Establishment and Application of an Indirect Enzyme-Linked Immunosorbent Assay for Measuring GPI-Anchored Protein 52 (P52) Antibodies in Babesia gibsoni-Infected Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites and Animal Experiments
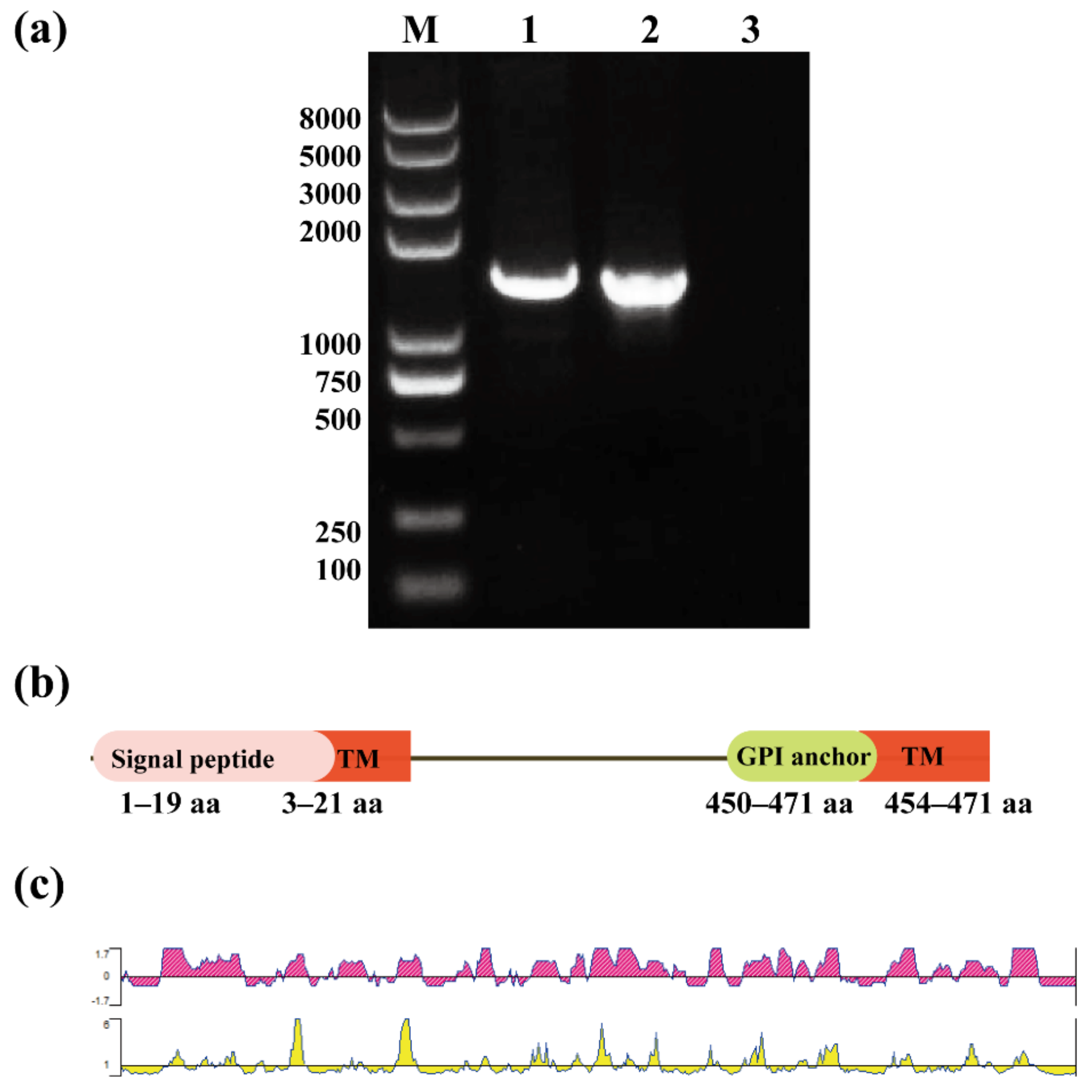
2.2. Bioinformatics Analysis
2.3. Extraction of Total RNA and gDNA
2.4. Cloning, Expression and Purification of rBgGPI52-WH
2.5. Production of Mouse Anti-rBgGPI52-WH Immune Serum
2.6. Preparation of B. gibsoni Lysates
2.7. Western Blot Analysis
2.8. Establishing iELISA Based on rBgGPI52-WH
2.9. Sensitivity and Speficity of the iELISA Assy
2.10. Repeatability of the iELISA Assay
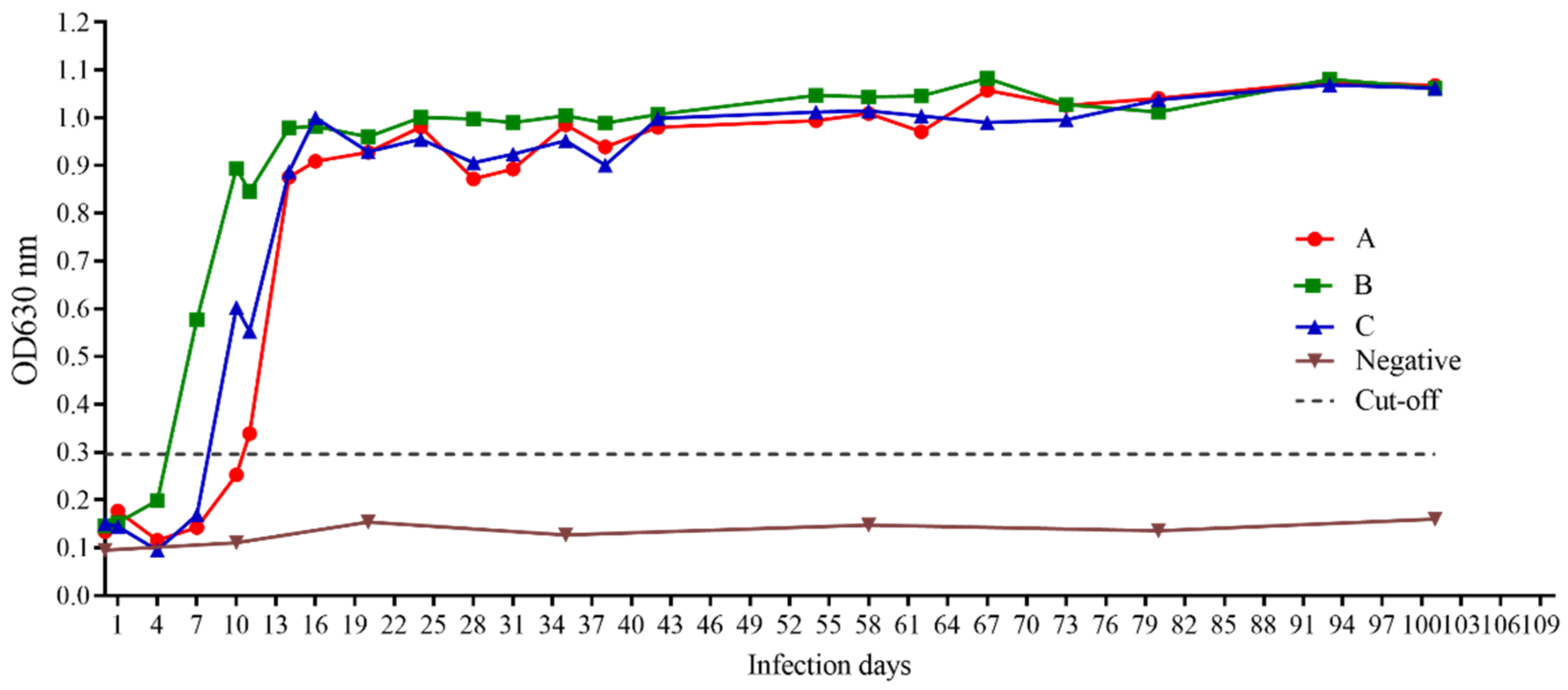
2.11. Detection of Antibodies against BgGPI52-WH in Experimental Infected Dog Samples
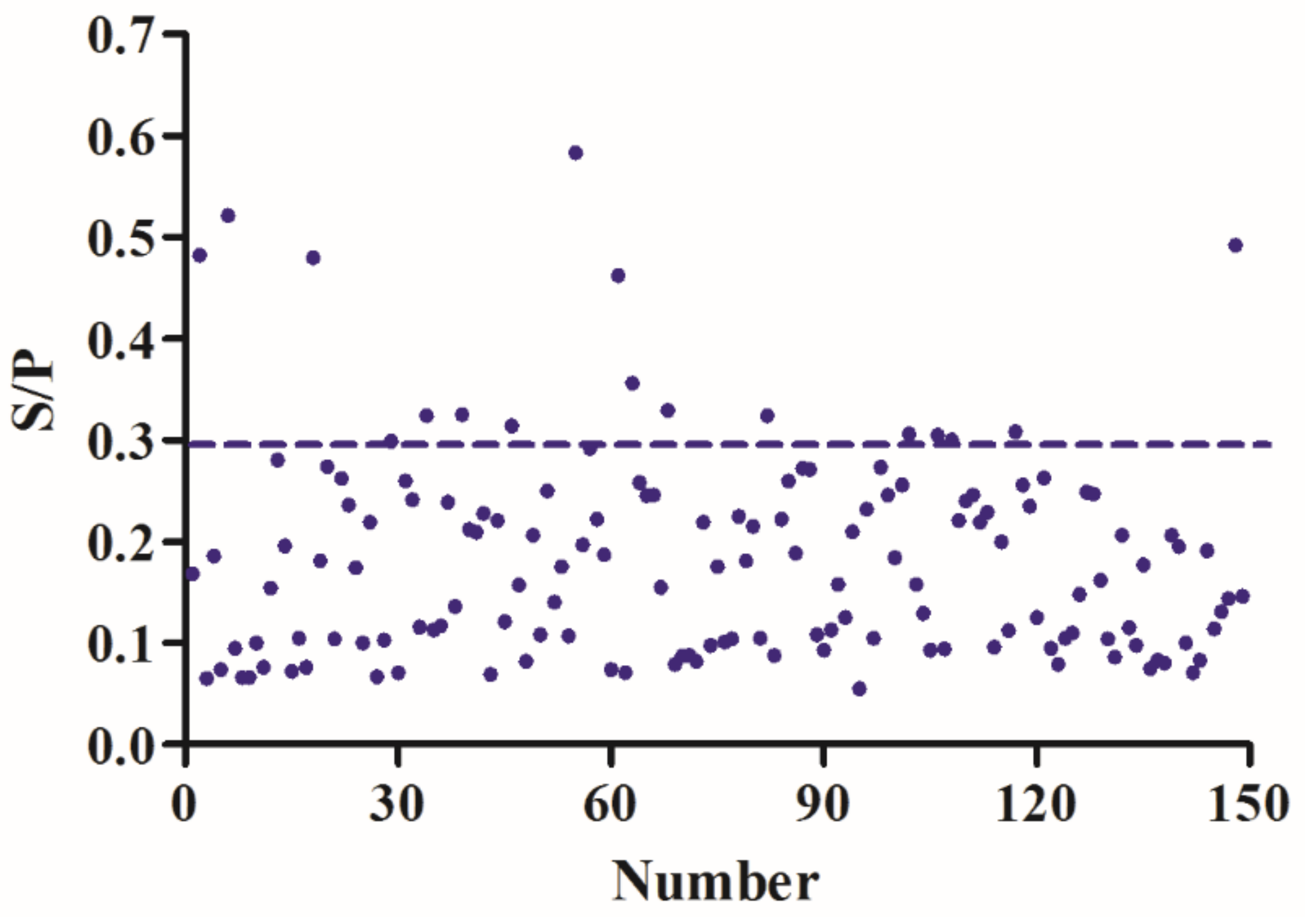
2.12. Evaluating the iELISA by Clinical Sample Testing
3. Results
3.1. Gene Sequence Analysis of BgGPI52-WH
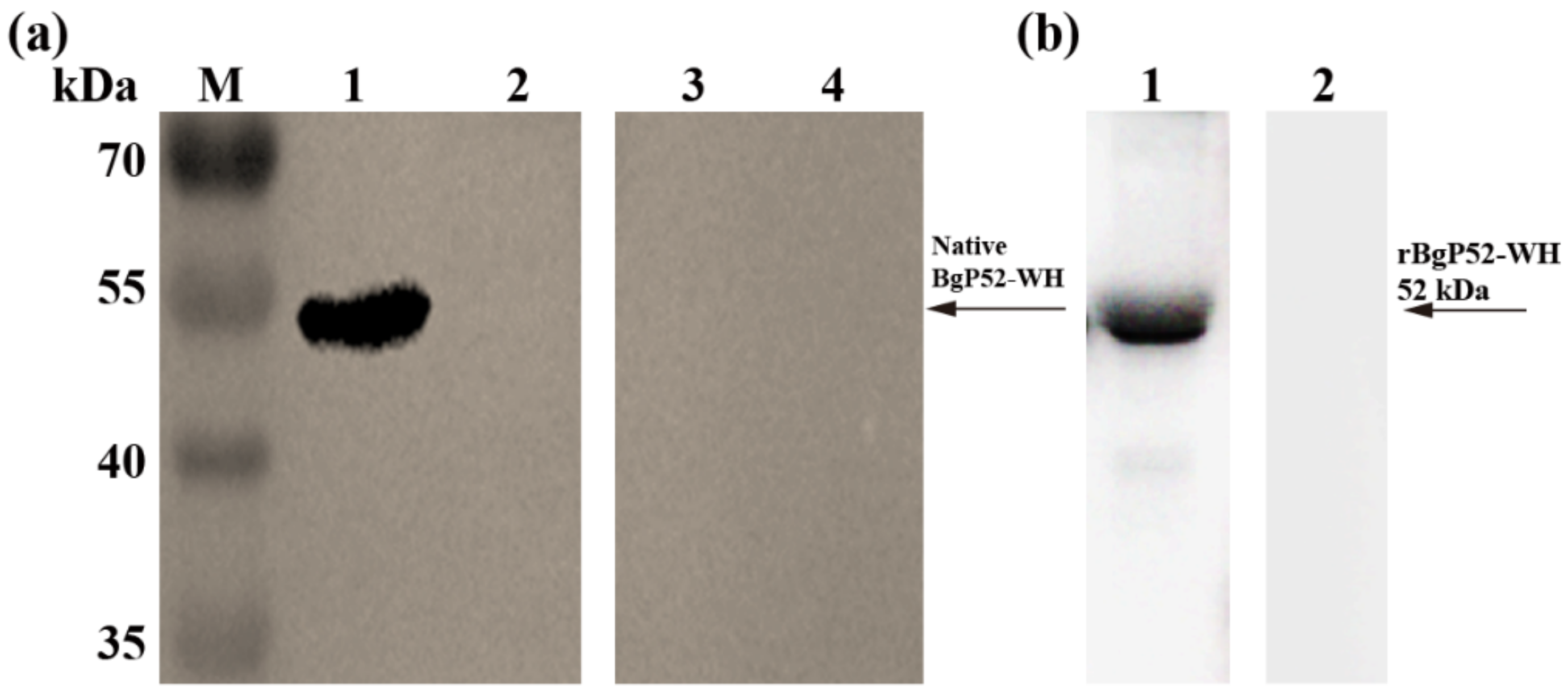
3.2. Cloning and Expression of BgGPI52-WH Recombinant Protein
3.3. Western Blot Analysis of rBgGPI52-WH Protein
3.4. Optimization of Experimental Conditions of Indirect ELISA
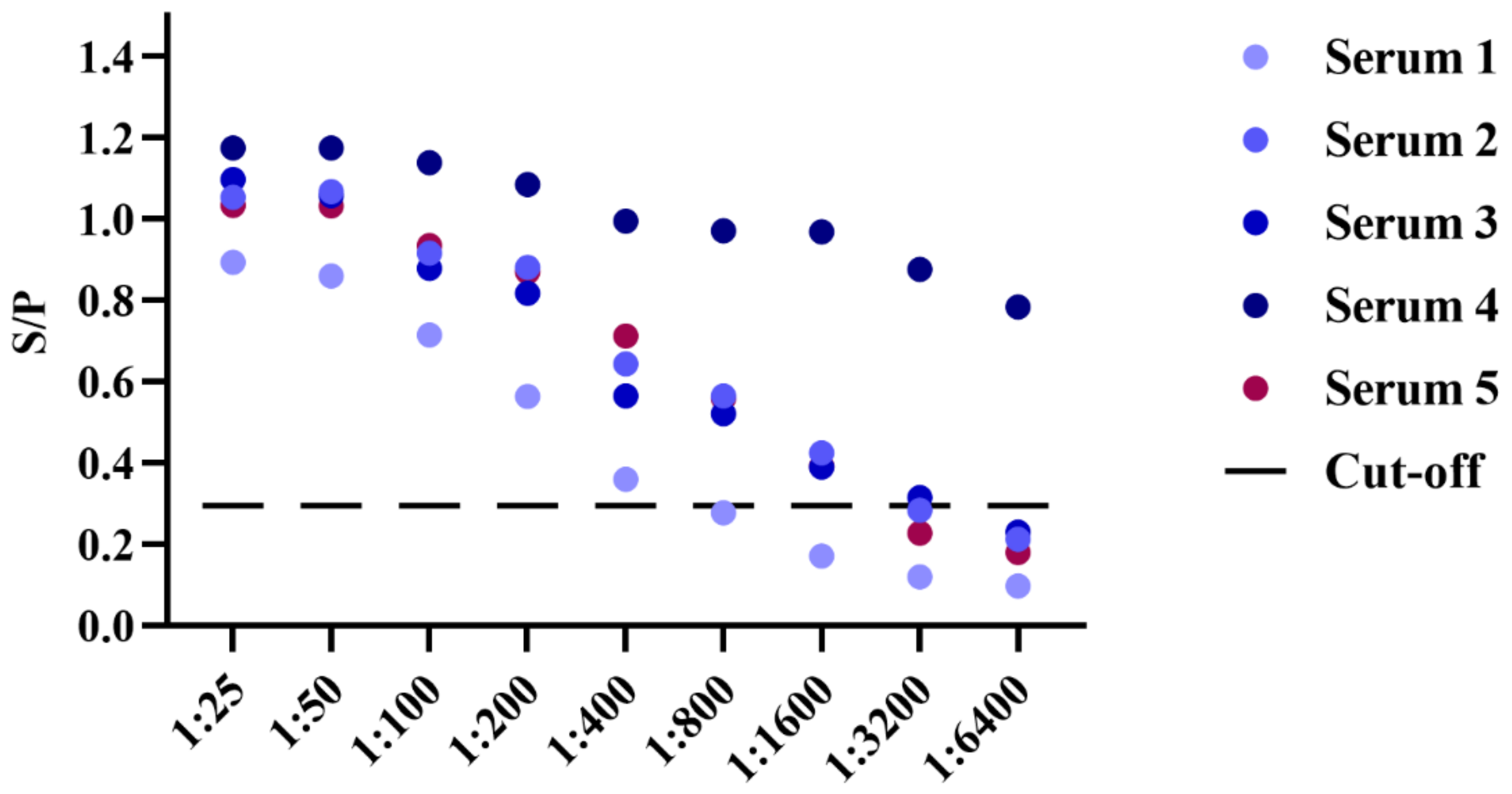
3.5. Sensitivity and Specificty Detection of BgGPI52-WH iELISA
3.6. Repeatability Detection of iELISA
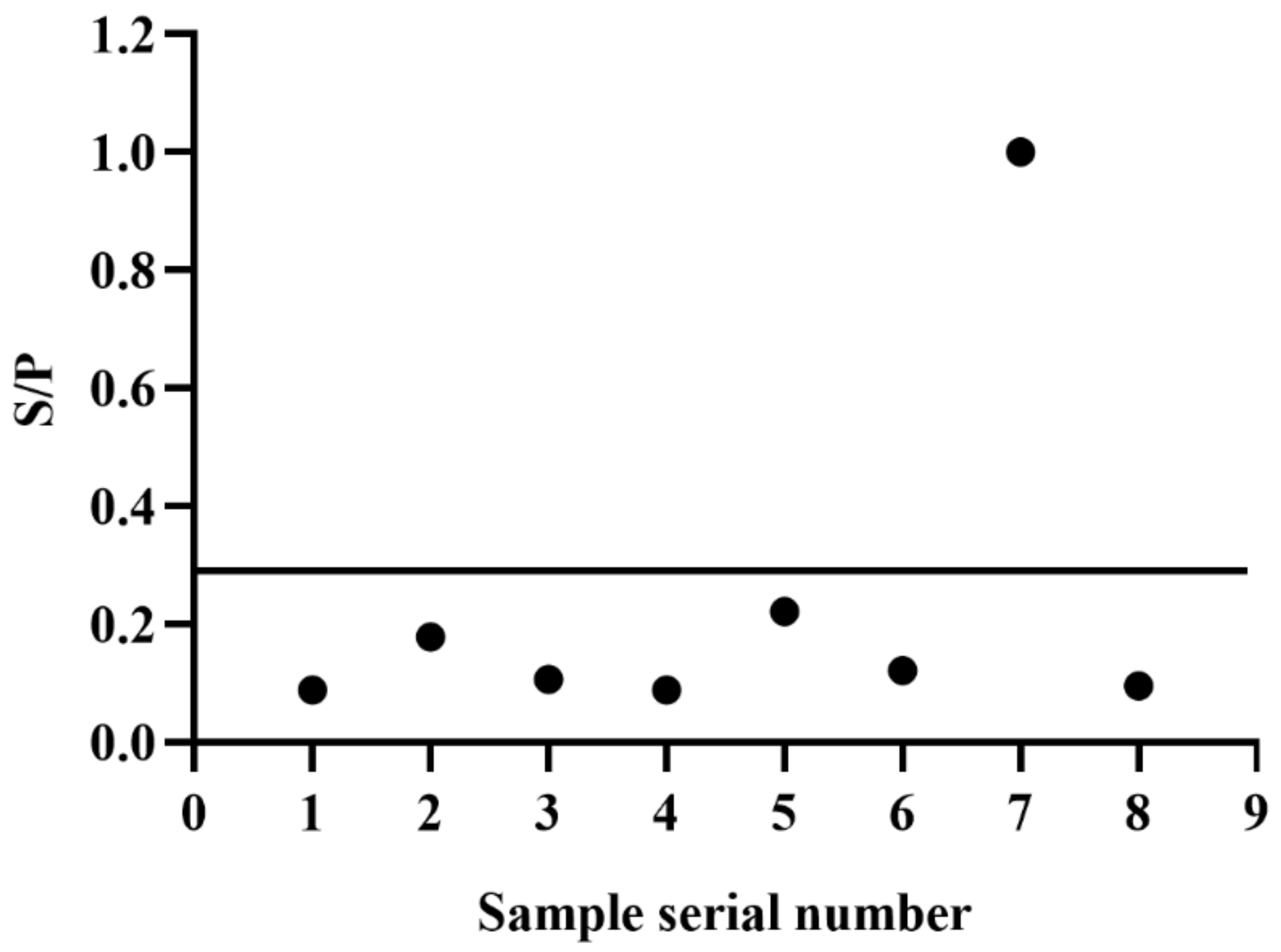
3.7. iELISA Evaluation Using Clinical and Experimental Infected Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, M.N.; Raina, O.K.; Sankar, M.; Rialch, A.; Tigga, M.N.; Kumar, G.R.; Banerjee, P.S. Molecular detection and genetic diversity of Babesia gibsoni in dogs in India. Infect. Genet. Evol. 2016, 41, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Irwin, P.J. Canine babesiosis. