Ultrasonographic Imaging Protocol and Sonoanatomy of the Lumbar Spine in Healthy Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levy, M.; Gaschen, L.; Rademacher, N.; Bragulla, H. Technique for ultrasound-guided intraarticular cervical articular process injection in the dog. Vet. Radiol. Ultrasound 2014, 55, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Brisson, B.A. Intervertebral Disc Disease in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 829–858. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.-F.; Fournet, A.; Bassanino, J.; Manassero, M.; Bedu, A.-S.; Leperlier, D. Ultrasonography is more accurate than percutaneous palpation for identifying targeted thoracolumbar intervertebral disc spaces in dogs. Vet. Radiol. Ultrasound 2018, 59, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Nanai, B.; Lyman, R.; Bichsel, P.S. Use of intraoperative ultrasonography in canine spinal cord lesions. Vet. Radiol. Ultrasound 2007, 48, 254–261. [Google Scholar] [CrossRef]
- Bonelli, M.; Tudury, E.; Santos, C.; Araújo, B.; Diogo, C.; Silva, A.; Costa, F. Intraoperative ultrasonography of the vertebral canal in dogs. Arq. Bras. Med. Veterinária Zootec. 2015, 67, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M. Intraoperative spinal ultrasonography in dogs: Normal findings and case-history reports. Vet. Radiol. Ultrasound 1993, 34, 264–268. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakayama, M.; Takase, K. Intraoperative spinal ultrasonography in two dogs with spinal disease. Vet. Radiol. Ultrasound 2006, 47, 99–102. [Google Scholar] [CrossRef]
- Zhou, H.; Miller, D.; Schulte, D.M.; Benes, L.; Bozinov, O.; Sure, U.; Bertalanffy, H. Intraoperative ultrasound assistance in treatment of intradural spinal tumours. Clin. Neurol. Neurosurg. 