Repurposing Drugs in Small Animal Oncology
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Advantage of Repurposing Drugs in Oncology Is Very Clear—Low Cost, Low Side Effects, Easy Access, and Worldwide Availability [16]
3. Repurposed Drugs Investigated in Clinical Setting in Dogs and Cats
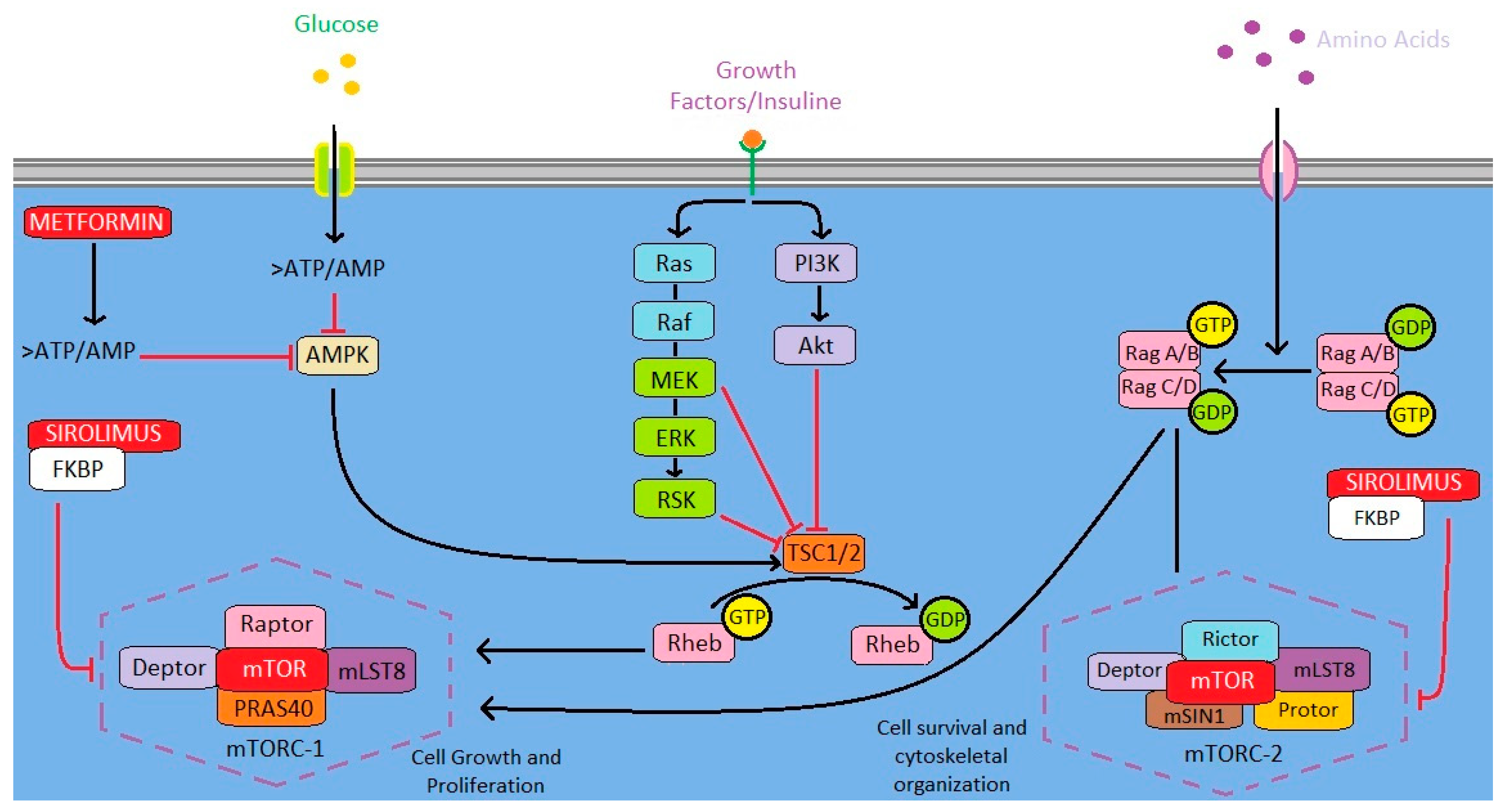
3.1. Auranofin
3.2. Desmopressin
3.3. Doxycycline
3.4. Losartan
3.5. Metformin
3.6. Sirolimus
3.7. Thalidomide
4. Possible Repurposed Drugs in Veterinary Oncology Not Yet Clinically Investigated in Dogs and Cats
4.1. Amlodipine
4.2. Amiloride
4.3. Propranolol
4.4. Statins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Xia, D.-K.; Hu, Z.-G.; Tian, Y.-F.; Zeng, F.-J. Statin use and prognosis of lung cancer: A systematic review and meta-analysis of observational studies and randomized controlled trials. Drug Des. Dev. Ther. 2019, 13, 405–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimers, M.S.; Bastiaannet, E.; van Herk-Sukel, M.; Lemmens, V.E.P.; Broek, C.B.M.V.D.; Van De Velde, C.J.H.; De Craen, A.J.M.; Liefers, G.J. Aspirin use after diagnosis improves survival in older adults with colon cancer: A retrospective cohort study. J. Am. Geriatr. Soc. 2012, 60, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Bastiaannet, E.; Sampieri, K.; Dekkers, O.M.; De Craen, A.J.M.; Van Herk-Sukel, M.P.P.; Lemmens, V.; Broek, C.B.M.V.D.; Coebergh, J.W.; Herings, R.M.C.; Van De Velde, C.J.H.; et al. Use of Aspirin postdiagnosis improves survival for colon cancer patients. Br. J. Cancer 2012, 106, 1564–1570. [Google Scholar] [CrossRef]
- Knapp, D.W.; Richardson, R.C.; Chan, T.C.; Bottoms, G.D.; Widmer, W.R.; DeNicola, D.B.; Teclaw, R.; Bonney, P.L.; Kuczek, T. Piroxicam Therapy in 34 Dogs With Transitional Cell Carcinoma of the Urinary Bladder. J. Vet. Intern. Med. 1994, 8, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Galván, D.C.; Ayyappan, A.P.; Bryan, B.A. Regression of primary cardiac angiosarcoma and metastatic nodules following propranolol as a single agent treatment. Oncoscience 2018, 5, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Banavali, S.; Pasquier, E.; Andre, N. Targeted therapy with propranolol and metronomic chemotherapy combination: Sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience 2015, 9, 499. [Google Scholar] [CrossRef]
- Huang, M.-R.; Ye, Y.-C.; Chen, S.-R.; Chai, J.-R.; Lu, J.-X.; Zhoa, L.; Gu, L.-J.; Wang, Z.-Y. Use of all-trans retinoic acid in the treatment of acute promyelocyte leukemia. Blood 1988, 72, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Meani, N.; Minardi, S.; Licciulli, S.; Gelmetti, V.; Coco, F.L.; Nervi, C.; Pelicci, P.G.; Müller, H.; Alcalay, M. Molecular signature of retinoic acid treatment in acute promyelocytic leukemia. Oncogene 2005, 24, 3358–3368. [Google Scholar] [CrossRef] [Green Version]
- Carella, A.M.; Beltrami, G.; Pica, G.; Carella, A.; Catania, G. Clarithromycin potentiates tyrosine kinase inhibitor treatment in patients with resistant chronic myeloid leukemia. Leuk. Lymphoma 2012, 53, 1409–1411. [Google Scholar] [CrossRef]
- Licht, J.D.; Shortt, J.; Johnstone, R. From anecdote to targeted therapy: The curious case of thalidomide in multiple myeloma. Cancer Cell 2014, 25, 9–11. [Google Scholar] [CrossRef]
- Hung, M.-S.; Chen, I.-C.; Lee, C.-P.; Huang, R.-J.; Chen, P.-C.; Tsai, Y.-H.; Yang, Y.-H. Statin improves survival in patients with EGFR-TKI lung cancer: A nationwide population-based study. PLoS ONE 2017, 12, e0171137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsou, Y.-A.; Chang, W.-C.; Lin, C.-D.; Chang, R.-L.; Tsai, M.-H.; Shih, L.-C.; Staniczek, T.; Wu, T.-F.; Hsu, H.-Y.; Chang, W.-D.; et al. Metformin increases survival in hypopharyngeal cancer patients with diabetes mellitus: Retrospective cohort study and cell-based analysis. Pharmaceuticals 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Dulskas, A.; Patasius, A.; Linkeviciute-Ulinskiene, D.; Zabuliene, L.; Urbonas, V.; Smailyte, G. Metformin increases cancer specific survival in colorectal cancer patients—National cohort study. Cancer Epidemiol. 2019, 62, 101587. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhou, J.; Gorak, E.J.; Quddus, F. Metformin Is Associated With Survival Benefit in Cancer Patients with Concurrent Type 2 Diabetes: A Systematic Review and Meta-Analysis. Oncologist 2013, 18, 1248–1255. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology—Patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015, 12, 732–742. [Google Scholar] [CrossRef]