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Uilenberg, G. Babesia—A historical overview. Vet. Parasitol. 2006, 138, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Casapulla, R.; Baldi, L.; Avallone, V.; Sannino, R.; Pazzanese, L.; Mizzoni, V. Canine piroplasmosis due to Babesia gibsoni: Clinical and morphological aspects. Vet. Rec. 1998, 142, 168–169. [Google Scholar] [CrossRef]
- Wozniak, E.J.; Barr, B.C.; Thomford, J.W.; Yamane, I.; McDonough, S.P.; Moore, P.F.; Naydan, D.; Robinson, T.W.; Conrad, P.A. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris). J. Parasitol. 1997, 83, 692–699. [Google Scholar] [CrossRef]
- Groves, M.G.; Yap, L.F. Babesia gibsoni (Patton, 1910) from a dog in Kuala Lumpur. Med. J. Malaya 1968, 22, 229. [Google Scholar]
- Kjemtrup, A.M.; Kocan, A.A.; Whitworth, L.; Meinkoth, J.; Birkenheuer, A.J.; Cummings, J.; Boudreaux, M.K.; Stockham, S.L.; Irizarry-Rovira, A.; Conrad, P.A. There are at least three genetically distinct small piroplasms from dogs. Int. J. Parasitol. 2000, 30, 1501–1505. [Google Scholar] [CrossRef]
- Wenxiang, L.; Xinshe, L. A case of Babesia gibsoni in a hunting dog. Chin. J. Vet. Sci. Technol. 1987, 5, 45–46. [Google Scholar]
- He, L.; Miao, X.; Hu, J.; Huang, Y.; He, P.; He, J.; Yu, L.; Malobi, N.; Shi, L.; Zhao, J. First Molecular Detection of Babesia gibsoni in Dogs from Wuhan, China. Front. Microbiol. 2017, 8, 1577. [Google Scholar] [CrossRef]
- Cannon, S.H.; Levy, J.K.; Kirk, S.K.; Crawford, P.C.; Leutenegger, C.M.; Shuster, J.J.; Liu, J.; Chandrashekar, R. Infectious diseases in dogs rescued during dogfighting investigations. Vet. J. 2016, 211, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lequin, R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, J.; Li, Z.; He, X.; Zhao, S.; Ma, Q.; Li, X.; Liu, J.; Liu, A.; Li, Y.; et al. A universal ELISA assay for detecting six strains of ovine Babesia species in China. Vet. Parasitol. 