2011, 113, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Galiano, K.; Obwegeser, A.; Bale, R.; Harlander, C.; Schatzer, R.; Schocke, M.; Gruber, H. Ultrasound-Guided and CT-Navigation-Assisted Periradicular and Facet Joint Injections in the Lumbar and Cervical Spine: A New Teaching Tool to Recognize the Sonoanatomic Pattern. Reg. Anesth. Pain Med. 2007, 32, 254–257. [Google Scholar] [CrossRef]
- Galiano, K.; Obwegeser, A.A.; Bodner, G.; Freund, M.; Maurer, H.; Kamelger, F.S.; Schatzer, R.; Ploner, F. Real-time Sonographic Imaging for Periradicular Injections in the Lumbar Spine. A sonographic anatomic study of a new technique. J. Ultrasound Med. 2005, 24, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Gofeld, M.; Bristow, S.J.; Chiu, S.C.; McQueen, C.K.; Bollag, L. Ultrasound-Guided Lumbar Transforaminal Injections. Feasibility and validation study. Spine 2012, 37, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, H.J.; Moon, D.E. Ultrasound-guided Pararadicular Injection in the Lumbar Spine: A Comparative Study of the Paramedian Sagittal and Paramedian Sagittal Oblique Approaches. Pain Pract. 2014, 15, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Loizides, A.; Gruber, H.; Peer, S.; Brenner, E.; Galiano, K.; Obernauer, J. A New Simplified Sonographic Approach for Pararadicular Injections in the Lumbar Spine: A CT-Controlled Cadaver Study. Am. J. Neuroradiol. 2011, 32, 828–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loizides, A.; Gruber, H.; Peer, S.; Galiano, K.; Bale, R.; Obernauer, J. Ultrasound Guided Versus CT-Controlled Pararadicular Injections in the Lumbar Spine: A Prospective Randomized Clinical Trial. Am. J. Neuroradiol. 2012, 34, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Simizu, S.; Kadota, R.; Takahasi, H. Ultrasound and Nerve Stimulation-Guided L5 Nerve Root Block. Spine 2009, 34, 2669–2673. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Pacheco, P.F.; Sellera, F.P.; Futema, F.; Cortopassi, S.R. The use of ultrasound to assist epidural injection in obese dogs. Vet. Anaesth. Analg. 2020, 47, 137–140. [Google Scholar] [CrossRef]