- Gonzalez-Fierro, A.; Dueñas-González, A. Drug repurposing for cancer therapy, easier said than done. Semin. Cancer Biol. 2019, 68, 123–131. [Google Scholar] [CrossRef]
- Strasser-Weippl, K.; Chavarri-Guerra, Y.; Villarreal-Garza, C.; Bychkovsky, B.; Debiasi, M.; Liedke, P.E.R.; Soto-Perez-De-Celis, E.; Dizon, D.; Cazap, E.; Lopes, G.D.L.; et al. Progress and remaining challenges for cancer control in Latin America and the Caribbean. Lancet Oncol. 2015, 16, 1405–1438. [Google Scholar] [CrossRef]
- Haitsma, G.; Patel, H.; Gurumurthy, P.; Postma, M.J. Access to anti-cancer drugs in India: Is there a need to revise reimbursement policies? Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 289–296. [Google Scholar] [CrossRef]
- Zhang, H.; Rose, B.J.; Pyuen, A.A.; Thamm, D.H. In vitro antineoplastic effects of auranofin in canine lymphoma cells. BMC Cancer 2018, 18, 522. [Google Scholar] [CrossRef] [Green Version]
- Endo-Munoz, L.; Bennett, T.C.; Topkas, E.; Wu, S.Y.; Thamm, D.H.; Brockley, L.; Cooper, M.; Sommerville, S.; Thomson, M.; O’Connell, K.; et al. Auranofin improves overall survival when combined with standard of care in a pilot study involving dogs with osteosarcoma. Vet. Comp. Oncol. 2019, 18, 206–213. [Google Scholar] [CrossRef]
- Parrales, A.; McDonald, P.; Ottomeyer, M.; Roy, A.; Shoenen, F.J.; Broward, M.; Bruns, T.; Thamm, D.; Weir, S.J.; Neville, K.A.; et al. Comparative oncology approach to drug repurposing in osteosarcoma. PLoS ONE 2018, 13, e0194224. [Google Scholar] [CrossRef]
- Rigobello, M.P.; Scutari, G.; Folda, A.; Bindoli, A. Mitochondrial thioredoxin reductase inhibition by gold(I) compounds and concurrent stimulation of permeability transition and release of cytochrome c. Biochem. Pharmacol. 2003, 67, 689–696. [Google Scholar] [CrossRef]
- Zhang, X.; Selvaraju, K.; Saei, A.A.; D’Arcy, P.; Zubarev, R.A.; Arnér, E.S.; Linder, S. Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 2019, 162, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.F.; Nelson, R.W.; Feldman, E.C.; Scott-Moncrieff, J.C.; Griffey, S.M. Central diabetes insipidus in dogs: 20 cases (1986–1995). J. Am. Vet. Med. Assoc. 1996, 209, 1884–1888. [Google Scholar]
- Vande Walle, J.; Stockner, M.; Raes, A.; Norgaard, J. Desmopressin 30 Years in Clinical Use: A Safety Review. Curr. Drug Saf. 2008, 2, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.E.; Vischer, U.M. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J. Thromb. Haemost. 2003, 1, 682–689. [Google Scholar] [CrossRef]
- Giron, S.; Tejera, A.M.; Ripoll, G.V.; Gomez, D.E. Desmopressin inhibits lung and lymph node metastasis in a mouse mammary carcinoma model of surgical manipulation. J. Surg. Oncol. 2002, 81, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Costantini, V.; Zacharski, L.R. The role of fibrin in tumor metastasis. Cancer Metastasis Rev. 1992, 11, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.; Woodman, R.C.; Poon, M.C.; Murohara, T.; Lefer, A.M.; Davenpeck, K.L.; Kubes, P. Desmopressin induces endothelial P-selectin expression and leukocyte rolling in postcapillary venules. Blood 1995, 86, 2760–2766. [Google Scholar] [CrossRef] [Green Version]
- Alonso, D.F.; Skilton, G.; Farías, E.F.; Joffé, E.B.D.K.; Gomez, D.E. Antimetastatic effect of desmopressin in a mouse mammary tumor model. Breast Cancer Res. Treat. 1999, 57, 271–275. [Google Scholar] [CrossRef]
- Ripoll G v Farina, H.G.; Yoshiji, H.; Gomez, D.E.; Alonso, D.F. Desmopressin reduces melanoma lung metastasis in transgenic mice overexpressing tissue inhibitor of metalloproteinases-1. In Vivo 2006, 20, 881–885. [Google Scholar]
- Wood, C.J.; Chu, M.L.; Selmic, L.E.; Mayhew, P.D.; Holt, D.E.; Martano, M.; Séguin, B.; Singh, A.; Boston, S.E.; Lux, C.; et al. Effect of perioperative desmopressin in cats with mammary carcinoma treated with bilateral mastectomy. Vet. Comp. Oncol. 2021, 19, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Hermo, G.A.; Torres, P.; Ripoll, G.V.; Scursoni, A.M.; Gomez, D.E.; Alonso, D.F.; Gobello, C. Perioperative desmopressin prolongs survival in surgically treated bitches with mammary gland tumours: A pilot study. Vet. J. 2008, 178, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hermo, G.A.; Turic, E.; Angelico, D.; Scursoni, A.M.; Gomez, D.E.; Gobello, C.; Alonso, D.F. Effect of adjuvant perioperative desmopressin in locally advanced canine mammary carcinoma and its relation to histologic grade. J. Am. Anim. Hosp. Assoc. 2011, 47, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorenmo, K.; Durham, A.C.; Evans, B.; Scavello, H.; Stefanovski, D. A prospective randomized trial of desmopressin in canine mammary carcinoma. Vet. Comp. Oncol. 2020, 18, 796–803. [Google Scholar] [CrossRef]