2021, 300, 109616. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.K.; Crawford, A.C.; Lindsay, D.; Sibley, L.D. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 1998, 66, 5322–5328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, R.; Harper, P.A.; Reichel, M.P.; Ellis, J.T. Progress in the serodiagnosis of Neospora caninum infections of cattle. Parasitol. Today 2000, 16, 110–114. [Google Scholar] [CrossRef]
- Schares, G.; Rauser, M.; Sondgen, P.; Rehberg, P.; Barwald, A.; Dubey, J.P.; Edelhofer, R.; Conraths, F.J. Use of purified tachyzoite surface antigen p38 in an ELISA to diagnose bovine neosporosis. Int. J. Parasitol. 2000, 30, 1123–1130. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Florin-Christensen, M.; Flores, D.A.; Echaide, I.; Suarez, C.E.; Schnittger, L. The glycosylphosphatidylinositol-anchored protein repertoire of Babesia bovis and its significance for erythrocyte invasion. Ticks Tick Borne Dis. 2014, 5, 343–348. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mayor, S. The GPI-anchor and protein sorting. Cell. Mol. Life Sci. CMLS 2001, 58, 1969–1987. [Google Scholar] [CrossRef]
- Cornillot, E.; Dassouli, A.; Pachikara, N.; Lawres, L.; Renard, I.; Francois, C.; Randazzo, S.; Bres, V.; Garg, A.; Brancato, J.; et al. A targeted immunomic approach identifies diagnostic antigens in the human pathogen Babesia microti. Transfusion 2016, 56, 2085–2099. [Google Scholar] [CrossRef] [Green Version]
- McGwire, B.S.; O’Connell, W.A.; Chang, K.P.; Engman, D.M. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: Implications for parasite virulence. J. Biol. Chem. 2002, 277, 8802–8809. [Google Scholar] [CrossRef] [Green Version]