- Gofeld, M. Ultrasound-Guided Caudad Epidural Access for the Lumbosacral Neurostimulation: Case Report and Technical Note. Neuromodul. Technol. Neural Interface 2011, 14, 68–71. [Google Scholar] [CrossRef]
- Grau, T. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg. Anesth. Pain Med. 2001, 26, 64–67. [Google Scholar] [CrossRef]
- Karmakar, M.K.; Li, X.; Ho, A.M.-H.; Kwok, W.H.; Chui, P.T. Real-time ultrasound-guided paramedian epidural access: Evaluation of a novel in-plane technique. Br. J. Anaesth. 2009, 102, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Machin, H.; Merlin, T.; Viscasillas, J. Use of ultrasonography to confirm epidural catheter position in a cat. Vet. Anaesth. Analg. 2018, 45, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Perlas, A.; Chaparro, L.E.; Chin, K.J. Lumbar Neuraxial Ultrasound for Spinal and Epidural Anesthesia. A systematic review and meta-analysis. Reg. Anesth. Pain Med. 2016, 41, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Viscasillas, J.; Gregori, T.; Castiñeiras, D.; Redondo, I.; Seymour, C. Description and evaluation of four ultrasound-guided approaches to aid spinal canal puncture in dogs. Vet. Anaesth. Analg. 2016, 43, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, J.; Ma, L.; Cai, Z.; Meng, C.; Qi, S.; Zhou, H. Ultrasound-guided Versus Fluoroscopy-controlled Lumbar Transforaminal Epidural Injections. A prospective randomized Clinical trial. Clin. J. Pain 2016, 32, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.