.; Carri´on, D.; Chapinal, N.; Sala, R. Increased sow nutrition during midgestation affects muscle fiber development and meat quality, with no consequences on growth performance. J. Anim. Sci. 2009, 87, 729–739. [Google Scholar] [CrossRef]
- Willis, S.K.; Wise, L.A.; Wesselink, A.K.; Rothman, K.J.; Mikkelsen, E.M.; Tucker, K.L.; Trolle, E.; Hatch, E.E. Glycemic load, dietary fiber, and added sugar and fecundability in 2 preconception cohorts. Am. J. Clin. Nutr. 2020, 112, 27–38. [Google Scholar] [CrossRef]
- Beltowski, J.; Semczuk, A. Liver X receptor (LXR) and the reproductive system—A potential novel target for therapeutic intervention. Pharmacol. Rep. 2010, 62, 15–27. [Google Scholar] [CrossRef]
- NIH Study Links High Cholesterol Levels to Lower Fertility. Available online: https://www.nih.gov/news-events/news-releases/nih-study-links-high-cholesterol-levels-lower-fertility (accessed on 4 March 2022).
- de Castro, M.B.T.; Farias, D.R.; Lepsch, J.; Mendes, R.H.; Ferreira, A.A.; Kac, G. High cholesterol dietary intake during pregnancy is associated with large for gestational age in a sample of low-income women of Rio de Janeiro, Brazil. Matern. Child Nutr. 2017, 13, e12361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, X.; Li, F.; Sun, H.; Zhang, J.; Li, R.; Gao, Y.; Dong, H.; Cai, C.; Zeng, G. Dietary cholesterol and egg intake are associated with the risk of gestational diabetes: A prospective study from Southwest China. BMC Pregnancy Childbirth 2022, 22, 45. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhou, P.; Liu, H.; Li, S.; Zhao, Y.; Deng, K.; Cao, D.D.; Che, L.Q.; Fang, Z.F.; Xu, S.Y.; et al. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Dom. Anim. 2016, 51, 492–500. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef] [Green Version]
- Lugarà, R.; Kreuzer, M.; Giller, K. Does a high-energy diet with or without Spirulina supplementation affect body weight and blood markers of pregnant sows and their piglets? Proc. Soc. Nutr. Physiol. 2021, 30, 80. [Google Scholar]
- Cowart, R.P. Parturition and dystocia in swine. In Large Animal Theriogenology; Youngquist, R.S., Threlfall, W.R., Eds.; Saunders: St. Louis, MO, USA, 2007; pp. 778–784. [Google Scholar]
- Wang, H.; Hu, C.; Cheng, C.; Cui, J.; Ji, Y.; Hao, X.; Li, Q.; Ren, W.; Deng, B.; Yin, Y.; et al. Unraveling the association of fecal microbiota and oxidative stress with stillbirth rate of sows. Theriogenology 2019, 136, 131–137. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary regulation of oxidative stress in chronic metabolic diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Quiniou, N.; Richard, S.; Mourot, J.; Etienne, M. Effect of dietary fat or starch supply during gestation and/or lactation on the performance of sows, piglets’ survival and on the performance of progeny after weaning. Animal 2008, 2, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Laws, J.; Amusquivar, E.; Laws, A.; Herrera, E.; Lean, I.J.; Dodds, P.F.; Clarke, L. Supplementation of sow diets with oil during gestation: Sow body condition, milk yield and milk composition. Livest. Sci. 2009, 123, 88–96. [Google Scholar] [CrossRef]