- Orlean, P.; Menon, A.K. Thematic review series: Lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: How we learned to stop worrying and love glycophospholipids. J. Lipid Res. 2007, 48, 993–1011. [Google Scholar] [PubMed] [Green Version]
- Suzuki, K.G.; Kasai, R.S.; Hirosawa, K.M.; Nemoto, Y.L.; Ishibashi, M.; Miwa, Y.; Fujiwara, T.K.; Kusumi, A. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 2012, 8, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Yu, J.J.; Seshan, K.R.; Reichard, U.; Cole, G.T. A parasitic phase-specific adhesin of Coccidioides immitis contributes to the virulence of this respiratory Fungal pathogen. Infect. Immun. 2002, 70, 3443–3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekutis, C.; Ferguson, D.J.; Grigg, M.E.; Camps, M.; Boothroyd, J.C. Surface antigens of Toxoplasma gondii: Variations on a theme. Int. J. Parasitol. 2001, 31, 1285–1292. [Google Scholar] [CrossRef]
- Wahlgren, M.; Goel, S.; Akhouri, R.R. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat. Rev. Microbiol. 2017, 15, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Florin-Christensen, M.; Schnittger, L.; Dominguez, M.; Mesplet, M.; Rodriguez, A.; Ferreri, L.; Asenzo, G.; Wilkowsky, S.; Farber, M.; Echaide, I.; et al. Search for Babesia bovis vaccine candidates. Parassitologia 2007, 49 (Suppl. S1), 9–12. [Google Scholar]
- Bono, M.F.; Mangold, A.J.; Baravalle, M.E.; Valentini, B.S.; Thompson, C.S.; Wilkowsky, S.E.; Echaide, I.E.; Farber, M.D.; Torioni de Echaide, S.M. Efficiency of a recombinant MSA-2c-based ELISA to establish the persistence of antibodies in cattle vaccinated with Babesia bovis. Vet. Parasitol. 2008, 157, 203–210. [Google Scholar] [CrossRef]
