Social Behavior and Group Formation in Male Asian Elephants (Elephas maximus): The Effects of Age and Musth in Wild and Zoo-Housed Animals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Subjects
2.2. Observation Protocols
2.3. Data Analysis
2.3.1. Effect of Age on Musth
2.3.2. Effect of Age and Musth Status on Wild Social Group Composition
2.3.3. Factors Influencing Male Social Behavior
3. Results
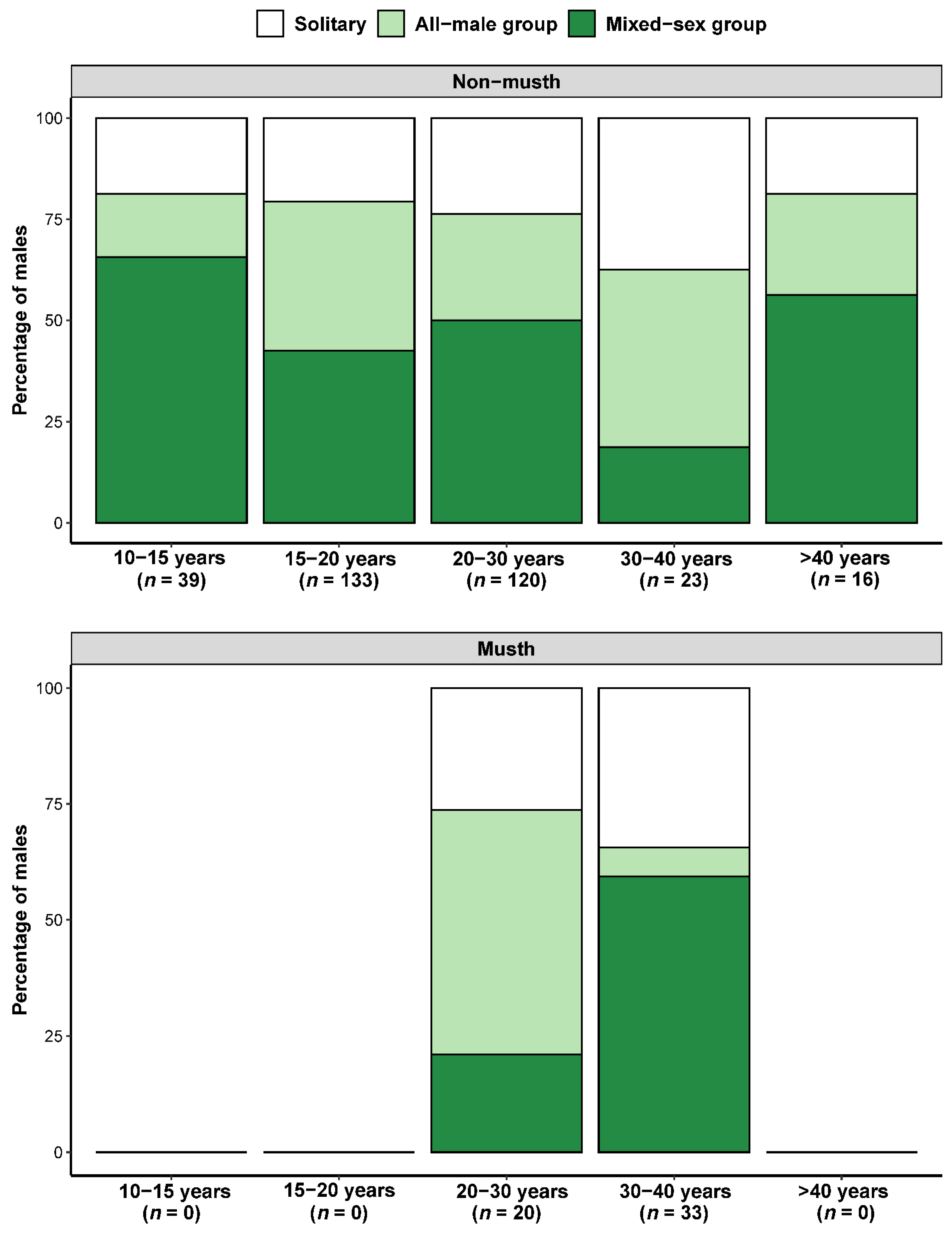
3.1. Effect of Age and Musth Status on Wild Social Group Composition
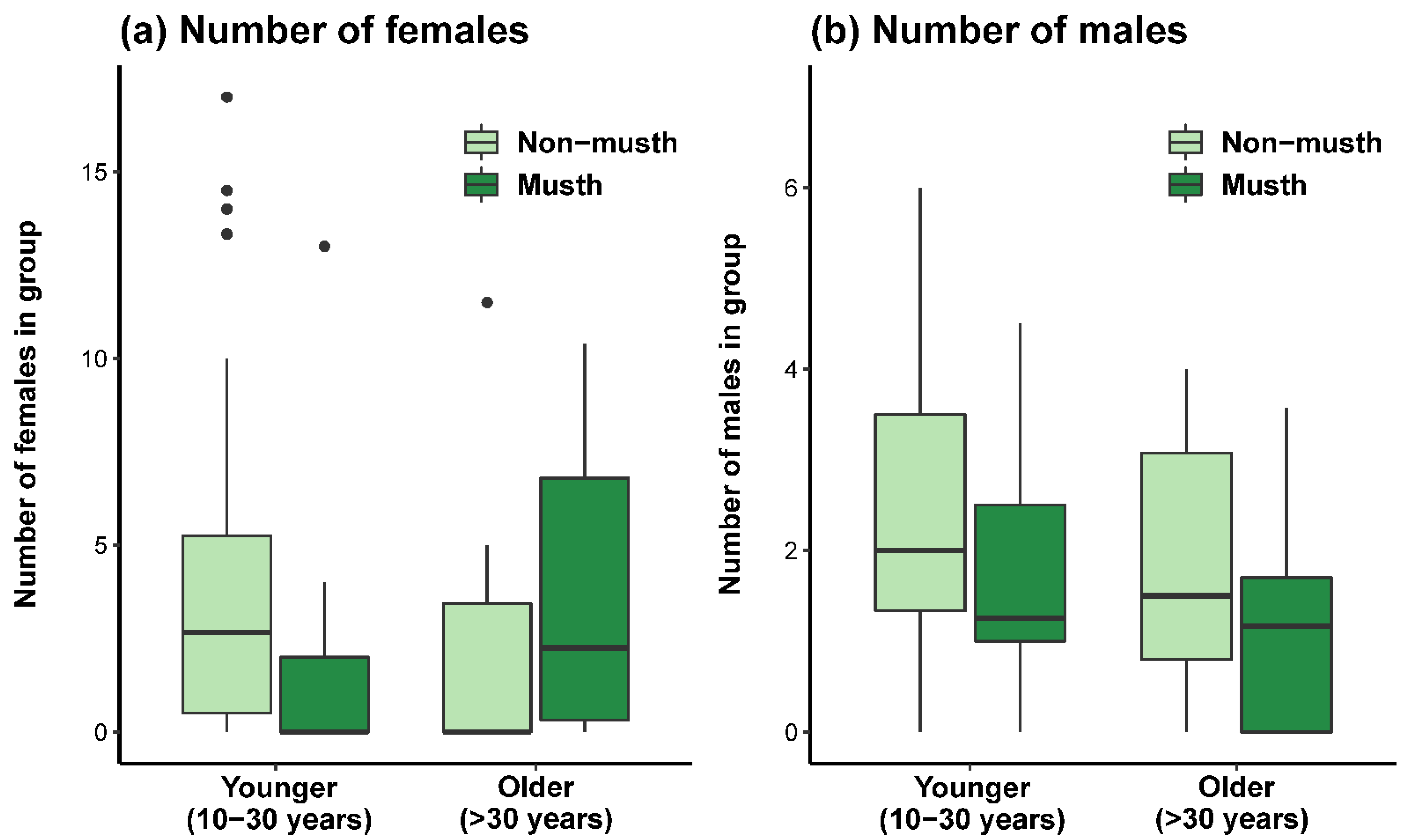
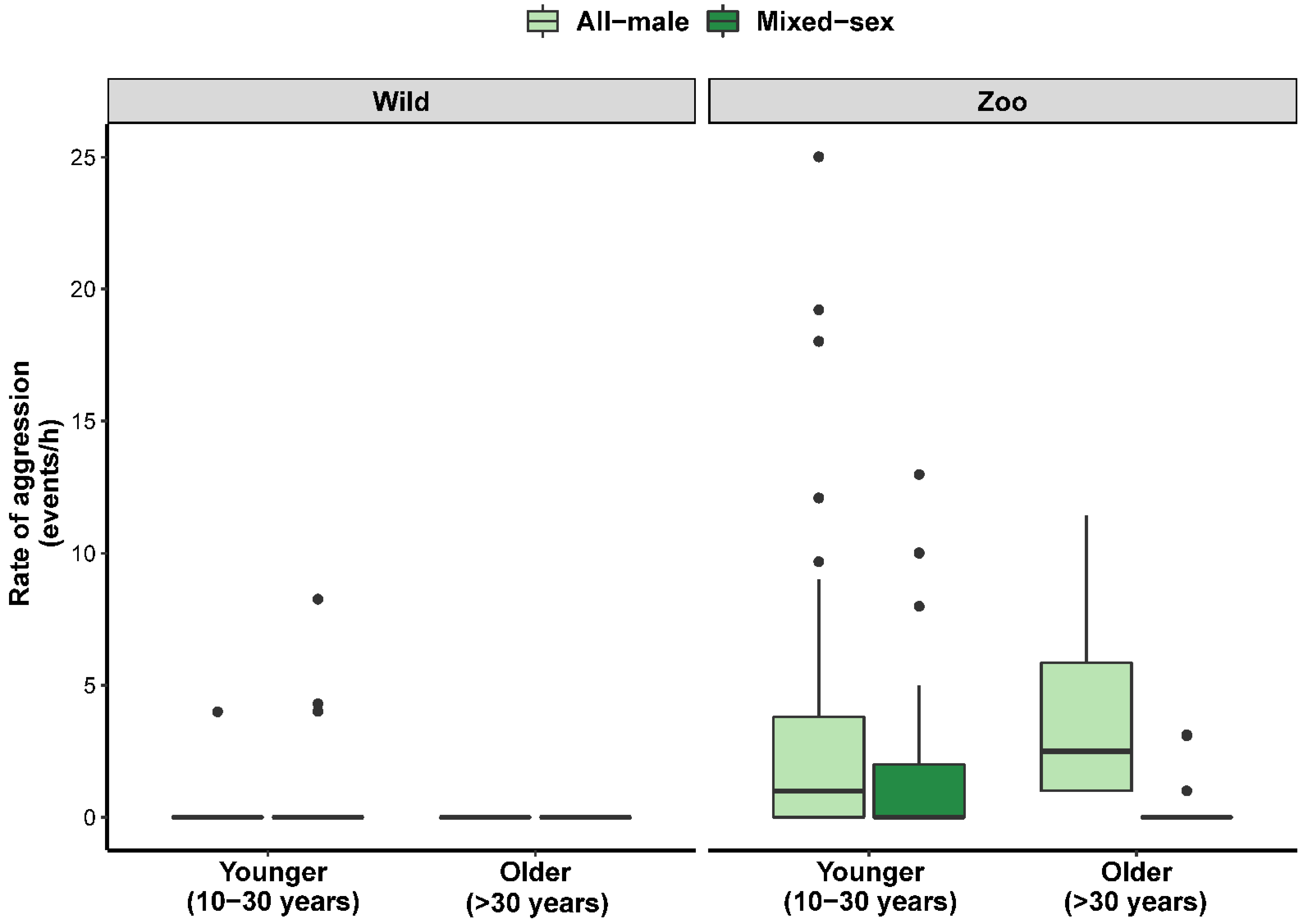
3.2. Factors Influencing Male Social Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chelliah, K.; Sukumar, R. Interplay of male traits, male mating strategies and female mate choice in the Asian elephant. Elephas Maximus Behav. 2015, 152, 1113–1144. [Google Scholar] [CrossRef]
- Chelliah, K.; Sukumar, R. The role of tusks, musth and body size in male-male competition among Asian elephants. Elephas Maximus Anim. Behav. 2013, 86, 1207–1214. [Google Scholar] [CrossRef]
- Sukumar, R. The Living Elephants: Evolutionary Ecology, Behavior, and Conservation; Oxford University Press: Oxford, UK, 2003; p. 478. [Google Scholar]
- de Silva, S.; Ranjeewa, A.D.G.; Kryazhimskiy, S. The dynamics of social networks among female Asian elephants. BMC Ecol. 2011, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandini, S.; Keerthipriya, P.; Vidya, T.N.C. Group size differences may mask similarities in social structure: A comparison of female elephant societies. Behav. Ecol. 2018, 29, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Srinivasaiah, N.; Kumar, V.; Vaidyanathan, S.; Sukumar, R. All-male groups in Asian elephants: A novel, adaptive social strategy in increasingly anthropogenic landscapes of southern India. Sci. Rep. 2019, 9, 8678. [Google Scholar] [CrossRef] [Green Version]
- Keerthipriya, P.; Nandini, S.; Vidya, T.N.C. Effects of male age and female presence on male associations in a large, polygynous mammal in southern India: The Asian elephant. Front. Ecol. Evol. 2021, 9, 616666. [Google Scholar] [CrossRef]
- Katugaha, H.I.E.; Silva, M.D.; Santiapillai, C. A long-term study on the dynamics of the elephant (Elephas maximus) population in Ruhuna National Park, Sri Lanka. Biol. Conserv. 1999, 89, 51–59. [Google Scholar] [CrossRef]
- Eisenberg, J.F.; McKay, G.M.; Jainudeen, M.R. Reproductive behavior of the Asiatic elephant (Elephas maximus maximus L.). Behaviour 1971, 38, 193–225. [Google Scholar]
- Jainudeen, M.R.; McKay, G.M.; Eisenberg, J.F. Observations on musth in the domesticated Asiatic elephant (Elephas maximus). Mammalia 1972, 36, 247–261. [Google Scholar] [CrossRef]
- Jainudeen, M.R.; Katongole, C.B.; Short, R.V. Plasma testosterone levels in relation to musth and sexual activity in the male Asiatic elephant. Elephas Maximus J. Reprod. Fertil. 1972, 29, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L. Comparative reproductive biology of elephants. In Reproductive Sciences in Animal Conservation: Progress and Prospects; Holt, W.V., Brown, J.L., Comizzoli, P., Eds.; Springer: New York, NY, USA, 2014; pp. 135–169. [Google Scholar] [CrossRef]