- Wagner, B.; Johnson, J.E.; Garcia-Tapia, D.; Honsberger, N.; King, V.; Strietzel, C.; Hardham, J.M.; Heinz, T.J.; Marconi, R.T.; Meeus, P.F.M. Comparison of effectiveness of cefovecin, doxycycline, and amoxicillin for the treatment of experimentally induced early Lyme borreliosis in dogs. BMC Vet. Res. 2015, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Wen, B.; Rikihisa, Y.; Mott, J.M.; Greene, R.; Kim, H.Y.; Zhi, N.; Couto, G.C.; Unver, A.; Bartsch, R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 1997, 35, 1852–1855. [Google Scholar] [CrossRef] [Green Version]
- Assayag, F.; Brousse, N.; Couturier, J.; Macintyre, E.; Mathiot, C.; Dewulf, S.; Froget, B.; Vincent-Salomon, A.; Decaudin, D. Experimental treatment of human diffuse large B-cell lymphoma xenografts by doxycycline alone or in combination with the anti-CD20 chimeric monoclonal antibody rituximab. Am. J. Hematol. 2009, 84, 387–388. [Google Scholar] [CrossRef]
- Pulvino, M.; Chen, L.; Oleksyn, D.; Li, J.; Compitello, G.; Rossi, R.; Spence, S.; Balakrishnan, V.; Jordan, C.; Poligone, B.; et al. Inhibition of COP9-signalosome (CSN) deneddylating activity and tumor growth of diffuse large B-cell lymphomas by doxycycline. Oncotarget 2015, 6, 14796–14813. [Google Scholar] [CrossRef] [Green Version]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Hussain, A.R.; Ahmed, S.O.; Ahmed, M.; Khan, O.; Al AbdulMohsen, S.; Platanias, L.C.; Al-Kuraya, K.S.; Uddin, S. Cross-talk between NFkB and the PI3-kinase/AKT pathway can be targeted in primary effusion lymphoma (PEL) cell lines for efficient apoptosis. PLoS ONE 2012, 7, e39945. [Google Scholar] [CrossRef] [PubMed]
- Ogut, D.; Reel, B.; Korkmaz, C.G.; Arun, M.Z.; Micili, S.C.; Ergur, B.U. Doxycycline down-regulates matrix metalloproteinase expression and inhibits NF-κb signalling in LPS-induced PC3 cells. Folia Histochem. Cytobiol. 2016, 54, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, S.; Pascual, G.; Feng, B.; Klann, K.; Behm, M.; Hotz-Wagenblatt, A.; Richter, K.; Zaoui, K.; Herpel, E.; Münch, C.; et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature 2022, 607, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Hume, K.R.; Sylvester, S.R.; Borlle, L.; Balkman, C.E.; McCleary-Wheeler, A.L.; Pulvino, M.; Casulo, C.; Zhao, J. Metabolic abnormalities detected in phase II evaluation of doxycycline in dogs with multicentric B-cell lymphoma. Front. Vet. Sci. 2018, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [Green Version]
- Hauge, A.; Rofstad, E.K. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020, 18, 207. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [Green Version]
- Seton-Rogers, S. Tumour microenvironment: Time to decompress. Nat. Rev. Cancer 2013, 13, 757. [Google Scholar]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total Neoadjuvant Therapy with FOLFIRINOX in Combination with Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.P.; Chow, L.; Das, S.; Haines, L.; Palmer, E.; Kurihara, J.N.; Coy, J.W.; Mathias, A.; Thamm, D.H.; Gustafson, D.L.; et al. Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined with the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma. Clin. Cancer Res. 2021, 28, 662–676. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Gardner, H.L.; Mathie, T.; Stingle, N.; Portela, R.; Pennell, M.L.; Clifford, C.A.; Rosenberg, M.P.; Vail, D.M.; Williams, L.E.; et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: A multi-institutional study. PLoS ONE 2015, 10, e0124889. [Google Scholar] [CrossRef] [PubMed]
- Laver, T.; London, C.A.; Vail, D.M.; Biller, B.J.; Coy, J.; Thamm, D.H. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet. Comp. Oncol. 2017, 16, E23–E29. [Google Scholar] [CrossRef]
- Kim, C.; Matsuyama, A.; Mutsaers, A.J.; Woods, J.P. Retrospective evaluation of toceranib (Palladia) treatment for canine metastatic appendicular osteosarcoma. Can. Vet. J. 2017, 58, 1059–1064. [Google Scholar]
- Winder, W.W.; Hardie, D.G. Endocrinology and Metabolism. 2018. Available online: www.physiology.org/journal/ajpendo (accessed on 20 August 2022).