- Carcy, B.; Precigout, E.; Schetters, T.; Gorenflot, A. Genetic basis for GPI-anchor merozoite surface antigen polymorphism of Babesia and resulting antigenic diversity. Vet. Parasitol. 2006, 138, 33–49. [Google Scholar] [CrossRef]
- Zhan, X.; Yu, L.; An, X.; Liu, Q.; Li, M.; Nie, Z.; Zhao, Y.; Wang, S.; Ao, Y.; Tian, Y.; et al. Evaluation of Babesia gibsoni GPI-anchored Protein 47 (BgGPI47-WH) as a Potential Diagnostic Antigen by Enzyme-Linked Immunosorbent Assay. Front. Vet. Sci. 2019, 6, 333. [Google Scholar] [CrossRef]
- Nathaly Wieser, S.; Schnittger, L.; Florin-Christensen, M.; Delbecq, S.; Schetters, T. Vaccination against babesiosis using recombinant GPI-anchored proteins. Int. J. Parasitol. 2019, 49, 175–181. [Google Scholar] [CrossRef]
- Goo, Y.K.; Xuan, X. New Molecules in Babesia gibsoni and their application for diagnosis, vaccine development, and drug discovery. Korean J. Parasitol. 2014, 52, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, B.; Wolf, C.; Greene, C.; Peterson, D.S. Sequence conservation in the rRNA first internal transcribed spacer region of Babesia gibsoni genotype Asia isolates. Vet. Parasitol. 2008, 152, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, Q.; Zhang, J.; Zhou, Y.; Zhang, H.; Gong, H.; Zhou, J. Seroprevalence survey of Babesia gibsoni infection and tick species in dogs in East China. Vet. Parasitol. 2015, 214, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Goo, Y.K.; Jia, H.; Aboge, G.O.; Terkawi, M.A.; Kuriki, K.; Nakamura, C.; Kumagai, A.; Zhou, J.; Lee, E.G.; Nishikawa, Y.; et al. Babesia gibsoni: Serodiagnosis of infection in dogs by an enzyme-linked immunosorbent assay with recombinant BgTRAP. Exp. Parasitol. 