- Brown, J.L.; Somerville, M.; Riddle, H.S.; Keele, M.; Duer, C.K.; Freeman, E.W. Comparative endocrinology of testicular, adrenal and thyroid function in captive Asian and African elephant bulls. Gen. Comp. Endocrinol. 2007, 151, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Chave, E.; Edwards, K.L.; Paris, S.; Prado, N.; Morfeld, K.A.; Brown, J.L. Variation in metabolic factors and gonadal, pituitary, thyroid, and adrenal hormones in association with musth in African and Asian elephant bulls. Gen. Comp. Endocrinol. 2019, 276, 1–13. [Google Scholar] [CrossRef] [PubMed]
- LaDue, C.A.; Vandercone, R.P.G.; Kiso, W.K.; Freeman, E.W. Behavioral characterization of musth in Asian elephants (Elephas maximus): Defining progressive stages of male sexual behavior in in-situ and ex-situ populations. Appl. Anim. Behav. Sci. 2022, 251, 105639. [Google Scholar] [CrossRef]
- LaDue, C.A.; Schulte, B.A.; Kiso, W.K.; Freeman, E.W. Musth and sexual selection in elephants: A review of signaling properties and potential fitness consequences. Behaviour 2022, 159, 207–242. [Google Scholar] [CrossRef]
- Hollister-Smith, J.A.; Poole, J.H.; Archie, E.A.; Vance, E.A.; Georgiadis, N.J.; Moss, C.J.; Alberts, S.C. Age, musth and paternity success in wild male African elephants, Loxodonta Africana. Anim. Behav. 2007, 74, 287–296. [Google Scholar] [CrossRef]
- Rasmussen, H.B.; Okello, J.B.A.; Wittemyer, G.; Siegismund, H.R.; Arctander, P.; Vollrath, F.; Douglas-Hamilton, I. Age- and tactic-related paternity success in male African elephants. Behav. Ecol. 2008, 19, 9–15. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Schulte, B.A. Applying knowledge of mammalian social organization, mating systems, and communication to management. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd ed.; Kleiman, D.G., Thompson, K.V., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 329–343. [Google Scholar]
- Schulte-Hostedde, A.I.; Mastromonaco, G.F. Integrating evolution in the management of captive zoo populations. Evol. Appl. 2015, 8, 413–422. [Google Scholar] [CrossRef]
- Keerthipriya, P.; Nandini, S.; Gautam, H.; Revathe, T.; Vidya, T.N.C. Musth and its effects on male–male and male–female associations in Asian elephants. J. Mammal. 2020, 101, 259–270. [Google Scholar] [CrossRef]
- Fernando, P.; Wikramanayake, E.D.; Janaka, H.K.; Jayasinghe, L.K.A.; Gunawardena, M.; Kotagama, S.W.; Weerakoon, D.; Pastorini, J. Ranging behavior of the Asian elephant in Sri Lanka. Mamm. Biol. 2008, 73, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, R.; Joshi, N.V.; Krishnamurthy, V. Growth in the Asian elephant. Proc. Indian Acad. Sci. (Anim. Sci.) 1988, 97, 561–571. [Google Scholar] [CrossRef]
- Mumby, H.S.; Chapman, S.N.; Crawley, J.A.H.; Mar, K.U.; Htut, W.; Soe, A.T.; Aung, H.H.; Lummaa, V. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evol. Biol. 2015, 15, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, L.E.L.; Krishnamurthy, V. How chemical signals integrate Asian elephant society: The known and the unknown. Zoo Biol. 2000, 19, 405–423. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Riddle, H.S.; Krishnamurthy, V. Mellifluous matures to malodorous in musth. Nature 2002, 415, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.R.; Comeskey, D.; Hunt, M.B.; Rasmussen, L.E.L. Chirality in elephant pheromones. Nature 2005, 438, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Chiyo, P.I.; Moss, C.J.; Alberts, S.C. The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS ONE 2012, 7, e31382. [Google Scholar] [CrossRef]
