Anti-Müllerian Hormone Inhibits FSH-Induced Cumulus Oocyte Complex In Vitro Maturation and Cumulus Expansion in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Collection and In Vitro Maturation of Cumulus Oocyte Complexes (COCs)
2.4. RNA Isolation and Quantitative Reverse-Transcription PCR Analysis
2.5. Detection of MPF and cAMP Contents in Oocytes
2.6. Evaluation of Cumulus Expansion
2.7. Measurement of Estrogen and Progesterone
2.8. Statistical Analysis
3. Results
3.1. Expression of Amh and Amhr2 in COCs, CCs and Cumulus-Free Oocytes
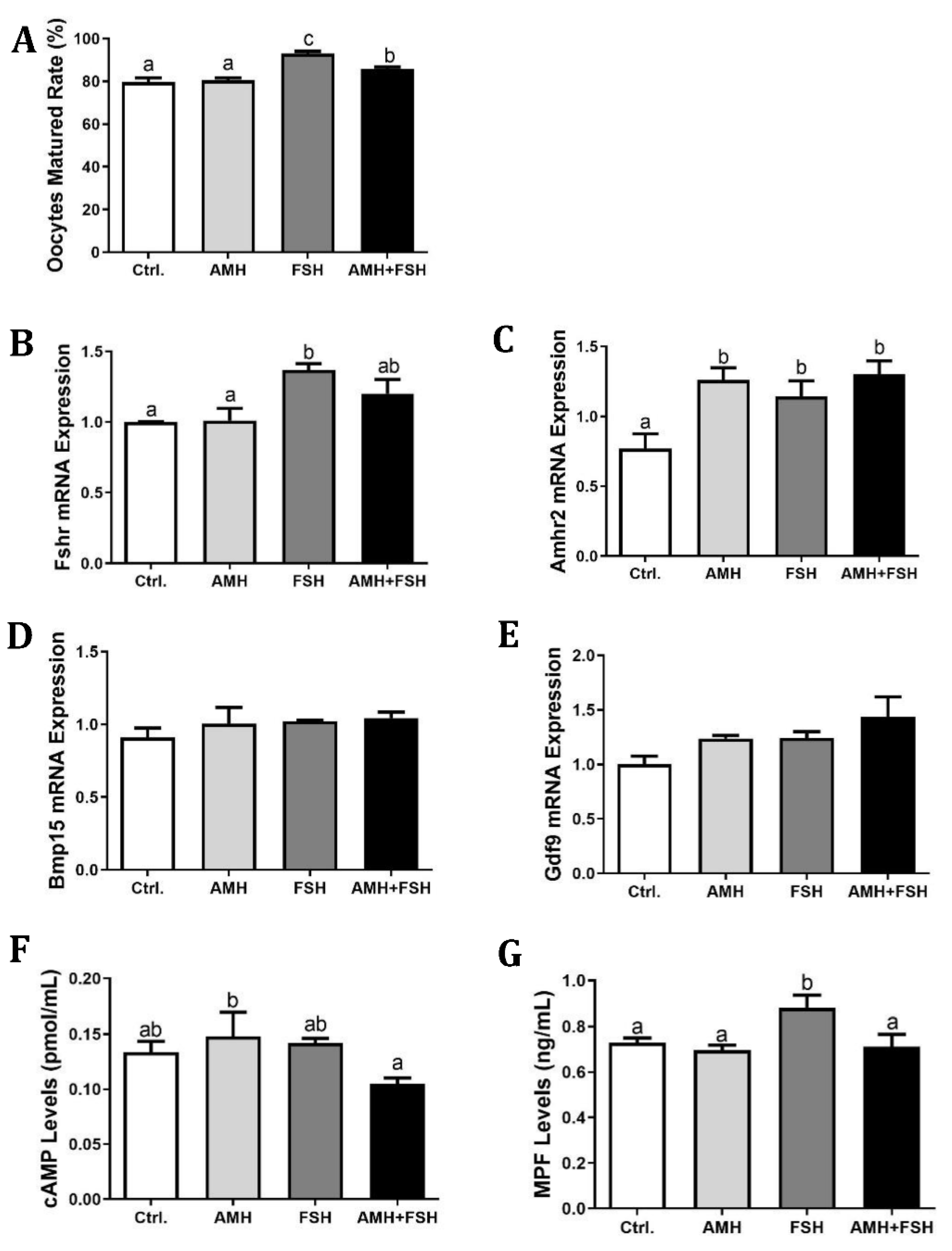
3.2. Effect of AMH on In Vitro Maturation of Cumulus Oocyte Complexes
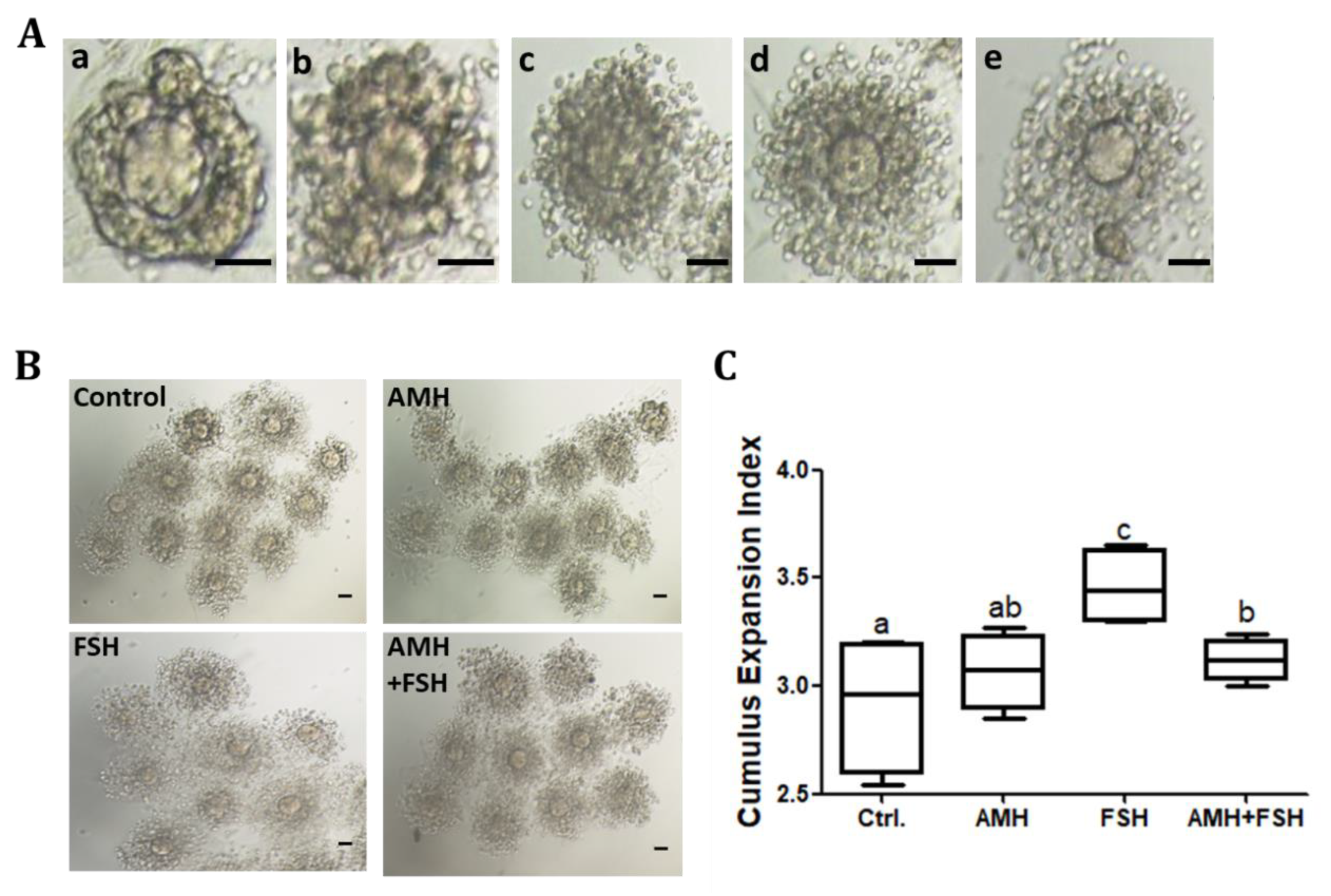
3.3. Effect of AMH on Cumulus Expansion of Cumulus Oocyte Complexes
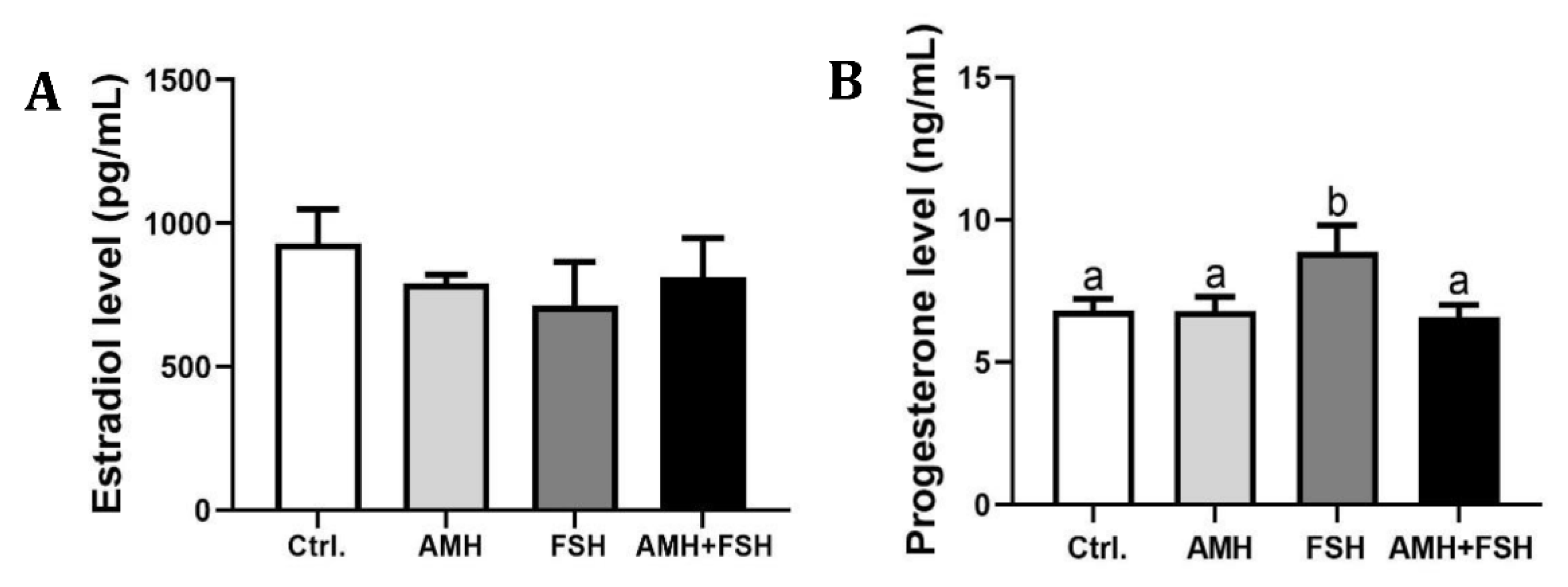
3.4. The Role of AMH in Regulation of Estradiol and Progesterone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cate, R.L.; Mattaliano, R.J.; Hession, C.; Tizard, R.; Farber, N.M.; Cheung, A.; Ninfa, E.G.; Frey, A.Z.; Gash, D.J.; Chow, E.P.; et al. Isolation of the bovine and human genes for müllerian inhibiting substance and expression of the human gene in animal cells. Cell 1986, 45, 685–698. [Google Scholar] [CrossRef]
- MacLaughlin, D.T.; Donahoe, P.K. Mullerian Inhibiting Substance: An Update. Adv. Exp. Med. Biol. 2002, 511, 25–38. [Google Scholar] [CrossRef]
- Lee, M.M.; Donahoe, P.K. Mullerian Inhibiting Substance: A Gonadal Hormone with Multiple Functions. Endocr. Rev. 1993, 14, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.M.; Behringer, R.R. A gene regulatory network for Müllerian duct regression. Environ. Epigenetics 2019, 5, dvz017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiçicioǧlu, C.; Kutlu, T.; Baglam, E.; Bakacak, Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil. Steril. 2006, 85, 592–596. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; A Visser, J. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Fallat, M.E.; Siow, Y.; Marra, M.; Cook, C.; Carrillo, A. Müllerian-inhibiting substance in follicular fluid and serum: A comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil. Steril. 