- Saeki, K.; Watanabe, M.; Tsuboi, M.; Sugano, S.; Yoshitake, R.; Tanaka, Y.; Ong, S.; Saito, T.; Matsumoto, K.; Fujita, N.; et al. Anti-tumour effect of metformin in canine mammary gland tumour cells. Vet. J. 2015, 205, 297–304. [Google Scholar] [CrossRef]
- Pierro, J.; Saba, C.; McLean, K.; Williams, R.; Karpuzoglu, E.; Prater, R.; Hoover, K.; Gogal, R. Anti-proliferative effect of metformin on a feline injection site sarcoma cell line independent of Mtor inhibition. Res. Vet. Sci. 2017, 114, 74–79. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, X.; Gao, P.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z.; Song, Y. The Potential Effect of Metformin on Cancer: An Umbrella Review. Front. Endocrinol. 2019, 10, 617. [Google Scholar] [CrossRef] [Green Version]
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar] [CrossRef]
- Yi, G.; He, Z.; Zhou, X.; Xian, L.; Yuan, T.; Jia, X.; Hong, J.; He, L.; Liu, J. PhenoLow concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int. J. Oncol. 2013, 43, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Martin, A.; Oliveras-Ferraros, C.; Menendez, J. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle 2009, 8, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Klose, K.; Packeiser, E.-M.; Müller, P.; Granados-Soler, J.L.; Schille, J.T.; Goericke-Pesch, S.; Kietzmann, M.; Escobar, H.M.; Nolte, I. Metformin and sodium dichloroacetate effects on proliferation, apoptosis, and metabolic activity tested alone and in combination in a canine prostate and a bladder cancer cell line. PLoS ONE 2021, 16, e0257403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ren, X.; Wang, Y.; Xu, E.; Wang, S.; Ge, R.; Liu, Y. Metformin inhibits the proliferation of canine mammary gland tumor cells through the AMPK/AKT/mTOR signaling pathway in vitro. Oncol. Lett. 2021, 22, 852. [Google Scholar] [CrossRef] [PubMed]
- Wypij, J.M. Pilot study of oral metformin in cancer-bearing cats. Vet. Comp. Oncol. 2015, 15, 345–354. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Menon, S.; Manning, B.D. Common corruption of the mTOR signaling network in human tumors. Oncogene 2008, 27, S43–S51. [Google Scholar] [CrossRef] [Green Version]
- Gupte, A.; Baker, E.K.; Wan, S.-S.; Stewart, E.; Loh, A.; Shelat, A.A.; Gould, C.M.; Chalk, A.M.; Taylor, S.; Lackovic, K.; et al. Systematic screening identifies dual PI3K and mTOR inhibition as a conserved therapeutic vulnerability in osteosarcoma. Clin. Cancer Res. 2015, 21, 3216–3229. [Google Scholar] [CrossRef] [Green Version]
- Komiya, T.; Memmott, R.M.; Blumenthal, G.M.; Bernstein, W.; Ballas, M.S.; De Chowdhury, R.; Chun, G.; Peer, C.J.; Figg, W.D.; Liewehr, D.J.; et al. A phase I/II study of pemetrexed with sirolimus in advanced, previously treated non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Liu, B.; Sun, X.; Rong, G.; Wang, W.; Li, H.; Guan, X.; Li, L.; Zhai, J.; Li, C.; et al. Safety and efficacy of sirolimus combined with endocrine therapy in patients with advanced hormone receptor-positive breast cancer and the exploration of biomarkers. Breast 2020, 52, 17–22. [Google Scholar] [CrossRef]
- Byeon, S.; Kang, M.J.; Choi, Y.J.; Kim, Y.J.; Kim, M.; Yun, J.; Yi, S.Y.; Kim, J.Y.; Kim, S.T.; Lee, J. Antitumor activity and safety of sirolimus for solid tumors with PIK3CA mutations: A multicenter, open-label, prospective single-arm study (KM 02-01, KCSG UN17-16). Transl. Cancer Res. 2020, 9, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Mazcko, C.N.; Cherukuri, A.; Berger, E.P.; Kisseberth, W.C.; Brown, M.E.; Lana, S.E.; Weishaar, K.; Flesner, B.K.; Bryan, J.N.; et al. Adjuvant sirolimus does not improve outcome in pet dogs receiving standard-of-care therapy for appendicular osteosarcoma: A prospective, randomized trial of 324 dogs. Clin. Cancer Res. 2021, 27, 3005–3016. [Google Scholar] [CrossRef]
- Gordon, I.K.; Ye, F.; Kent, M.S. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am. J. Vet. Res. 2008, 69, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Lenz, W. A short history of thalidomide embryopathy. Teratology 1988, 38, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor Activity of Thalidomide in Refractory Multiple Myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef] [Green Version]
- De Campos, C.B.; Lavalle, G.E.; Monteiro, L.N.; Pêgas, G.R.; Fialho, S.L.; Balabram, D.; Cassali, G.D. Adjuvant thalidomide and metronomic chemotherapy for the treatment of canine malignant mammary gland neoplasms. In Vivo 2018, 32, 1659–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, J.P.; Mathews, K.A.; Binnington, A.G. Thalidomide for the treatment of hemangiosarcoma in dogs. Vet. Comp. Oncol. 2004, 2, 108–109. [Google Scholar] [CrossRef]
- De Campos, C.B.; Lavalle, G.E.; Fialho Ligório, S.; Camargo Nunes, F.; Carneiro, R.A.; Amorim, R.L.; Cassali, G.D. Absence of significant adverse events following thalidomide administration in bitches diagnosed with mammary gland carcinomas. Vet. Rec. 2016, 179, 514. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Sabattini, S.; Vascellari, M.; Marconato, L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet. Comp. Oncol. 2018, 16, 497–504. [Google Scholar] [CrossRef]
- Polton, G.; Finotello, R.; Sabattini, S.; Rossi, F.; Laganga, P.; Vasconi, M.E.; Barbanera, A.; Stiborova, K.; Bley, C.R.; Marconato, L. Survival analysis of dogs with advanced primary lung carcinoma treated by metronomic cyclophosphamide, piroxicam and thalidomide. Vet. Comp. Oncol. 2018, 16, 399–408. [Google Scholar] [CrossRef]
- Bray, J.P.; Orbell, G.; Cave, N.; Munday, J.S. Does thalidomide prolong survival in dogs with splenic haemangiosarcoma? J. Small Anim. Pract. 2018, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.P.; Munday, J.S. Thalidomide reduces vascular endothelial growth factor immunostaining in canine splenic hemangiosarcoma. Vet. Sci. 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Boige, V.; Malka, D.; Ducreux, M. Therapeutic strategies using VEGF inhibitors in colorectal cancer. Bull. du Cancer 2005, 92, 29–36. [Google Scholar]