- Chiyo, P.I.; Wilson, J.W.; Archie, E.A.; Lee, P.C.; Moss, C.J.; Alberts, S.C. The influence of forage, protected areas, and mating prospects on grouping patterns of male elephants. Behav. Ecol. 2014, 25, 1494–1504. [Google Scholar] [CrossRef] [Green Version]
- Ekanayaka, S.K.K.; Campos-Arceiz, A.; Rupasinghe, M.; Pastorini, J.; Fernando, P. Patterns of crop raiding by Asian elephants in a human-dominated landscape in southeastern Sri Lanka. Gajah 2011, 34, 20–25. [Google Scholar]
- Sukumar, R.; Gadgil, M. Male-female differences in foraging on crops by Asian elephants. Anim. Behav. 1988, 36, 1233–1235. [Google Scholar] [CrossRef]
- LaDue, C.A.; Eranda, I.; Jayasinghe, C.; Vandercone, R.P.G. Mortality patterns of Asian elephants in a region of human–Elephant conflict. J. Wildl. Manag. 2021, 85, 794–802. [Google Scholar] [CrossRef]
- Williams, C.; Tiwari, S.K.; Goswami, V.R.; de Silva, S.; Kumar, A.; Baskaran, N.; Yoganand, K.; Menon, V. Elephas maximus. In The IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2020. [Google Scholar]
- Menon, V.; Tiwari, S.K. Population status of Asian elephants Elephas maximus and key threats. Int. Zoo Yearb. 2019, 53, 17–30. [Google Scholar] [CrossRef]
- Mumby, H.S.; Plotnik, J.M. Taking the elephants’ perspective: Remembering elephant behavior, cognition and ecology in human-elephant conflict mitigation. Front. Ecol. Evol. 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- AsERSM. Asian Elephant Range States Meeting Final Report; Ministry of Environment and Forestry, Government of Indonesia: Jakarta, Indonesia, 2017; p. 67. [Google Scholar]
- Conley, S. Conservation philosophy and activities of the International Elephant Foundation. Int. Zoo Yearb. 2019, 53, 208–216. [Google Scholar] [CrossRef]
- Hutchins, M. Variation in nature: Its implications for zoo elephant management. Zoo Biol. 2006, 25, 161–171. [Google Scholar] [CrossRef]
- Sukumar, R. A brief review of the status, distribution and biology of wild Asian elephants. Int. Zoo Yearb. 2006, 40, 1–8. [Google Scholar] [CrossRef]
- Nordin, C. Asian Elephant-2016 North American Regional Studbook; Saint Louis Zoo: St. Louis, MO, USA, 2017; p. 149. [Google Scholar]
- Hartley, M.; Wood, A.; Yon, L. Facilitating the social behaviour of bull elephants in zoos. Int. Zoo Yearb. 2019, 53, 62–77. [Google Scholar] [CrossRef]
- Vidya, T.N.C.; Sukumar, R. Social organization of the Asian elephant (Elephas maximus) in southern India inferred from microsatellite DNA. J. Ethol. 2005, 23, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Poole, J.H. Rutting behavior in African elephants: The phenomenon of musth. Behaviour 1987, 102, 283–316. [Google Scholar] [CrossRef]
- Schreier, A.L.; Readyhough, T.S.; Moresco, A.; Davis, M.; Joseph, S. Social dynamics of a newly integrated bachelor group of Asian elephants (Elephas maximus): Welfare implications. J. Appl. Anim. Welf. Sci. 2021, 1–18. [Google Scholar] [CrossRef]