1997, 67, 962–965. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Margalioth, E.J.; Gal, M.; Ben-Chetrit, A.; Algur, N.; Zylber-Haran, E.; Brooks, B.; Huerta, M.; Spitz, I.M. Serum anti-Mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum. Reprod. 2005, 20, 1814–1819. [Google Scholar] [CrossRef]
- Fanchin, R.; Mendez Lozano, D.H.; Frydman, N.; Gougeon, A.; di Clemente, N.; Frydman, R.; Taieb, J. Anti-Müllerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J. Clin. Endocrinol. Metab. 2007, 92, 1796–1802. [Google Scholar] [CrossRef] [Green Version]
- Kallio, S.; Aittomaki, K.; Piltonen, T.; Veijola, R.; Liakka, A.; Vaskivuo, T.E.; Dunkel, L.; Tapanainen, J.S. Anti-Müllerian hormone as a predictor of follicular reserve in ovarian insufficiency: Special emphasis on FSH-resistant ovaries. Hum. Reprod. 2012, 27, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Visser, J.A.; Schipper, I.; Laven, J.S.; Themmen, A.P. Anti-Müllerian hormone: An ovarian reserve marker in primary ovarian insufficiency. Nat. Rev. Endocrinol. 2012, 8, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.H.; Campbell, A.J.; Farrand, S.; McLennan, I.S. Anti-Müllerian hormone level in older women: Detection of granulosa cell tumor recurrence. Int. J. Gynecol. Cance. 2012, 22, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.H.; Lee, M.M.; Fuller, A.F., Jr.; Kehas, D.J.; Donahoe, P.K.; MacLaughlin, D.T. Diagnostic utility of Müllerian inhibiting substance determination in patients with primary and recurrent granulosa cell tumors. Gynecol. Oncol. 1999, 73, 51–55. [Google Scholar] [CrossRef]
- Jimenez-Krassel, F.; Scheetz, D.; Neuder, L.; Ireland, J.; Pursley, J.; Smith, G.; Tempelman, R.; Ferris, T.; Roudebush, W.; Mossa, F.; et al. Concentration of anti-Müllerian hormone in dairy heifers is positively associated with productive herd life. J. Dairy Sci. 2015, 98, 3036–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico, C.; Fabre, S.; Médigue, C.; di