- Teo, S.K.; Evans, M.G.; Brockman, M.J.; Ehrhart, J.; Morgan, J.M.; Stirling, D.I.; Thomas, S.D. Safety profile of thalidomide after 53 weeks of oral administration in beagle dogs. Toxicol. Sci. 2001, 59, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Dou, X.; Zou, M.; Lu, H.; Wang, K.; Liu, Q.; Liu, Y.; Wang, W.; Jin, M.; Kong, D. Anticancer Effects of Amlodipine Alone or in Combination with Gefitinib in Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 902305. [Google Scholar] [CrossRef]
- Yoshida, J.; Ishibashi, T.; Nishio, M. Antitumor effects of amlodipine, a Ca2+ channel blocker, on human epidermoid carcinoma A431 cells in vitro and in vivo. Eur. J. Pharmacol. 2004, 492, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Sparkes, A.; Briscoe, K.; Carter, J.; Sala, S.C.; Jepson, R.E.; Reynolds, B.S.; Scansen, B. ISFM Consensus Guidelines on the Diagnosis and Management of Hypertension in Cats. J. Feline Med. Surg. 2017, 19, 288–303. [Google Scholar] [CrossRef]
- Wang, R.; Brattain, M.G. The maximal size of protein to diffuse through the nuclear pore is larger than 60 kDa. FEBS Lett. 2007, 581, 3164–3170. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, A.; Swift, R.; Arthurs, C.; Marote, G.; Abramo, F.; McKay, J.; Thomson, C.; Beltran, M.; Millar, M.; Priestnall, S.; et al. Quantitative expression and co-localization of Wnt signalling related proteins in feline squamous cell carcinoma. PLoS ONE 2016, 11, e0161103. [Google Scholar] [CrossRef] [Green Version]
- Alqudah, M.A.; Al-Samman, R.; Azaizeh, M.; Alzoubi, K.H. Amlodipine inhibits proliferation, invasion, and colony formation of breast cancer cells. Biomed. Rep. 2022, 16, 50. [Google Scholar] [CrossRef]
- Zheng, Y.-T.; Yang, H.-Y.; Li, T.; Zhao, B.; Shao, T.-F.; Xiang, X.-Q.; Cai, W.-M. Amiloride sensitizes human pancreatic cancer cells to erlotinib in vitro through inhibition of the PI3K/AKT signaling pathway. Acta Pharmacol. Sin. 2015, 36, 614–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, A.C.; Inkol, J.M.; Luu, A.K.; Mutsaers, A.J. Effects of the potassium-sparing diuretic amiloride on chemotherapy response in canine osteosarcoma cells. J. Vet. Intern. Med. 2019, 33, 800–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Lee, Y.J. Amiloride augments TRAIL-induced apoptotic death by inhibiting phosphorylation of kinases and phosphatases associated with the P13K-Akt pathway. Oncogene 2005, 24, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicastro, E.S.; Majdalani, M.G.; Abello, M.S.; Doiny, D.G.; Falconi, E.C.; Díaz, C.J.; Moltedo, J.M. Experience using propranolol for the management of supraventricular tachycardia in patients younger than 1 year. Arch. Argent Pediatr. 2020, 118, 273–276. [Google Scholar] [PubMed]
- Prichard, B.N.C.; Gillam, P.M.S. Treatment of Hypertension with Propranolol. Br. Med. J. 1969, 1, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef] [Green Version]
- Thaker, P.H.; Han, L.Y.; A Kamat, A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.N.; Bankson, J.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Cole, S.W.; Nagaraja, A.; Lutgendorf, S.K.; Green, P.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Rains, S.L.; Amaya, C.N.; Bryan, B.A. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience 2017, 4, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Nagaraja, A.S.; Rodriguez-Aguayo, C.; Sadaoui, N.C.; Stone, R.L.; Matsuo, K.; Dalton, H.J.; Previs, R.A.; Jennings, N.B.; et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015, 6, 4266–4273. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Calderón, D.M.; Assao, A.; Garcia, N.G.; Coutinho-Camillo, C.M.; Roffé, M.; Germano, J.N.; Oliveira, D.T. Beta adrenergic receptor activation inhibits oral cancer migration and invasiveness. Arch. Oral Biol. 2020, 118, 104865. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.B.; Armaiz-Pena, G.; Takahashi, R.; Lin, Y.G.; Trevino, J.; Li, Y.; Jennings, N.; Arevalo, J.; Lutgendorf, S.K.; Gallick, G.E.; et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J. Biol. Chem. 2007, 282, 29919–29926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, X.; Che, X.; Zhao, W.; Zhang, D.; Bi, T.; Wang, G. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol. Rep. 2010, 24, 1669–1676. [Google Scholar]
- Hajighasemi, F.; Hajighasemi, S. Effect of propranolol on angiogenic factors in human hematopoietic cell lines in vitro. Iran. Biomed. J. 2009, 13, 223–228. [Google Scholar] [PubMed]
- Yang, E.V.; Kim, S.J.; Donovan, E.L.; Chen, M.; Gross, A.C.; Marketon, J.I.; Barsky, S.H.; Glaser, R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav. Immun. 2009, 23, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.V.; Sood, A.K.; Chen, M.; Li, Y.; Eubank, T.D.; Marsh, C.B.; Jewell, S.; Flavahan, N.A.; Morrison, C.; Yeh, P.E.; et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006, 66, 10357–10364. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, J.H.; Jeong, K.J.; Lee, J.; Han, J.W.; Choi, W.S.; Kim, Y.K.; Kang, J.; Park, C.G.; Lee, H.Y. Retracted: Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1α protein-dependent mechanism. Int. J. Cancer 2011, 128, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Street, J.; Pouchy, C.; Carre, M.; Gifford, A.J.; Murray, J.; Norris, M.D.; Trahair, T.; Andre, N.; Kavallaris, M. B-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer 2013, 108, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, E.; Ciccolini, J.; Carre, M.; Giacometti, S.; Fanciullino, R.; Pouchy, C.; Montero, M.-P.; Serdjebi, C.; Kavallaris, M.; André, N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget 2011, 2, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Ramu, A.; Spanier, R.; Rahamimoff, H.; Fuks, Z. Restoration of doxorubicin responsiveness in doxorubicin-resistant P388 murine leukaemia cells. Br. J. Cancer 1984, 50, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Deng, G.-H.; Zhang, J.; Wang, Y.; Xia, X.-Y.; Luo, X.-M.; Deng, Y.-T.; He, S.-S.; Mao, Y.-Y.; Peng, X.-C.; et al. The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 2015, 52, 130–142. [Google Scholar] [CrossRef]