- Finnell, S.; Glaeser, S. Asian Elephant Musth Scale-Visible Signs; Oregon Zoo: Portland, OR, USA, 2016. [Google Scholar]
- Rasmussen, L.E.L.; Krishnamurthy, V. Urinary, temporal gland, and breath odors from Asian elephants of Mudumalai National Park. Gajah 2001, 20, 1–7. [Google Scholar]
- Varma, S.; Baskaran, N.; Sukumar, R. Field Key for Elephant Population Estimation and Age and Sex Classification: Resource Material for Synchronized Elephant Population Count Using Block Count, Line Transect Dung Count Method and Waterhole Count; Centre for Ecological Sciences, Indian Institute of Science: Bangalore, India, 2012; p. 24. [Google Scholar]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMonitor app. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- Bateson, M.; Martin, P. Measuring Behaviour: An Introductory Guide, 4th ed.; Cambridge University Press: Cambridge, UK, 2021; p. 238. [Google Scholar]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c); R package version 2.2-2; 2019. Available online: https://cran.r-project.org/web/packages/AICcmodavg/index.html (accessed on 5 May 2022).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bartón, K. MuMIn: Multi-Model Inference; 1.43.15; 2019. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 5 May 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer-Verlag: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, H. Delayed competitive breeding in roving males. J. Theor. Biol. 1994, 166, 127–133. [Google Scholar] [CrossRef]
- LaDue, C.A.; Scott, N.L.; Margulis, S.W. A survey of musth among captive male elephants in North America: Updated results and implications for management. J. Elephant Manag. Assoc. 2014, 25, 18–24. [Google Scholar]
- Scott, N.L.; Riddle, H. Assessment of musth in captivity: A survey of factors affecting the frequency and duration of musth in captive male elephants Elephas Maximus-Loxodonta Africana. J. Elephant Manag. Assoc. 2003, 14, 11–15. [Google Scholar]
- Kurt, F.; Garaï, M.E. The Asian Elephant in Captivity: A Field Study; Cambridge University Press India Pvt. Ltd.: New Delhi, India, 2007; p. 352. [Google Scholar]
- Silva, I.D.; Kuruwita, V.Y. Hematology, plasma, and serum biochemistry values in free-ranging elephants (Elephas maximus ceylonicus) in Sri Lanka. J. Zoo Wildl. Med. 1993, 24, 434–439. [Google Scholar]
- Dickerman, R.D.; Pernikoff, D.; Zachariah, N.Y.; McConathy, W.J.; Gracy, R.W.; Raven, P.V. Creatinine kinase and lactic dehydrogenase isozyme measurements in male Asian elephants (Elephas maximus) during musth and nonmusth. Clin. Chem. 1994, 40, 989. [Google Scholar]
- Slotow, R.; van Dyk, G.; Poole, J.; Page, B.; Klocke, A. Older bull elephants control young males. Nature 2000, 408, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Ruckstuhl, K.E.; Neuhaus, P. Sexual segregation in ungulates: A comparative test of three hypotheses. Biol. Rev. 2002, 77, 77–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arivazhagan, C.; Sukumar, R. Constructing age structures of Asian elephant populations: A comparison of two field methods of age estimation. Gajah 2008, 29, 11–16. [Google Scholar]
- Sukumar, R. Ecology of the Asian elephant in southern Indian. I. Movement and habitat utilization patterns. J. Trop. Ecol. 1989, 5, 1–18. [Google Scholar] [CrossRef]