Clemente, N.; Clément, F.; Bontoux, M.; Touzé, J.L.; Dupont, M.; Briant, E.; Rémy, B.; et al. Anti-müllerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 2009, 80, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.; Carvalho, P.; Rozner, A.; Vieira, L.; Hackbart, K.; Bender, R.; Dresch, A.; Verstegen, J.; Shaver, R.; Wiltbank, M. Relationship between circulating anti-Müllerian hormone (AMH) and superovulatory response of high-producing dairy cows. J. Dairy Sci. 2015, 98, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Liang, A.; Salzano, A.; D’Esposito, M.; Comin, A.; Montillo, M.; Yang, L.; Campanile, G.; Gasparrini, B. Anti-Mullerian hormone (AMH) concentration in follicular fluid and mRNA expression of AMH receptor type II and LH receptor in granulosa cells as predictive markers of good buffalo (Bubalus Bubalis) donors. Theriogenology 2016, 86, 963–970. [Google Scholar] [CrossRef]
- Rico, C.; Drouilhet, L.; Salvetti, P.; Dalbiès-Tran, R.; Jarrier, P.; Touzé, J.L.; Pillet, E.; Ponsart, C.; Fabre, S.; Monniaux, D. Determination of anti-Müllerian hormone concentrations in blood as a tool to select Holstein donor cows for embryo production: From the laboratory to the farm. Reprod. Fertil. Dev. 2012, 24, 932–944. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Bisinotto, R.S.; Lima, F.S.; Greco, L.F.; Morrison, A.; Kumar, A.; Thatcher, W.W.; Santos, J.E.P. Plasma anti-Müllerian hormone in adult dairy cows and associations with fertility. J. Dairy Sci. 2014, 97, 6888–6900. [Google Scholar] [CrossRef] [Green Version]
- Baarends, W.M.; Uilenbroek, J.T.; Kramer, P.; Hoogerbrugge, J.W.; van Leeuwen, E.C.; Themmen, A.P.; Grootegoed, J.A. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 1995, 136, 4951–4962. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Visser, J.A.; Themmen, A.P. Regulation of ovarian function: The role of anti-Müllerian hormone. Reproduction 2002, 124, 601–609. [Google Scholar] [CrossRef]