- Kalinichenko, V.V.; Mokyr, M.B.; Graf, L.H.; Cohen, R.L.; Chambers, D.A. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J. Immunol. 1999, 163, 2492–2499. [Google Scholar] [PubMed]
- Zhou, L.; Li, Y.; Li, X.; Chen, G.; Liang, H.; Wu, Y.; Tong, J.; Ouyang, W. Propranolol attenuates surgical stress–induced elevation of the regulatory T cell response in patients undergoing radical mastectomy. J. Immunol. 2016, 196, 3460–3469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, Y.; Piterman, O.; Liscovitch, M. Inhibition of phosphatidic acid phosphohydrolase activity by sphingosine. Dual action of sphingosine in diacylglycerol signal termination. FEBS Lett. 1990, 277, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mischiati, C.; Melloni, E.; Corallini, F.; Milani, D.; Bergamini, C.; Vaccarezza, M. Potential Role of PKC Inhibitors in the Treatment of Hematological Malignancies. Curr. Pharm. Des. 2008, 14, 2075–2084. [Google Scholar] [CrossRef]
- Zhong, S.; Yu, D.; Zhang, X.; Chen, X.; Yang, S.; Tang, J.; Zhao, J.; Wang, S. β-Blocker use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Eur. J. Cancer Prev. 2016, 25, 440–448. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Gandini, S.; Benemei, S.; Lotti, T.; Marchionni, N.; Rossari, S.; Gori, A.; Geppetti, P. The effect of beta-blocker treatment in patients with cutaneous melanoma. J. Clin. Oncol. 2011, 29, 8524. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Botteri, E.; Pennacchioli, E.; Geppetti, P.; Gandini, S. Propranolol for Off-label TREATMENT of Patients With Melanoma. JAMA Oncol. 2018, 4, e172908. [Google Scholar] [CrossRef]
- Bhattacharyya, G.S.; Babu, K.G.; Bondarde, S.A.; Biswas, G.; Ranade, A.; Parikh, P.M.; Bascomb, N.F.; Malhotra, H. Effect of coadministered beta blocker and COX-2 inhibitor to patients with pancreatic cancer prior to receiving albumin-bound (Nab) paclitaxel. J. Clin. Oncol. 2015, 33, 302. [Google Scholar] [CrossRef]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.-M.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: A phase II randomized trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef]
- Gandhi, S.; Pandey, M.R.; Attwood, K.; Ji, W.; Witkiewicz, A.K.; Knudsen, E.S.; Allen, C.; Tario, J.D.; Wallace, P.K.; Cedeno, C.D.; et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: Safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 2021, 27, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Cranmer, L.D.; Loggers, E.T.; Pollack, S.M. Propranolol for the treatment of vascular sarcomas. J. Exp. Pharmacol. 2018, 10, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, L.; Garofoli, M.; Di Fonte, R.; Fucci, L.; Volpicella, M.; Strippoli, S.; Guida, M.; Azzariti, A. The β-adrenergic receptor antagonist propranolol offsets resistance mechanisms to chemotherapeutics in diverse sarcoma subtypes: A pilot study. Sci. Rep. 2020, 10, 10465. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.; Amaya, C.N.; Rains, S.; Chow, M.; Dickerson, E.B.; Bryan, B.A. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015, 151, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Duckett, M.M.; Phung, S.K.; Nguyen, L.; Khammanivong, A.; Dickerson, E.B.; Dusenbery, K.; Lawrence, J. The adrenergic receptor antagonists propranolol and carvedilol decrease bone sarcoma cell viability and sustained carvedilol reduces clonogenic survival And increases radiosensitivity in canine osteosarcoma cells. Vet. Comp. Oncol. 2019, 18, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Gorden, B.H.; Saha, J.; Khammanivong, A.; Schwartz, G.K.; Dickerson, E.B. Lysosomal drug sequestration as a mechanism of drug resistance in vascular sarcoma cells marked by high CSF-1R expression. Vasc. Cell 2014, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Saha, J.; Kim, J.H.; Amaya, C.N.; Witcher, C.; Khammanivong, A.; Korpela, D.M.; Brown, D.R.; Taylor, J.; Bryan, B.A.; Dickerson, E.B. Propranolol Sensitizes Vascular Sarcoma Cells to Doxorubicin by Altering Lysosomal Drug Sequestration and Drug Efflux. Front. Oncol. 2021, 10, 614288. [Google Scholar] [CrossRef]
- Kim, J.-H.; Graef, A.J.; Dickerson, E.B.; Modiano, J.F. Pathobiology of hemangiosarcoma in dogs: Research advances and future perspectives. Vet. Sci. 2015, 2, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Faulhaber, E.A.; Janik, E.; Thamm, D.H. Adjuvant carboplatin for treatment of splenic hemangiosarcoma in dogs: Retrospective evaluation of 18 cases (2011–2016) and comparison with doxorubicin-based chemotherapy. J. Vet. Intern. Med. 2021, 35, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Wendelburg, K.M.; Price, L.L.; Burgess, K.E.; Lyons, J.A.; Lew, F.H.; Berg, J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012). J. Am. Vet. Med. Assoc. 2015, 247, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, J.; He, C.; Fang, M. Angiosarcoma: A review of diagnosis and current treatment. Am. J. Cancer Res. 2019, 9, 2303–2313. [Google Scholar] [PubMed]
- Marconato, L.; Chalfon, C.; Finotello, R.; Polton, G.; Vasconi, M.E.; Annoni, M.; Stefanello, D.; Mesto, P.; Capitani, O.; Agnoli, C.; et al. Adjuvant anthracycline-based vs metronomic chemotherapy vs no medical treatment for dogs with metastatic splenic hemangiosarcoma: A multi-institutional retrospective study of the Italian Society of Veterinary Oncology. Vet. Comp. Oncol. 2019, 17, 537–544. [Google Scholar] [CrossRef]
- Brown, M.S.; Faust, J.R.; Goldstein, J.L.; Kaneko, I.; Endo, A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem. 1978, 253, 1121–1128. [Google Scholar] [CrossRef]
- Matusewicz, L.; Meissner, J.; Toporkiewicz, M.; Sikorski, A.F. The effect of statins on cancer cells—Review. Tumor Biol. 2015, 36, 4889–4904. [Google Scholar] [CrossRef]