2008, 118, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Hafalla, J.C.; Silvie, O.; Matuschewski, K. Cell biology and immunology of malaria. Immunol. Rev. 2011, 240, 297–316. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Kennedy, P.J.; Ellis, J.T. A guide to in silico vaccine discovery for eukaryotic pathogens. Brief. Bioinform. 2013, 14, 753–774. [Google Scholar] [CrossRef]
- Man, S.; Fu, Y.; Guan, Y.; Feng, M.; Qiao, K.; Li, X.; Gao, H.; Cheng, X. Evaluation of a Major Surface Antigen of Babesia microti Merozoites as a Vaccine Candidate against Babesia Infection. Front. Microbiol. 2017, 8, 2545. [Google Scholar] [CrossRef]
- Karasova, M.; Tothova, C.; Grelova, S.; Fialkovicova, M. The Etiology, Incidence, Pathogenesis, Diagnostics, and Treatment of Canine Babesiosis Caused by Babesia gibsoni Infection. Animals 2022, 12, 739. [Google Scholar] [CrossRef]
- Goo, Y.K.; Aboge, G.O.; Terkawi, M.A.; Jia, H.; Yamagishi, J.; Sunaga, F.; Namikawa, K.; Cha, S.Y.; Jang, H.K.; Kim, S.; et al. Four promising antigens, BgP32, BgP45, BgP47, and BgP50, for serodiagnosis of Babesia gibsoni infection were classified as B. gibsoni merozoite surface protein family. Parasitol. Int. 2012, 61, 364–368. [Google Scholar] [CrossRef]
- Liu, M.; Igarashi, I.; Xuan, X. Babesia gibsoni . Trends Parasitol. 2022, in press. [Google Scholar] [CrossRef]
- Kirk, S.K.; Levy, J.K.; Crawford, P.C. Efficacy of Azithromycin and Compounded Atovaquone for Treatment of Babesia gibsoni in Dogs. J. Vet. Intern. Med. 2017, 31, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Chen, J.J.; Sahu, G.K.; Tyring, S.; Ramsey, K.; Indrikovs, A.J.; Petersen, J.R.; Paar, D.; Cloyd, M.W. Detection of antibodies to human immunodeficiency virus (HIV) that recognize conformational epitopes