- Behringer, R.R.; Cate, R.L.; Froelick, G.J.; Palmiter, R.D.; Brinster, R.L. Abnormal sexual development in transgenic mice chronically expressing Müllerian inhibiting substance. Nature 1990, 345, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Behringer, R.R.; Finegold, M.J.; Cate, R.L. Müllerian-inhibiting substance function during mammalian sexual development. Cell 1994, 79, 415–425. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; et al. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Grossman, M.P.; Nakajima, S.T.; Fallat, M.E.; Siow, Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil. Steril. 2008, 89, 1364–1370. [Google Scholar] [CrossRef]
- Chang, H.M.; Klausen, C.; Leung, P.C. Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil. Steril. 2013, 100, 585–592. [Google Scholar] [CrossRef]
- Yang, M.; Cushman, R.; Fortune, J. Anti-Müllerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol. Hum. Reprod. 2017, 23, 282–291. [Google Scholar] [CrossRef]
- Grøndahl, M.; Nielsen, M.E.; Canto, M.D.; Fadini, R.; Rasmussen, I.; Westergaard, L.; Kristensen, S.G.; Yding Andersen, C. Anti-Müllerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod. Biomed. 2011, 22, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Koide, S.S.; Donahoe, P.K. Müllerian inhibiting substance as oocyte meiosis inhibitor. Mol. Cell Endocrinol. 1986, 47, 225–234. [Google Scholar] [CrossRef]
- Tsafriri, A.; Picard, J.-Y.; Josso, N. Immunopurified Anti-Müllerian Hormone does not Inhibit Spontaneous Resumption of Meiosis in Vitro of Rat Oocytes1. Biol. Reprod. 1988, 38, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shao, L.; Xu, Y.; Cui, Y.; Liu, J.; Chian, R.-C. Effect of Anti-Mullerian Hormone in Culture Medium on Quality of Mouse Oocytes Matured In Vitro. PLoS ONE 2014, 9, e99393. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fagbohun, C.F.; Downs, S.M. Maturation of the Mouse Oocyte-Cumulus Cell Complex: Stimulation by Lectins1. Biol. Reprod. 1990, 42, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.Y.; Schmidt, K.T.; Kristensen, S.G.; Rosendahl, M.; Byskov, A.G.; Ernst, E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum. Reprod. 