- Cafforio, P.; Dammacco, F.; Gernone, A.; Silvestris, F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis 2005, 26, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Helbig, G.; Hołowiecki, J. Ras signaling pathway as a target for farnesyltransferase inhibitors—A new, promising prospects in the treatment for malignant disorders. Wiadomości Lek. 2004, 57, 462–467. [Google Scholar]
- Tu, Y.-S.; Kang, X.-L.; Zhou, J.-G.; Lv, X.-F.; Tang, Y.-B.; Guan, Y.-Y. Involvement of Chk1–Cdc25A-cyclin A/CDk2 pathway in simvastatin induced S-phase cell cycle arrest and apoptosis in multiple myeloma cells. Eur. J. Pharmacol. 2011, 670, 356–364. [Google Scholar] [CrossRef]
- Kochuparambil, S.T.; Al-Husein, B.; Goc, A.; Soliman, S.; Somanath, P.R. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Ther. 2011, 336, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Sirvent, P.; Mercier, J.; Lacampagne, A. New insights into mechanisms of statin-associated myotoxicity. Curr. Opin. Pharmacol. 2008, 8, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.C.; Park, M.J.; Ye, S.-K.; Kim, C.-W.; Kim, Y.-N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006, 168, 1107–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westover, E.J.; Covey, D.F.; Brockman, H.L.; Brown, R.E.; Pike, L.J. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects: Studies with cholesterol enantiomers. J. Biol. Chem. 2003, 278, 51125–51133. [Google Scholar] [CrossRef] [Green Version]
- Ringerike, T.; Blystad, F.D.; Levy, F.O.; Madshus, I.H.; Stang, E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J. Cell Sci. 2002, 115, 1331–1340. [Google Scholar] [CrossRef]
- Menter, D.G.; Ramsauer, V.P.; Harirforoosh, S.; Chakraborty, K.; Yang, P.; Hsi, L.; Newman, R.A.; Krishnan, K. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS ONE 2011, 6, e28813. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Smalley, S.; Sadarangani, A.; Chen-Lin, K.; Oliva, B.; Branes, J.; Carvajal, J.; Gejman, R.; Owen, G.I.; Cuello, M. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. J. Cell. Mol. Med. 2010, 14, 1180–1193. [Google Scholar]
- Dulak, J.; Jozkowicz, A. Anti-Angiogenic and Anti-Inflammatory Effects of Statins: Relevance to Anti-Cancer Therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar] [CrossRef]
- Wojtkowiak, J.W.; Sane, K.M.; Kleinman, M.; Sloane, B.F.; Reiners, J.J.; Mattingly, R.R. Aborted autophagy and nonapoptotic death induced by farnesyl transferase inhibitor and lovastatin. J. Pharmacol. Exp. Ther. 2011, 337, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.-B.; Zhang, J.-W.; Gao, J.-B.; Qi, Y.-W.; Gao, Y.; Xu, L.; Ma, Y.; Wei, Z.-Z. Atorvastatin induces autophagy in MDA-MB-231 breast cancer cells. Ultrastruct. Pathol. 2018, 42, 409–415. [Google Scholar] [CrossRef]
- Asakura, K.; Izumi, Y.; Yamamoto, M.; Yamauchi, Y.; Kawai, K.; Serizawa, A.; Mizushima, T.; Ohmura, M.; Kawamura, M.; Wakui, M.; et al. The cytostatic effects of lovastatin on ACC-MESO-1 cells. J. Surg. Res. 2011, 170, e197–e209. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Liu, Y.-L.; Lin, Y.-C.; Shun, C.-T.; Wu, M.-S.; Chen, C.-C. Inhibition of Autophagy Enhances Anticancer Effects of Atorvastatin in Digestive Malignancies. Cancer Res. 2010, 70, 7699–7709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misirkic, M.; Janjetovic, K.; Vucicevic, L.; Tovilovic, G.; Ristic, B.; Vilimanovich, U.; Harhaji-Trajkovic, L.; Sumarac-Dumanovic, M.; Micic, D.; Bumbasirevic, V.; et al. Inhibition of AMPK-dependent autophagy enhances in vitro antiglioma effect of simvastatin. Pharmacol. Res. 2012, 65, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, Z.; DeWitt, J.P.; Xie, L.; Chen, Y.; Li, X.; Han, L.; Li, D.; Xia, J.; Zhang, Y.; et al. Fluvastatin Prevents Lung Adenocarcinoma Bone Metastasis by Triggering Autophagy. eBioMedicine 2017, 19, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Kenific, C.M.; Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015, 25, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Mehibel, M.; Ortiz-Martinez, F.; Voelxen, N.; Boyers, A.; Chadwick, A.; Telfer, B.; Mueller-Klieser, W.; West, C.; Critchlow, S.E.; Williams, K.J.; et al. Statin-induced metabolic reprogramming in head and neck cancer: A biomarker for targeting monocarboxylate transporters. Sci. Rep. 2018, 8, 16804. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; He, Y.; Zhao, P.; Hu, Y.; Tao, J.; Chen, J.; Huang, Y. Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β3/FAK pathway and tumor-associated macrophage repolarization using legumain-activatable delivery. Theranostics 2019, 9, 265. [Google Scholar] [CrossRef]
- Farooqi, M.A.M.; Malhotra, N.; Mukherjee, S.D.; Sanger, S.; Dhesy-Thind, S.K.; Ellis, P.; Leong, D.P. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0209486. [Google Scholar] [CrossRef]
- Abdullah, M.I.; de Wolf, E.; Jawad, M.J.; Richardson, A. The poor design of clinical trials of statins in oncology may explain their failure—Lessons for drug repurposing. Cancer Treat. Rev. 2018, 69, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Ung, M.H.; MacKenzie, T.A.; Onega, T.L.; Amos, C.I.; Cheng, C. Statins associate with improved mortality among patients with certain histological subtypes of lung cancer. Lung Cancer 2018, 126, 89–96. [Google Scholar] [CrossRef]
- Seckl, M.J.; Ottensmeier, C.; Cullen, M.; Schmid, P.; Ngai, Y.; Muthukumar, D.; Thompson, J.; Harden, S.V.; Middleton, G.; Fife, K.M.; et al. Multicenter, phase III, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (LUNGSTAR). J. Clin. Oncol. 2017, 35, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Lee, S.-H.; Yoo, N.J.; Hyung, L.S.; Moon, Y.J.; Yun, T.; Kim, H.T.; Lee, J.S. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non–small cell lung cancer. Clin. Cancer Res. 2011, 17, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneau, A.; Rico, C.; Boerboom, D.; Paquet, M. Statins downregulate YAP and TAZ and exert anti-cancer effects in canine mammary tumour cells. Vet. Comp. Oncol. 2021, 20, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Mosallanejad, B.; Avizeh, R.; Jalali, M.R.; Pourmahdi, M. Comparative evaluation between chitosan and atorvastatin on serum lipid profile changes in hyperlipidemic cats. Iran. J. Vet. Res. 2016, 17, 36–40. [Google Scholar] [CrossRef]