- Tudisco, R.; Musco, N.; Pero, M.E.; Morittu, V.M.; Grossi, M.; Mastellone, V.; Cavaliere, G.; Wanapat, M.; Infascelli, F.; Lombardi, P. Influence of dietary hydrogenated palm oil supplementation on serum biochemistry and progesterone levels in dairy goats. Anim. Nutr. 2019, 5, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Bickerstaffe, R.; Annison, E.F. The desaturase activity of goat and sow mammary tissue. Comp. Biochem. Physiol. 1970, 35, 653–665. [Google Scholar] [CrossRef]
- Palombo, V.; Loor, J.J.; D’Andrea, M.; Vailati-Riboni, M.; Krogh, U.; Theil, P.K. Transcriptional profiling of swine mammary gland during the transition from colostrogenesis to lactogenesis using RNA sequencing. BMC Genom. 2018, 19, 322. [Google Scholar] [CrossRef] [PubMed]
- Shimkus, A.; Shimkiene, A.; Jouzaitiene, V.; Zavodnik, L.; Juozaitis, A.; Muzikevicius, A. Influence of blue algae spirulina platensis on the productivity of sows. Comptes Rendus Acad. Bulg. Sci. 2009, 62, 405–410. [Google Scholar]
- Manzocchi, E.; Guggenbühl, B.; Kreuzer, M.; Giller, K. Effects of the substitution of soybean meal by spirulina in a hay-based diet for dairy cows on milk composition and sensory perception. J. Dairy Sci. 2020, 103, 11349–11362. [Google Scholar] [CrossRef]
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Naidu, S.J.; Arangasamy, A.; Selvaraju, S.; Binsila, B.K.; Reddy, I.J.; Ravindra, J.P.; Bhatta, R. Maternal influence on the skewing of offspring sex ratio: A review. Anim. Prod. Sci. 2022. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Z.; Wu, Z.; Hong, L. Sex Manipulation Technologies Progress in Livestock: A Review. Front. Vet. Sci. 2020, 7, 481. [Google Scholar] [CrossRef]
- Alexenko, A.P.; Mao, J.; Ellersieck, M.R.; Davis, A.M.; Whyte, J.J.; Rosenfeld, C.S.; Roberts, R.M. The contrasting effects of ad libitum and restricted feeding of a diet very high in saturated fats on sex ratio and metabolic hormones in mice. Biol. Reprod. 2007, 77, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S.; Grimm, K.M.; Livingston, K.A.; Brokman, A.M.; Lamberson, W.E.; Roberts, R.M. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc. Natl. Acad. Sci. USA 2003, 100, 4628–4632. [Google Scholar] [CrossRef] [Green Version]
- Fountain, E.D.; Mao, J.; Whyte, J.J.; Mueller, K.E.; Ellersieck, M.R.; Will, M.J.; Roberts, R.M.; Macdonald, R.; Rosenfeld, C.S. Effects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in mice. Biol. Reprod. 2008, 8, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulliver, C.E.; Friend, M.A.; King, B.J.; Wilkins, J.F.; Clayton, E.H. A higher proportion of female lambs when ewes were fed oats and cottonseed meal prior to and following conception. Anim. Prod. Sci. 2013, 53, 464–471. [Google Scholar] [CrossRef]