-L.; Peeters, D.; Busoni, V. Ultrasonographic percutaneous anatomy of the caudal lumbar region and ultrasound-guided lumbar puncture in the dog. Vet. Radiol. Ultrasound 2010, 51, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Finn-Bodner, S.T.; Hudson, J.A.; Coates, J.R.; Sorjonen, D.C.; Simpson, S.T.; Cox, N.R.; Wright, J.C.; Garrett, P.D.; Steiss, J.E.; Vaughn, D.M.; et al. Ultrasonographic anatomy of the normal canine spinal cord and correlation with histopathology after induced spinal cord trauma. Vet. Radiol. Ultrasound 1995, 36, 39–48. [Google Scholar] [CrossRef]
- Gregori, T.; Viscasillas, J.; Benigni, L. Ultrasonographic anatomy of the sacrococcygeal region and ultrasound-guided epidural injection at the sacrococcygeal space in dogs. Vet. Rec. 2014, 175, 68. [Google Scholar] [CrossRef]
- Liotta, A.; Busoni, V.; Carrozzo, M.V.; Sandersen, C.; Gabriel, A.; Bolen, G. Feasibility of ultrasound-guided epidural access at the lumbo-sacral space in dogs. Vet. Radiol. Ultrasound 2014, 56, 220–228. [Google Scholar] [CrossRef]
- Lopes, É.R.; Bellegard, G.M.; Cury, F.S.; Abreu, F.A.; Ambrósio, C.E.; Carregaro, A.B.; Hage, M.C.F. Evaluation of the applicability of musculoskeletal ultrasonography of the thoracolumbar and lumbar spine segment of healthy dogs. Pesqui. Veterinária Bras. 2018, 38, 2278–2283. [Google Scholar] [CrossRef]
- Graff, S.M.; Wilson, D.V.; Guiot, L.P.; Nelson, N.C. Comparison of three ultrasound guided approaches to the lumbar plexus in dogs: A cadaveric study. Vet. Anaesth. Analg. 2015, 42, 394–404. [Google Scholar] [CrossRef]
- MacKenzie, S.D.; Caswell, J.L.; Brisson, B.A.; Gaitero, L.; Chalmers, H.J. Comparison between computed tomography, fluoroscopy, and ultrasonography for guiding percutaneous injection of the canine intervertebral disc. Vet. Radiol. Ultrasound 2014, 55, 571–581. [Google Scholar] [CrossRef]
- McNally, D.; Naish, C.; Halliwell, M. Intervertebral disc structure: Observation by a novel use of ultrasound imaging. Ultrasound Med. Biol. 2000, 26, 751–758. [Google Scholar] [CrossRef]
- Naish, C.; Mitchell, R.; Innes, J.; Halliwell, M.; McNally, D. Ultrasound Imaging of the Intervertebral Disc. Spine 2003, 28, 107–113. [Google Scholar] [CrossRef] [PubMed]