- Evans, K.E.; Harris, S. Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. Anim. Behav. 2008, 76, 779–787. [Google Scholar] [CrossRef]
- Goldenberg, S.Z.; de Silva, S.; Rasmussen, H.B.; Douglas-Hamilton, I.; Wittemyer, G. Controlling for behavioural state reveals social dynamics among male African elephants, Loxodonta africana. Anim. Behav. 2014, 95, 111–119. [Google Scholar] [CrossRef]
- Allen, C.R.B.; Brent, L.J.N.; Motsentwa, T.; Weiss, M.N.; Croft, D.P. Importance of old bulls: Leaders and followers in collective movements of all-male groups in African savannah elephants (Loxodonta africana). Sci. Rep. 2020, 10, 13996. [Google Scholar] [CrossRef]
- Poole, J.H.; Lee, P.C.; Njiraini, N.; Moss, C.J. Longevity, competition, and musth: A long-term perspective on male reproductive strategies. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H., Lee, P.C., Eds.; The University of Chicago Press: Chicago, IL, USA, 2011; pp. 272–286. [Google Scholar]
- Sukumar, R. The Asian Elephant: Ecology and Management; Cambridge University Press: Cambridge, UK, 1989; p. 255. [Google Scholar]
- Nandini, S.; Keerthipriya, P.; Vidya, T.N.C. Seasonal variation in female Asian elephant social structure in Nagarahole-Bandipur, southern India. Anim. Behav. 2017, 134, 135–145. [Google Scholar] [CrossRef]
- Brown, J.L.; Corea, R.; Dangolla, A.; Easwaran, E.K.; Mikota, S.; Oo, Z.M.; Sarma, K.; Thitaram, C. Management and care of captive Asian elephant bulls in musth. Gajah 2020, 52, 60–63. [Google Scholar]
- Rajaram, A. Musth in elephants. Resonance 2006, 11, 18–27. [Google Scholar] [CrossRef]
- Santiapillai, C.; Read, B.; Jacobson, G.; Wijeyamohan, S.; Rambukpotha, S. A paradigm shift in the management of musth among bull elephants in captivity in Sri Lanka. Ceylon J. Sci. (Biol. Sci.) 2011, 40, 25–32. [Google Scholar] [CrossRef]
- Gore, M.; Hutchins, M.; Ray, J. A review of injuries caused by elephants in captivity: An examination of predominant factors. Int. Zoo Yearb. 2006, 40, 51–62. [Google Scholar] [CrossRef]
- Readyhough, T.S.; Joseph, S.; Davis, M.; Moresco, A.; Schreier, A.L. Impacts of socialization on bull Asian elephant (Elephas maximus) stereotypical behavior. J. Zool. Bot. Gard. 2022, 3, 113–130. [Google Scholar] [CrossRef]
- Keerthipriya, P. Associations, Dominance Interactions, and Musth in Male ASIAN Elephants in Nagarahole and Bandipur National Parks, Southern India; Jawaharlal Nehru Centre for Advanced Scientific Research: Bangalore, India, 2018. [Google Scholar]
- Schulte, B.A.; Rasmussen, L.E.L. Signal-receiver interplay in the communication of male condition by Asian elephants. Anim. Behav. 1999, 57, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.; Moore, R.; Harris, S. The social and ecological integration of captive-raised adolescent male African elephants (Loxodonta africana) into a wild population. PLoS ONE 2013, 8, e55933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, B.A.; LaDue, C.A. The chemical ecology of elephants: 21st century additions to our understanding and future outlooks. Animals 2021, 11, 2860. [Google Scholar] [CrossRef]