of glycoproteins 160 and 41 often allows for early diagnosis of HIV infection. J. Infect. Dis. 2002, 186, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenberger, R.M.; Stefanic, S.; Naucke, T.J.; Sarkunas, M.; Zamokas, G.; Grimm, F.; Deplazes, P. An ELISA for the early diagnosis of acute canine babesiosis detecting circulating antigen of large Babesia spp. Vet. Parasitol. 2017, 243, 162–168. [Google Scholar] [CrossRef] [PubMed]

| Function Category | Website/Software |
|---|---|
| Nucleotide sequence | https://www.ncbi.nlm.nih.gov/, accessed on 12 December 2019 |
| http://piroplasmadb.org/piro/, accessed on 12 December 2019 | |
| http://www.uniprot.org/, accessed on 12 December 2019 | |
| Sequence alignment | http://mafft.cbrc.jp/alignment/server/index.htmL, accessed on 12 December 2019 |
| http://www.ncbi.nlm.nih.gov/blast/, accessed on 12 December 2019 | |
| SignalP | http://www.cbs.dtu.dk/services/SignalP/, accessed on 12 December 2019 |
| Transmembrane prediction | https://embnet.vital-it.ch/software/TMPRED_form.html, accessed on 12 December 2019 |
| ProtScale | https://web.expasy.org/protscale/, accessed on 12 December 2019 |
| GPI anchor site prediction | http://mendel.imp.ac.at/gpi/plant_server.htm, accessed on 12 December 2019 |
| http://gpi.unibe.ch/, accessed on 12 December 2019 | |
| http://gpcr.biocomp.unibo.it/predgpi/, accessed on12 December 2019 | |
| B cell epitope prediction | DNASTAR |
| Primer design | Clone Manager |
| Primers | Primer Sequences (5′–3′) | Restriction Enzyme |
|---|---|---|
| BgGPI52-WH-F | 5′-ATGAGACTAGTTCGTGCATTCC-3′ | |
| BgGPI52-WH-R | 5′-TTAAAATACAGCGACAGCCACAG-3′ | |
| BgGPI52-WH-BamH I-F | 5′-CAGGATCCACTGGTGATGGGAATATGACAG-3′ | BamH I |
| BgGPI52-WH-Xho I-R | 5′-TCCTCGAGTTAAAATACAGCGACAGCCACAG-3′ | Xho I |