- Clayton, E.H.; Friend, M.A.; Wilkins, J.F. Increasing the proportion of female lambs by feeding Merino ewes a diet high in omega-6 fatty acids around mating. Anim. Prod. Sci. 2016, 56, 1174–1184. [Google Scholar] [CrossRef]
- Green, M.P.; Spate, L.D.; Parks, T.E.; Kimura, K.; Murphy, C.N.; Williams, J.E.; Kerley, M.S.; Green, J.A.; Keisler, D.H.; Roberts, R.M. Nutritional skewing of conceptus sex in sheep: Effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA). Reprod. Biol. Endocrinol. 2008, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gestation Diet | Lactation Diet | ||||
|---|---|---|---|---|---|
| CTR | HED | CTRL | HEDL | Spirulina | |
| Ingredients (g/kg as fed) | |||||
| Hydrogenated palm oil | - | 150.00 | - | 150.00 | - |
| Saccharose | - | 200.00 | - | 200.00 | - |
| d-Fructose | - | 150.00 | - | 150.00 | - |
| Cholesterol | - | 2.00 | - | 2.00 | - |
| Corn germ | 590.00 | 298.00 | 488.00 | 200.00 | - |
| Wheat | 300.00 | - | 300.00 | - | - |
| Soybean meal | 45.00 | 130.00 | 145.00 | 217.00 | - |
| Lignocellulose | 26.60 | 29.00 | 26.60 | 29.00 | - |
| Monocalcium phosphate | 13.00 | 20.00 | 11.20 | 20.00 | - |
| Calcium carbonate | 11.00 | 6.00 | 11.60 | 6.00 | - |
| NaCl | 6.20 | 6.00 | 6.20 | 6.00 | - |
| Vitamin and mineral premix † | 5.00 | 5.00 | 5.00 | 5.00 | - |
| l-Lysine | 2.30 | 2.50 | 3.60 | 5.40 | - |
| dl-Methionine | - | 1.00 | 0.70 | 1.90 | - |
| dl-Tryptophan | 0.20 | 0.40 | 0.50 | 1.00 | - |
| l-Threonine | 0.70 | 1.30 | 1.80 | 3.40 | - |
| Valine | - | - | - | 2.20 | - |
| Chemical composition (g/kg dry matter if not stated otherwise) | |||||
| Total ash | 47.80 | 47.14 | 54.94 | 54.32 | 90.20 |
| Crude protein | 110.00 | 93.52 | 150.95 | 162.33 | 638.00 |
| Ether extract | 26.83 | 158.17 | 29.14 | 143.40 | 47.06 |
| Starch | 462.00 | 93.00 | 408.00 | 74.50 | 51.00 |
| Total sugars | 46.50 | 359.00 | 51.50 | 326.00 | <0.5 |
| Crude fiber | 6.71 | 8.67 | 6.17 | 8.07 | n.a. |
| Cholesterol | 0.03 | 0.48 | 0.02 | 0.45 | n.a |
| Gross energy (MJ/kg dry matter) | 13.41 | 17.17 | 12.96 | 16.75 | n.a. |
| Fatty acid composition (g/100 g total FA)§ | |||||
| C12:0 | 0.14 | 0.64 | 0.30 | 0.08 | 0.03 |
| C14:0 | 0.15 | 0.91 | 0.12 | 0.11 | 0.17 |
| C16:0 | 15.96 | 30.69 | 12.32 | 5.92 | 42.97 |
| iso C16:0 | 0.06 | 0.00 | 0.06 | 0.00 | 1.93 |
| C16:1 n-7 | 0.15 | 0.01 | 0.12 | 0.03 | 5.44 |
| C16:1 x | 0.34 | 0.00 | 0.04 | 0.00 | 0.05 |
| C17:1 | 0.03 | 0.00 | 0.04 | 0.03 | 0.33 |
| C18:1 cis-9 | 26.04 | 3.31 | 21.82 | 2.86 | 1.62 |
| C18:1 cis-11 | 0.88 | 0.11 | 0.67 | 0.12 | 0.73 |
| C18:2 n-6 (LA) | 47.60 | 5.04 | 45.25 | 5.07 | 16.58 |
| C18:3 n-3 (ALA) | 1.93 | 0.34 | 2.28 | 0.68 | 0.43 |
| C18:3 n-6 (GLA) | 0.00 | 0.00 | 0.00 | 0.00 | 23.23 |
| C20:0 | 0.49 | 0.89 | 0.64 | 2.00 | 0.09 |
| C20:1 n-9 | 0.42 | 0.04 | 0.34 | 0.06 | 0.12 |