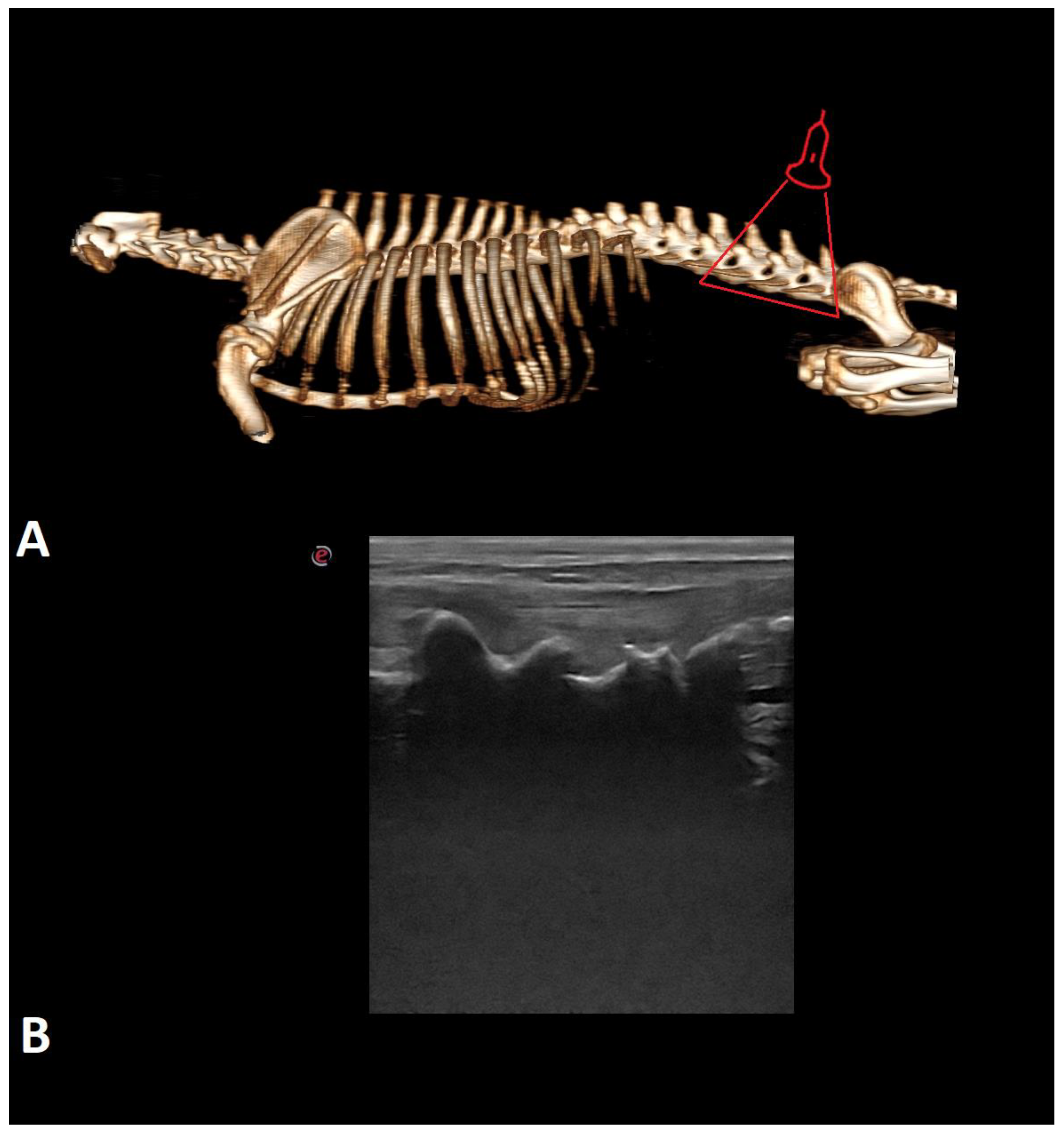

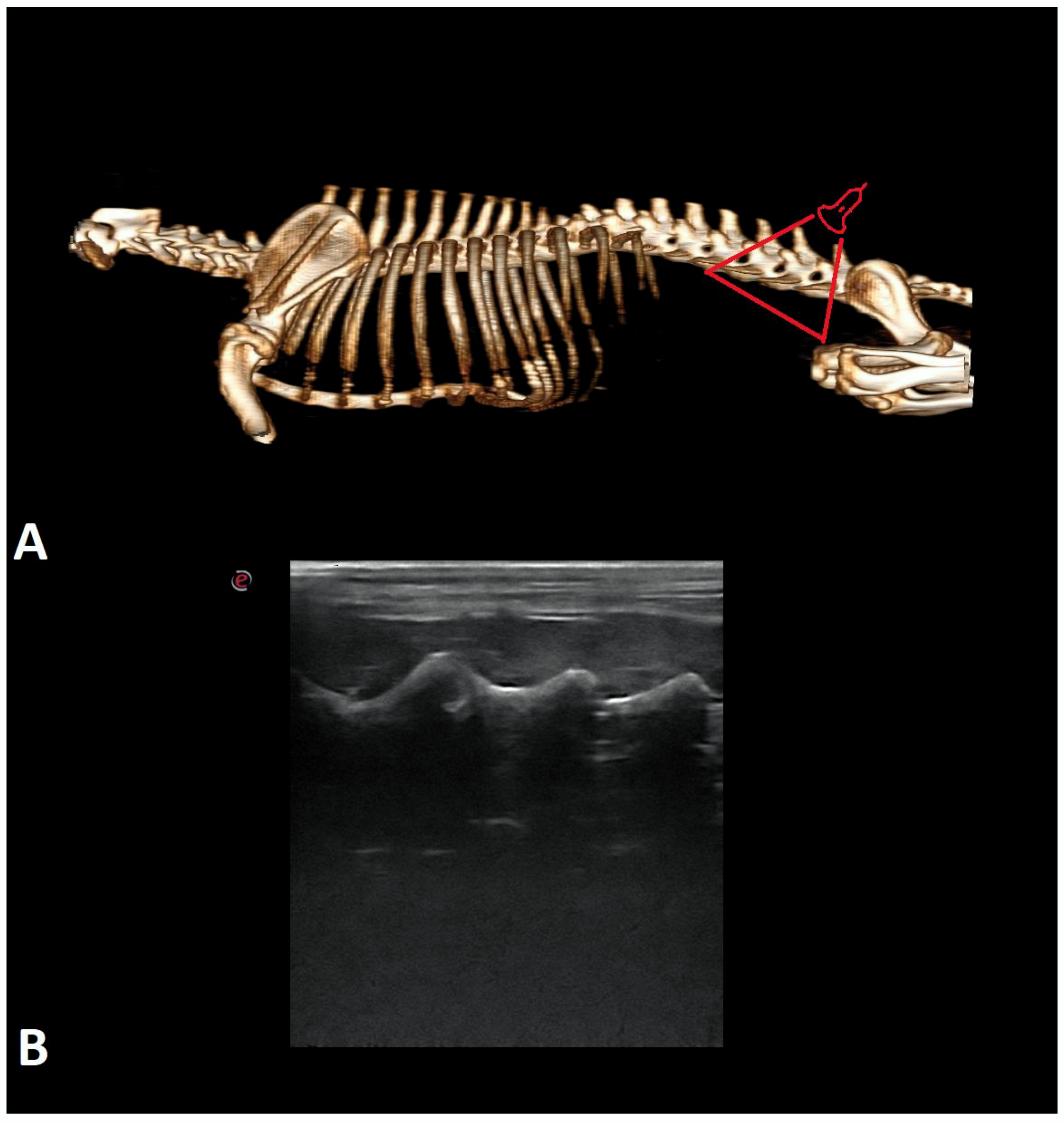
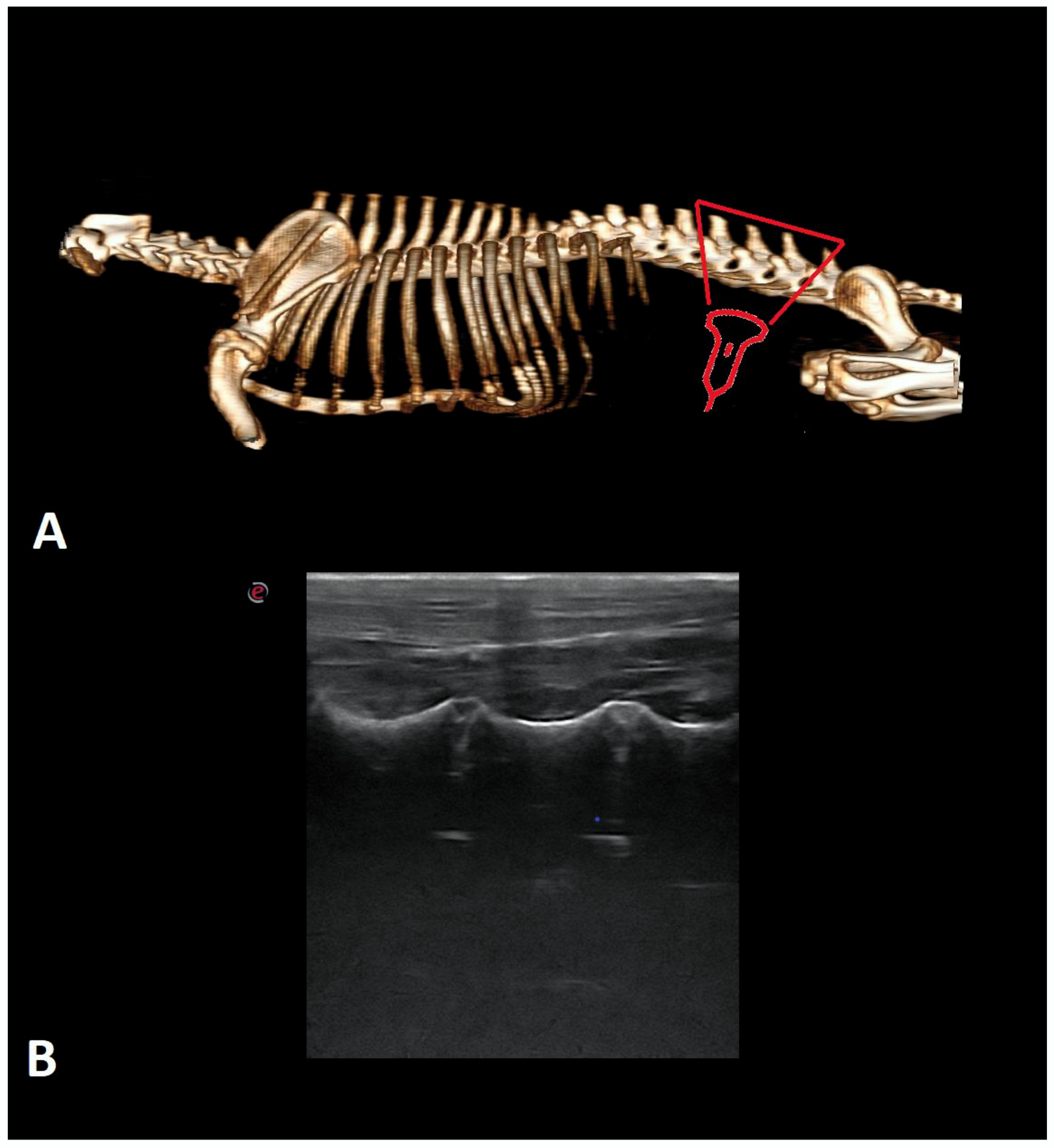





| Breed | Number of Cases |
|---|---|
| Mixed Breed | 9 |
| Yorkshire Terrier | 3 |
| German Shepherd | 2 |
| Labrador Retriever | 2 |
| Chihuahua | 1 |
| Cocker Spaniel | 1 |
| Dachshund | 1 |
| Irish Seter | 1 |
| Maltese | 1 |
| Miniature Schnauzer | 1 |
| Polish Hunting Dog | 1 |
| Structure | Visibility (Number/% of Cases) |
|---|---|
| Sacral crest | 2/8.7 |
| Spinous processes | 2/8.7 |
| Intertransverse ligament | 10/43.48 |
| Vertebral canal floor | 11/47.83 |
| Articular processes | 12/52.17 |
| Transverse processes | 15/65.22 |
| Vertebral body | 23/100 |
| Intervertebral discs | 23/100 |
| Structure | Visibility (Number/% of Cases) |
|---|---|
| Vertebral arch | 1/4.35 |
| Supraspinous ligament | 1/4.35 |
| Dorsal limit of the vertebral canal | 4/17.39 |
| Transverse processes | 6/26.09 |
| Spinalis and semispinalis muscle | 6/26.09 |
| Articular processes | 7/30.43 |
| Multifidus muscle | 7/30.43 |
| Iliocostalis and longissimus muscles | 7/30.43 |
| Spinous processes | 9/39.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abako, J.; Holak, P.; Bajon, J.; Zhalniarovich, Y. Ultrasonographic Imaging Protocol and Sonoanatomy of the Lumbar Spine in Healthy Dogs. Animals 2022, 12, 1187. https://doi.org/10.3390/ani12091187
Abako J, Holak P, Bajon J, Zhalniarovich Y. Ultrasonographic Imaging Protocol and Sonoanatomy of the Lumbar Spine in Healthy Dogs. Animals. 2022; 12(9):1187. https://doi.org/10.3390/ani12091187
Chicago/Turabian StyleAbako, Justyna, Piotr Holak, Joanna Bajon, and Yauheni Zhalniarovich. 2022. "Ultrasonographic Imaging Protocol and Sonoanatomy of the Lumbar Spine in Healthy Dogs" Animals 12, no. 9: 1187. https://doi.org/10.3390/ani12091187