2010, 25, 1282–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedem, A.; Yung, Y.; Yerushalmi, G.M.; Haas, J.; Maman, E.; Hanochi, M.; Hemi, R.; Orvieto, R.; Dor, J.; Hourvitz, A. Anti Mül-lerian Hormone (AMH) level and expression in mural and cumulus cells in relation to age. J. Ovarian. Res. 2014, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.H.; Hwang, J.L.; Seow, K.M.; Huang, L.W.; Hsieh, B.C.; Chen, H.J.; Tzeng, C.R.; Bai, C.H. Effect of incubation with dif-ferent concentrations and durations of FSH for in-vitro maturation of murine oocytes. Reprod. Biomed. 2011, 23, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ge, J.; Wei, J.; Liu, J.; Liu, C.; Ma, C.; Zhao, X.; Wei, Q.; Ma, B. Effect of FSH on E2/GPR30-mediated mouse oocyte maturation in vitro. Cell Signal. 2020, 66, 109464. [Google Scholar] [CrossRef]
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Brown, K.; Simpson, E.R.; Mason, H.D. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil. Steril. 2011, 96, 1246–1251. [Google Scholar] [CrossRef]
- Buccione, R.; Vanderhyden, B.C.; Caron, P.J.; Eppig, J.J. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev. Biol. 1990, 138, 16–25. [Google Scholar] [CrossRef]
- Shimada, M.; Umehara, T.; Hoshino, Y. Roles of epidermal growth factor (EGF)-like factor in the ovulation process. Reprod. Med. Biol. 2016, 15, 201–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yang, J.; Li, J.; Zhang, M. CREB activity is required for epidermal growth factor-induced mouse cumulus expansion. Mol. Reprod. Dev. 2019, 86, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Němcová, L.; Nagyová, E.; Petlach, M.; Tománek, M.; Procházka, R. Molecular Mechanisms of Insulin-Like Growth Factor 1 Promoted Synthesis and Retention of Hyaluronic Acid in Porcine Oocyte-Cumulus Complexes. Biol. Reprod. 2007, 76, 1016–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Seibel, M.M.; MacLaughlin, D.T.; Donahoe, P.K.; Ransil, B.J.; A Hametz, P.; Richards, C.J. The inhibitory effects of müllerian-inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 1992, 75, 911–917. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer | Sequence 5′–3′ | Product Length (bp) | Accession No. |