| C20:2 n-6 | 0.11 | 0.01 | 0.05 | 0.00 | 0.30 |
| C20:3 n-6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.38 |
| C22:0 | 0.22 | 0.35 | 0.35 | 1.15 | 0.00 |
| ∑ Saturated FA | 22.02 | 90.34 | 29.11 | 91.11 | 49.22 |
| ∑ Monounsaturated FA | 28.02 | 4.26 | 23.3 | 3.14 | 8.85 |
| ∑ Polyunsaturated FA | 49.96 | 5.40 | 47.62 | 5.75 | 41.93 |
| ∑ n-6 FA | 47.71 | 5.05 | 45.30 | 5.07 | 40.40 |
| ∑ n-3 FA | 1.90 | 0.34 | 2.27 | 0.68 | 0.45 |
| n-6/n-3 FA ratio | 25.13 | 14.74 | 19.68 | 7.44 | 73.10 |
| Diet (D) | CTR | HED | SEM | Significance | ||||
|---|---|---|---|---|---|---|---|---|
| Spirulina (Sp) | - | + | - | + | D | Sp | D × Sp | |
| Gestation length (d) 1 | 116.20 | 115.80 | 116.20 | 114.25 | 1.437 | n.s. | n.s. | n.s. |
| Litter size (piglets)1 | ||||||||
| Total † | 13.20 | 12.40 | 11.66 | 12.60 | 2.070 | n.s. | n.s. | n.s. |
| Born alive | 11.20 | 11.82 | 10.12 | 11.15 | 2.498 | n.s. | n.s. | n.s. |
| Weaned | 10.15 | 10.35 | 9.00 | 9.25 | 2.442 | # | n.s. | n.s. |
| Sex ratio (male:female) 1 | 1.50 | 1.12 | 0.83 | 1.77 | 0.453 | n.s. | n.s. | # |
| Total litter weight (kg) 1,‡ | 20.62 | 19.02 | 19.44 | 18.55 | 3.233 | n.s. | n.s. | n.s. |
| Average birth weight (kg) 1,‡ | 1.670 | 1.580 | 1.740 | 1.560 | 0.130 | n.s. | n.s. | n.s. |
| Mortality (%)1 | ||||||||
| Foetal deaths | 12.31 | 4.11 | 13.48 | 16.36 | 6.159 | n.s. | n.s. | n.s. |
| Until weaning | 8.58 | 9.43 | 10.63 | 19.06 | 6.331 | n.s. | n.s. | n.s. |
| Milk yield (kg)2 | ||||||||
| Total | 325.22 | 283.38 | 280.88 | 292.35 | 65.834 | n.s. | n.s. | n.s. |
| Per piglet per day | 1.07 | 0.75 | 1.01 | 0.98 | 0.159 | n.s. | n.s. | n.s. |
| Diet (D) | CTR | HED | SEM | Significance | ||||
|---|---|---|---|---|---|---|---|---|
| Spirulina (Sp) | - | + | - | + | D | Sp | D × Sp | |
| Fat (g/100 g) | 9.77 | 9.07 | 8.26 | 6.21 | 1.870 | n.s. | n.s. | n.s. |
| Protein (g/100 g) | 15.92 | 16.37 | 15.81 | 15.29 | 0.751 | n.s. | n.s. | n.s. |
| Lactose (g/100 g) | 0.88 | 1.07 | 1.05 | 1.19 | 0.148 | n.s. | n.s. | n.s. |
| Estimated energy content (MJ/kg) | 8.36 | 8.21 | 7.77 | 6.87 | 0.873 | n.s. | n.s. | n.s. |
| IgA (mg/mL) | 6.92 | 7.53 | 6.37 | 7.31 | 1.330 | n.s. | n.s. | n.s. |
| IgG (mg/mL) | 57.61 | 56.03 | 60.91 | 58.26 | 8.462 | n.s. | n.s. | n.s. |
| IgM (mg/mL) | 2.68 | 3.11 | 2.29 | 2.49 | 0.511 | n.s. | n.s. | n.s. |
| Fatty acid (FA) composition (g/100 g total FA)† | ||||||||
| C14:0 | 1.61 | 1.41 | 1.68 | 1.47 | 0.329 | n.s. | # | n.s. |
| C15:0 | 0.09 | 0.07 | 0.14 | 0.11 | 0.038 | * | n.s. | n.s. |
| C16:0 | 25.17 | 25.44 | 25.00 | 25.53 | 1.542 | n.s. | n.s. | n.s. |
| iso C16:0 | 0.89 | 0.93 | 0.88 | 0.94 | 0.514 | n.s. | n.s. | n.s. |
| C16:1 n-7 | 2.91 | 2.73 | 2.85 | 3.43 | 0.465 | n.s. | n.s. | n.s. |
| C17:0 | 0.21 | 0.19 | 0.26 | 0.20 | 0.064 | n.s. | # | n.s. |
| C17:1 | 0.17 | 0.17 | 0.21 | 0.19 | 0.048 | # | n.s. | n.s. |
| C18:0 | 10.02 | 10.61 | 12.86 | 12.33 | 3.610 | ** | n.s. | n.s. |