- Stoeger, A.S. Elephant sonic and infrasonic sound production, perception, and processing. In Neuroendocrine Regulation of Animal Vocalization: Mechanisms and Anthropogenic Factors in Animal Communication; Rosenfeld, C.S., Hoffmann, F., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2021; pp. 189–199. [Google Scholar]
- de Silva, S. Acoustic communication in the Asian elephant. Elephas Maximus Maximus Behav. 2010, 147, 825–852. [Google Scholar]
- Taylor, L.A.; Vollrath, F.; Lambert, B.; Lunn, D.; Douglas-Hamilton, I.; Wittemyer, G. Movement reveals reproductive tactics in male elephants. J. Anim. Ecol. 2020, 89, 57–67. [Google Scholar] [CrossRef]
- Fernando, P.; De Silva, M.K.C.R.; Jayasinghe, L.K.A.; Janaka, H.K.; Pastorini, J. First country-wide survey of the endangered Asian elephant: Towards better conservation and management in Sri Lanka. Oryx 2021, 55, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, R. The management of large mammals in relation to male strategies and conflict with people. Biol. Conserv. 1991, 55, 93–102. [Google Scholar] [CrossRef]
- Schulte, B.A. Social structure and helping behavior in captive elephants. Zoo Biol. 2000, 19, 447–459. [Google Scholar] [CrossRef]
- Scott, N.L.; LaDue, C.A. The behavioral effects of exhibit size versus complexity in African elephants: A potential solution for smaller spaces. Zoo Biol. 2019, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Thevarajah, S.J.; Readyhough, T.S.; Davis, M.; Moresco, A.; Joseph, S.; Schreier, A.L. Nighttime behavior and the length of social relationships in male Asian elephants. J. Appl. Anim. Welf. Sci. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]


| Category | Send | Receive | Behavior | Definition |
|---|---|---|---|---|
| Aggression | ✓ | Bite | Use teeth to contact another elephant | |
| ✓ | Head-butt | Use forehead and/or base of trunk to contact another elephant | ||
| ✓ | Push | Other than with the head or trunk, use a part of the body to move another elephant (includes tusk and kick) | ||
| ✓ | Spar | Elephants face each other with raised chins, pulling and pushing with intertwined trunks; contact tusk(s)/tush(es) with another elephant, accompanied by a forward lunging motion; focal animal initiates interaction | ||
| ✓ | Trunk swing | Throw trunk out quickly in direction of elephant within one body length away without contact | ||
| Prosocial behavior | ✓ | ✓ | Approach | Within one body length of another elephant, locomote towards elephant, without recipient moving away |
| ✓ | ✓ | Rub | Use head, body, and/or leg(s) to contact another elephant for more than one sec | |
| ✓ | ✓ | Trunk entwine | Wrap trunks mutually (other than in sparring context) | |
| ✓ | ✓ | Trunk touch | Trunk tip touches or attempts to touch another elephant on one of the following: anus, body, ear, genitals, head, mouth, temporal gland, trunk, other | |
| Dominance behavior | ✓ | Chase | Rapid pursuit of another elephant that is moving away from focal animal | |
| ✓ | Displace | Move to within one body length of another elephant, who apparently leaves as a result of the proximity | ||
| ✓ | Hoard | Prevent another elephant from using a resource, either actively (through quick contact) or passively (body positioning) (e.g., food, water, mud wallow) | ||