|---|---|---|---|---|
| Amh | Forward | TACTCGGGACACCCGCTATT | 110 | NM_007445.3 |
| Reverse | TCAGGGTGGCACCTTCTCT | |||
| Amhr2 | Forward | GCAGCACAAGTATCCCCAAAC | 204 | NM_001356575.1 |
| Reverse | GTCTCGGCATCCTTGCATCTC | |||
| Fshr | Forward | AGGTACAGCTCTGCCATGCT | 171 | NM_013523.3 |
| Reverse | GTACGAGGAGGGCCATAACA | |||
| Gdf9 | Forward | TGGAACACTTGCTCAAATCGG | 106 | XM_006532220.5 |
| Reverse | GACATGGCCTCCTTTACCACA | |||
| Bmp15 | Forward | GAAAATGGTGAGGCTGGTAAAG | 153 | NM_009757.5 |
| Reverse | AGATGAAGTTGATGGCGGTAAA | |||
| Ptx3 | Forward | TTTGGAAGCGTGCATCCTGT | 186 | NM_008987.3 |
| Reverse | GTTCTCCTTTCCACCCACCA | |||
| Ptgs2 | Forward | GTTCATCCCTGACCCCCAAG | 193 | NM_011198.4 |
| Reverse | TCCATCCTTGAAAAGGCGCA | |||
| Tnfaip6 | Forward | GCTCAACAGGAGTGAGCGAT | 166 | NM_009398.2 |
| Reverse | CTGACCGTACTTGAGCCGAA | |||
| Has2 | Forward | GACGACAGGCACCTTACCAA | 116 | NM_008216.3 |
| Reverse | TGCTGGTTCAGCCATCTCAG | |||
| β-actin | Forward | TAAAGACCTCTATGCCAACACAGT | 241 | NM_007393.5 |
| Reverse | CACGATGGAGGGGCCGGACTCATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Li, Z.; Zhao, X.; Hua, L.; Liu, S.; He, C.; Yang, L.; Davis, J.S.; Liang, A. Anti-Müllerian Hormone Inhibits FSH-Induced Cumulus Oocyte Complex In Vitro Maturation and Cumulus Expansion in Mice. Animals 2022, 12, 1209. https://doi.org/10.3390/ani12091209
Yu X, Li Z, Zhao X, Hua L, Liu S, He C, Yang L, Davis JS, Liang A. Anti-Müllerian Hormone Inhibits FSH-Induced Cumulus Oocyte Complex In Vitro Maturation and Cumulus Expansion in Mice. Animals. 2022; 12(9):1209. https://doi.org/10.3390/ani12091209
Chicago/Turabian StyleYu, Xue, Zan Li, Xinzhe Zhao, Liping Hua, Shuanghang Liu, Changjiu He, Liguo Yang, John S. Davis, and Aixin Liang. 2022. "Anti-Müllerian Hormone Inhibits FSH-Induced Cumulus Oocyte Complex In Vitro Maturation and Cumulus Expansion in Mice" Animals 12, no. 9: 1209. https://doi.org/10.3390/ani12091209