| C18:1 cis-9 | 36.97 | 38.76 | 37.33 | 37.99 | 1.682 | n.s. | n.s. | n.s. |
| C18:1 cis-11 | 3.06 | 3.24 | 2.63 | 3.19 | 0.331 | # | * | n.s. |
| C18:1 cis-13 | 0.12 | 0.15 | 0.12 | 0.15 | 0.019 | n.s. | * | n.s. |
| C18:2 n-6 (LA) | 14.28 | 12.39 | 11.31 | 9.59 | 2.013 | * | n.s. | n.s. |
| C18:3 n-6 (GLA) | 0.12 | 0.19 | 0.11 | 0.14 | 0.094 | n.s. | * | n.s. |
| C18:3 n-3 (ALA) | 0.65 | 0.48 | 0.59 | 0.53 | 0.164 | n.s. | * | n.s. |
| C20:0 | 0.13 | 0.15 | 0.20 | 0.17 | 0.024 | * | n.s. | # |
| C20:1 cis-9 | 0.53 | 0.53 | 0.44 | 0.48 | 0.232 | # | n.s. | n.s. |
| C20:2 n-6 | 0.33 | 0.31 | 0.20 | 0.19 | 0.051 | *** | n.s. | n.s. |
| C20:3 n-6 | 0.18 | 0.17 | 0.16 | 0.19 | 0.033 | n.s. | n.s. | n.s. |
| C20:4 n-6 | 1.26 | 1.27 | 1.87 | 1.58 | 0.795 | n.s. | n.s. | n.s. |
| C20:5 n-3 | 0.13 | 0.11 | 0.15 | 0.15 | 0.090 | n.s. | n.s. | n.s. |
| C22:5 n-3 | 0.17 | 0.16 | 0.20 | 0.19 | 0.046 | n.s. | n.s. | n.s. |
| ∑SFA | 38.63 | 39.15 | 40.90 | 41.14 | 1.596 | * | n.s. | n.s. |
| ∑MUFA | 44.05 | 45.94 | 44.14 | 45.76 | 1.899 | n.s. | n.s. | n.s. |
| ∑PUFA | 17.21 | 15.29 | 15.15 | 12.89 | 1.992 | n.s. | n.s. | n.s. |
| MUFA/SFA ratio | 1.14 | 1.18 | 1.08 | 1.13 | 0.065 | n.s. | n.s. | n.s. |
| PUFA/SFA ratio | 0.45 | 0.40 | 0.38 | 0.31 | 0.059 | # | n.s. | n.s. |
| UFA/SFA ratio | 1.61 | 1.57 | 1.45 | 1.45 | 0.094 | * | n.s. | n.s. |
| ∑ n-3 FA | 1.02 | 0.84 | 1.10 | 0.96 | 0.114 | n.s. | n.s. | n.s. |
| ∑ n-6 FA | 16.01 | 14.29 | 13.87 | 11.63 | 1.908 | n.s. | n.s. | n.s. |
| n-6/n-3 FA ratio | 15.62 | 17.00 | 12.42 | 12.60 | 1.372 | ** | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugarà, R.; Grześkowiak, Ł.; Zentek, J.; Meese, S.; Kreuzer, M.; Giller, K. A High-Energy Diet and Spirulina Supplementation during Pre-Gestation, Gestation, and Lactation do Not Affect the Reproductive and Lactational Performance of Primiparous Sows. Animals 2022, 12, 1171. https://doi.org/10.3390/ani12091171
Lugarà R, Grześkowiak Ł, Zentek J, Meese S, Kreuzer M, Giller K. A High-Energy Diet and Spirulina Supplementation during Pre-Gestation, Gestation, and Lactation do Not Affect the Reproductive and Lactational Performance of Primiparous Sows. Animals. 2022; 12(9):1171. https://doi.org/10.3390/ani12091171
Chicago/Turabian StyleLugarà, Rosamaria, Łukasz Grześkowiak, Jürgen Zentek, Susanne Meese, Michael Kreuzer, and Katrin Giller. 2022. "A High-Energy Diet and Spirulina Supplementation during Pre-Gestation, Gestation, and Lactation do Not Affect the Reproductive and Lactational Performance of Primiparous Sows" Animals 12, no. 9: 1171. https://doi.org/10.3390/ani12091171
APA StyleLugarà, R., Grześkowiak, Ł., Zentek, J., Meese, S., Kreuzer, M., & Giller, K. (2022). A High-Energy Diet and Spirulina Supplementation during Pre-Gestation, Gestation, and Lactation do Not Affect the Reproductive and Lactational Performance of Primiparous Sows. Animals, 12(9), 1171. https://doi.org/10.3390/ani12091171