| Average Value | SD | CV% | |
|---|---|---|---|
| Serum 1 | 0.943 | 0.038 | 4.03 |
| Serum 2 | 0.989 | 0.028 | 2.83 |
| Serum 3 | 0.501 | 0.043 | 8.58 |
| Serum 4 | 0.088 | 0.003 | 3.41 |
| Serum 5 | 0.121 | 0.007 | 5.79 |
| Average Value | SD | CV% | |
|---|---|---|---|
| Serum 1 | 0.992 | 0.043 | 4.335 |
| Serum 2 | 0.997 | 0.025 | 2.508 |
| Serum 3 | 0.588 | 0.013 | 2.192 |
| Serum 4 | 0.057 | 0.004 | 7.144 |
| Serum 5 | 0.133 | 0.010 | 7.519 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhan, X.; Li, D.; Zhao, J.; Wei, H.; Alzan, H.; He, L. Establishment and Application of an Indirect Enzyme-Linked Immunosorbent Assay for Measuring GPI-Anchored Protein 52 (P52) Antibodies in Babesia gibsoni-Infected Dogs. Animals 2022, 12, 1197. https://doi.org/10.3390/ani12091197
Liu Q, Zhan X, Li D, Zhao J, Wei H, Alzan H, He L. Establishment and Application of an Indirect Enzyme-Linked Immunosorbent Assay for Measuring GPI-Anchored Protein 52 (P52) Antibodies in Babesia gibsoni-Infected Dogs. Animals. 2022; 12(9):1197. https://doi.org/10.3390/ani12091197
Chicago/Turabian StyleLiu, Qin, Xueyan Zhan, Dongfang Li, Junlong Zhao, Haiyong Wei, Heba Alzan, and Lan He. 2022. "Establishment and Application of an Indirect Enzyme-Linked Immunosorbent Assay for Measuring GPI-Anchored Protein 52 (P52) Antibodies in Babesia gibsoni-Infected Dogs" Animals 12, no. 9: 1197. https://doi.org/10.3390/ani12091197
APA StyleLiu, Q., Zhan, X., Li, D., Zhao, J., Wei, H., Alzan, H., & He, L. (2022). Establishment and Application of an Indirect Enzyme-Linked Immunosorbent Assay for Measuring GPI-Anchored Protein 52 (P52) Antibodies in Babesia gibsoni-Infected Dogs. Animals, 12(9), 1197. https://doi.org/10.3390/ani12091197