| ✓ | Lead | Be followed within one body length of another elephant for at least two body lengths in distance | ||
| ✓ | Steal | Take resource from another elephant that is actively consuming that resource (e.g., food, water, mud wallow), preventing them from using it | ||
| ✓ | Back-up | Conspecific walks backward to within one body length of focal elephant (or rump turned toward focal elephant in proximity) | ||
| ✓ | Leave | Conspecific moves away from focal elephant that is within one body length | ||
| ✓ | Share | While consuming a resource, another elephant allows focal animal to use same resource (e.g., food, water, mud wallow) | ||
| Submissive behavior | ✓ | Back-up | Walk backward to within one body length of conspecific (or turn rump towards elephant in proximity) | |
| ✓ | Bite | Another elephant uses teeth to contact focal elephant | ||
| ✓ | Chase | Conspecific rapidly pursues focal elephant, and the focal elephant is moving away from the initiator | ||
| ✓ | Displace | Conspecific moves to within one body length of focal elephant, who apparently leaves as a result of the proximity | ||
| ✓ | Head-butt | Conspecific uses forehead and/or base of the trunk to contact focal elephant | ||
| ✓ | Hoard | Conspecific prevents focal elephant from using a resource, either actively (through quick contact) or passively (body positioning) (e.g., food, water, mud wallow) | ||
| ✓ | Lead | Follow within one body length of another elephant for at least two body lengths in distance | ||
| ✓ | Mount | Another elephant places forelegs on back of focal elephant | ||
| ✓ | Push | Other than with the head or trunk, conspecific uses a part of the body to move focal elephant (includes tusk and kick) | ||
| ✓ | Spar | Elephants face each other with raised chins, pulling and pushing with intertwined trunks; contact tusk(s)/tush(es) with another elephant, accompanied by a forward lunging motion; conspecific initiates interaction | ||
| ✓ | Steal | Conspecific takes a resource from focal elephant that is actively consuming that resource (e.g., food, water, mud wallow), preventing them from using it | ||
| ✓ | Trunk swing | Conspecific throws trunk out quickly in the direction of focal elephant less than one body length away without contact | ||
| ✓ | Trunk over back | Conspecific places at least two-thirds of trunk over the back, head, or neck of the focal elephant |
| Fixed Effect | Estimate | SE | t-Value | |
|---|---|---|---|---|
| (a) Number of females in group | Intercept | 5.680 | 1.155 | 4.918 |
| Musth | 3.305 | 1.393 | 2.373 | |
| 15–20 year | –1.948 | 1.461 | –1.334 | |
| 20–30 year | –2.953 | 1.426 | –2.070 | |
| 30–40 year | –5.060 | 1.785 | –2.834 | |
| >40 year | 1.785 | 3.055 | 0.584 | |
| Musth: 15–20 year | — | — | — | |
| Musth: 20–30 year | –4.195 | 1.804 | –2.325 | |
| Musth: 30–40 year | — | — | — | |
| Musth: >40 year | — | — | — | |
| (b) Number of males in group | Intercept | 2.088 | 0.451 | 4.626 |
| Musth | –0.791 | 0.759 | –1.042 | |
| 15–20 year | –0.173 | 0.571 | –0.304 | |
| 20–30 year | 0.632 | 0.565 | –1.119 | |
| 30–40 year | –0.056 | 0.739 | –0.075 | |
| >40 year | 0.198 | 1.194 | –0.166 | |
| Musth: 15–20 year | — | — | — | |