- Bonaparte, A.; Tansey, C.; Wiebe, M.; Espinoza, H.E.; Patlogar, J.E.; Murphy, L.A.; Nakamura, R.K. The effect of atorvastatin on haemostatic parameters in apparently healthy dogs. J. Small Anim. Pract. 2019, 60, 565–570. [Google Scholar] [CrossRef]
- Herron, C.; Brueckner, C.; Chism, J.; Kemp, D.; Prescott, J.; Smith, G.; Melich, D.; Oleas, N.; Polli, J. Toxicokinetics and toxicity of atorvastatin in dogs. Toxicol. Appl. Pharmacol. 2015, 289, 117–123. [Google Scholar] [CrossRef]


| Repurposed Drug | Action | Dose | Clinical Trial | Clinical Trial Results | Statistics | References |
|---|---|---|---|---|---|---|
| Auranofin | Inhibition of thioredoxin reductase with increased reactive oxygen species, oxidative stress, and cancer cell death | 6 mg/dog < 15 kg PO q 3 days 9 mg/dog > 15 kg PO q 3 days | Dogs with osteosarcoma | Standard-care treatment (surgery + carboplatin) + auranofin (n = 40; OS = 329 days) * | p = 0.036 | Endo-Munoz et al. (2019) [20] |
| Historical control group with standard care (n = 26; OS = 240 days) | ||||||
| Desmopressin | Anti-metastatic (inhibition of metastatic emboli formation and subsequent adherence of these emboli to target metastatic sites) | 1 μg/kg IV 30 min preoperatively and 1 μg/kg IV 24 h postoperatively | Dogs with stage III or IV mammary carcinoma | Surgery + placebo (n = 10; DFI = 85 days; OS = 333 days) | p < 0.01 (DFI) and p = 0.05 (OS) | Hermo et al. (2008) [33] |
| Surgery + desmopressin (n = 11; DFI = 608 days; OS > 600 days) | ||||||
| 1 μg/kg IV 30 min preoperatively and 1 μg/kg IV 24 h postoperatively | Dogs with stage III or IV mammary carcinoma | Surgery + placebo (n = 10; DFI = 88 days; OS = 237 days) | p < 0.01 | Hermo et al. (2011) [34] | ||
| surgery + desmopressin (n = 18; DFI = 608 days; OS = 809 days) | ||||||
| 3 mcg/kg SC preoperatively and 3 mcg/kg SC 24 h postoperatively | Dogs with mammary carcinoma | Surgery + placebo (n = 12; OS = 754 days) | p < 0.73 | Sorenmo et al. (2020) [35] | ||
| Surgery + desmopressin (n = 12; OS = 818 days) | ||||||
| 1 μg/kg IV 30 min preoperatively and 1 μg/kg IV 24 h postoperatively | Cats with mammary carcinoma | Surgery (n = 45; DFI = 966 days) | p = 0.9 | Wood et al. (2021) [32] | ||
| Surgery + desmopressin (n = 15; DFI not reached) | ||||||
| Doxycicline | Inhibition of MMP9 and NF-κB (anti-proliferative effect) | 7.5 or 10 mg/kg PO q 12 h | Dogs with lymphoma | Doxycicline (n = 13; no objective response but one dog achieved stable disease for 6 weeks) | - | Hume et al. (2018) [44] |
| Losartan | Anti-metastatic (inhibition of CCL2-CCR2, monocyte recruitment, and tumor-associated macrophages) | 10 mg/kg PO q 12 h. | Dogs with metastatic osteosarcoma | Toceranib + Losartan (n = 28; 50% of clinical benefit and 25% of objective response) | - | Regan et al. (2021) [52] |
| Metformin | Cell cycle arrest (AMPK activation leading to m-TOR inhibition and increased expression of p53) | 10 mg/kg PO q 12 h. | Cats with various neoplasms (5 carcinomas, 2 cutaneous lymphomas and 2 injection site sarcomas) | 9 cats Metformin (n = 9; 2 cats with skin SCC had modest measurable response) | - | Wypij (2015) [66] |
| Sirolimus (rapamycin) | Cell cycle arrest (m-TOR inhibition) | 0.1 mg/kg on either a Monday through Friday schedule or Monday–Wednesday–Friday schedule for 4 consecutive weeks | Dogs with osteosarcoma | Standard-care treatment (surgery + carboplatin) (n = 157; OS = 282 days) | p > 0.05 | LeBlanc et al. (2021) [73] |
| Standard-care + sirolimus (n = 152; OS = 280 days) | ||||||
| Thalidomide | Anti-angiogenic properties, decreased VEGF and TNF-alpha | 20 mg/kg PO q 24 h for 3 months, followed by 10 mg/kg PO q 24 h. | Dogs with stage V mammary carcinoma | Surgery (n = 5; OS = 150 days) | p < 0.0001 (except between groups treated with surgery or surgery + maximum tolerated dose of chemotherapy) | Campos et al. (2018) [77] |
| Surgery + maximum tolerated dose of chemotherapy (n = 3; OS = 148 days) | ||||||
| Surgery + maximum tolerated dose of chemotherapy + metronomic chemotherapy (n = 6; OS = 376.5 days) | ||||||
| Surgery + maximum tolerated dose of chemotherapy + thalidomide (n = 13; OS = 463 days) | ||||||
| 2 mg/kg PO q 24 h | Dogs with inflammatory mammary carcinoma | Piroxicam + thalidomide + toceranib (n = 14; OS = 59 days) | p = 0.032 | Rossi et al. (2018) [80] | ||
| Radiotherapy + piroxicam + thalidomide + toceranib (n = 4; OS = 180 days) | ||||||
| 8.7 mg/kg PO q 24 h | Dogs with stage II (n = 10) and III (n = 5) splenic haemangiosarcoma | Surgery and thalidomide (OS = 172 days) | - | Bray et al. (2018) [82] | ||
| 2 mg/kg PO q 24 h | Dogs with advanced primary lung carcinoma | Metronomic chemotherapy (low-dose cyclophosphamide + piroxicam + thalidomide) (n = 25; OS = 139 days) | p < 0.007 (difference between metronomic/thalidomide group and the three remaining) | Polton et al. (2018) [81] | ||
| Surgery (n = 36; OS = 92 days) | ||||||
| Maximum tolerated dose of chemotherapy (n = 11; OS = 61 days) | ||||||
| No oncologic treatment (n = 19; OS = 60 days) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, A.; Horta, R.S.; Vieira, R.A.M.; Hume, K.R.; Dobson, J. Repurposing Drugs in Small Animal Oncology. Animals 2023, 13, 139. https://doi.org/10.3390/ani13010139
Giuliano A, Horta RS, Vieira RAM, Hume KR, Dobson J. Repurposing Drugs in Small Animal Oncology. Animals. 2023; 13(1):139. https://doi.org/10.3390/ani13010139
Chicago/Turabian StyleGiuliano, Antonio, Rodrigo S. Horta, Rafael A. M. Vieira, Kelly R. Hume, and Jane Dobson. 2023. "Repurposing Drugs in Small Animal Oncology" Animals 13, no. 1: 139. https://doi.org/10.3390/ani13010139
APA StyleGiuliano, A., Horta, R. S., Vieira, R. A. M., Hume, K. R., & Dobson, J. (2023). Repurposing Drugs in Small Animal Oncology. Animals, 13(1), 139. https://doi.org/10.3390/ani13010139