| Musth: 20–30 year | –0.433 | 0.965 | –0.448 | |
| Musth: 30–40 year | — | — | — | |
| Musth: >40 year | — | — | — |
| WILD | Est. | SE | t-Value | ZOO | Est. | SE | t-Value | |
|---|---|---|---|---|---|---|---|---|
| Rate aggression | Intercept | 0.922 | 0.252 | 3.660 | Intercept | 0.545 | 1.565 | 0.987 |
| 15–20 years | −0.751 | 0.314 | −2.393 | Age | 0.118 | 0.105 | 1.123 | |
| 20–30 years | −0.916 | 0.310 | −2.954 | Group (mixed) | −0.964 | 1.910 | −0.505 | |
| 30–40 years | −0.922 | 0.354 | −2.605 | Age:Group | 0.167 | 0.111 | 1.508 | |
| 40+ years | −0.922 | 0.532 | −1.731 | |||||
| Rate prosocial behavior | Intercept | 3.985 | 1.541 | 2.586 | Intercept | 6.387 | 3.806 | 1.678 |
| Eles present | 0.596 | 0.106 | 5.646 | Early musth | −34,250 | 7711 | −4.441 | |
| Full musth | 29,260 | 24,320 | 1.203 | |||||
| Post-musth | 13,230 | 9.044 | 1.463 | |||||
| Age | 0.0174 | 0.244 | 0.071 | |||||
| Group (mixed) | 4.292 | 4.452 | 0.964 | |||||
| Eles present | 3.968 | 0.806 | 4.921 | |||||
| Early musth:Age | 2920 | 657.5 | 4.442 | |||||
| Full musth:Age | −2418 | 2010 | −1.202 | |||||
| Early:Group | 34,250 | 7712 | 4.441 | |||||
| Full:Group | −29,270 | 24,320 | −1.204 | |||||
| Age:Group | −0.406 | 0.257 | −1.577 | |||||
| Early:Group:Age | −2920 | 657.5 | −4.442 | |||||
| Full:Age:Group | 2418.0 | 2010 | 1.203 | |||||
| Rate dominance behavior | Intercept | 2.102 | 0.403 | 5.218 | Intercept | 3.257 | 0.423 | 7.707 |
| Rate submissive behavior | Intercept | 1.316 | 0.360 | 3.652 | Intercept | 2.297 | 1.498 | 1.534 |
| Early musth | −19,420 | 3853 | −5.041 | |||||
| Full musth | −4.456 | 12,210 | −0.365 | |||||
| Post-musth | −0.254 | 4.079 | −0.062 | |||||
| Age | 0.079 | 0.100 | 0.791 | |||||
| Group (mixed) | −0.206 | 1.973 | −0.104 | |||||
| Eles present | 1656 | 328.5 | 5.042 | |||||
| Early musth:Age | 367.9 | 1009 | 0.364 | |||||
| Full musth:Age | 19,420 | 3853 | 5.041 | |||||
| Early:Group | 4453 | 12,210 | 0.365 | |||||
| Full:Group | −0.138 | 0.116 | −1.191 | |||||
| Age:Group | −1656 | 328.5 | −5.042 | |||||
| Early:Group:Age | −367.9 | 1009 | −0.364 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaDue, C.A.; Vandercone, R.P.G.; Kiso, W.K.; Freeman, E.W. Social Behavior and Group Formation in Male Asian Elephants (Elephas maximus): The Effects of Age and Musth in Wild and Zoo-Housed Animals. Animals 2022, 12, 1215. https://doi.org/10.3390/ani12091215
LaDue CA, Vandercone RPG, Kiso WK, Freeman EW. Social Behavior and Group Formation in Male Asian Elephants (Elephas maximus): The Effects of Age and Musth in Wild and Zoo-Housed Animals. Animals. 2022; 12(9):1215. https://doi.org/10.3390/ani12091215
Chicago/Turabian StyleLaDue, Chase A., Rajnish P. G. Vandercone, Wendy K. Kiso, and Elizabeth W. Freeman. 2022. "Social Behavior and Group Formation in Male Asian Elephants (Elephas maximus): The Effects of Age and Musth in Wild and Zoo-Housed Animals" Animals 12, no. 9: 1215. https://doi.org/10.3390/ani12091215
APA StyleLaDue, C. A., Vandercone, R. P. G., Kiso, W. K., & Freeman, E. W. (2022). Social Behavior and Group Formation in Male Asian Elephants (Elephas maximus): The Effects of Age and Musth in Wild and Zoo-Housed Animals. Animals, 12(9), 1215. https://doi.org/10.3390/ani12091215







