Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 1) Peanut Meal
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Heating Processing Methods, and Chemical Analysis
2.2. In Situ Procedures and Calculations
2.3. Intestinal Digestibility Procedures
2.4. Statistical Analysis
- -
- Control versus processing treatments;
- -
- Effect of xylose;
- -
- Linear effect of heating time;
- -
- Quadratic effect of heating time;
- -
- Interaction between xylose and heating time.
3. Results
3.1. Autoclave
3.2. Conventional Oven
3.3. Microwave Oven
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Bianco Benedeti, P.; Valadares Filho, S.C.; Chizzotti, M.L.; Marcondes, M.I.; de Sales Silva, F.A. Development of Equations to Predict Carcass Weight, Empty Body Gain, and Retained Energy of Zebu Beef Cattle. Animal 2021, 15, 100028. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.G.; Drennan, M.J.; Moloney, A.P. Comparison of Supplementary Concentrate Levels with Grass Silage, Separate or Total Mixed Ration Feeding, and Duration of Finishing in Beef Steers. Livest. Sci. 2006, 103, 169–180. [Google Scholar] [CrossRef]
- Filho, S.d.V.; Silva, L.F.C.e.; Gionbelli, M.P.; Rotta, P.P.; Marcondes, M.I.; Chizzotti, M.L.; Prados, L.F. Nutrient Requirements of Zebu and Crossbred Cattle—BR CORTE, 3rd ed.; UFV, DZO: Viçosa, MG, Brazil, 2016. [Google Scholar]
- NASEM—National Academies of Sciences Engineering Medicine. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-27335-0. [Google Scholar]
- Waltz, D.M.; Stern, M.D. Evaluation of Various Methods for Protecting Soya-Bean Protein from Degradation by Rumen Bacteria. Anim. Feed. Sci. Technol. 1989, 25, 111–122. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Toone, C.D.; Ludden, P.A. Effects of Supplemental Ruminally Degradable Protein versus Increasing Amounts of Supplemental Ruminally Undegradable Protein on Site and Extent of Digestion and Ruminal Characteristics in Lambs Fed Low-Quality Forage. J. Anim. Sci. 2007, 85, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Church, D.C. (Ed.) The Ruminant Animal: Digestive Physiology and Nutrition; Prentice Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Boisen, S.; Hvelplund, T.; Weisbjerg, M.R. Ideal Amino Acid Profiles as a Basis for Feed Protein Evaluation. Livest. Prod. Sci. 2000, 64, 239–251. [Google Scholar] [CrossRef]
- Herd, R.M.; Archer, J.A.; Arthur, P.F. Reducing the Cost of Beef Production through Genetic Improvement in Residual Feed Intake: Opportunity and Challenges to Application. J. Anim. Sci. 2003, 81, E9–E17. [Google Scholar]
- Petit, H.V.; Tremblay, G.F.; Marcotte, M.; Audy, R. Degradability and Digestibility of Full-Fat Soybeans Treated with Different Sugar and Heat Combinations. Can. J. Anim. Sci. 1999, 79, 213–220. [Google Scholar] [CrossRef]
- Kanzler, C.; Haase, P.T. Melanoidins Formed by Heterocyclic Maillard Reaction Intermediates via Aldol Reaction and Michael Addition. J. Agric. Food Chem. 2019, 68, 332–339. [Google Scholar] [CrossRef]
- Marounek, M.; Brezina, P. Heat-induced Formation of Soluble Maillard Reaction Products and Its Influence on Utilization of Glucose by Rumen Bacteria. Arch. Tierernaehrung 1993, 43, 45–51. [Google Scholar] [CrossRef]
- Camire, M.E.; Krumhar, K. Chemical and Nutritional Changes in Foods During Extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–57. [Google Scholar] [CrossRef]
- Can, A.; Yilmaz, A. Usage of Xylose or Glucose as Non-Enzymatic Browning Agent for Reducing Ruminal Protein Degradation of Soybean Meal. Small Rumin. Res. 2002, 46, 173–178. [Google Scholar] [CrossRef]
- Satter, L.D. Protein Supply from Undegraded Dietary Protein. J. Dairy Sci. 1986, 69, 2734–2749. [Google Scholar] [CrossRef]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Samadi; Yu, P. Dry and Moist Heating-Induced Changes in Protein Molecular Structure, Protein Subfraction, and Nutrient Profiles in Soybeans. J. Dairy Sci. 2011, 94, 6092–6102. [Google Scholar] [CrossRef]
- Bachmann, M.; Kuhnitzsch, C.; Michel, S.; Thierbach, A.; Bochnia, M.; Greef, J.M.; Martens, S.D.; Steinhöfel, O.; Zeyner, A. Effect of Toasting Grain Silages from Field Peas (Pisum sativum) and Field Beans (Vicia faba) on In Vitro Gas Production, Methane Production, and Post-Ruminal Crude Protein Content. Anim. Nutr. 2020, 6, 342–352. [Google Scholar] [CrossRef]
- Paya, H.; Taghizadeh, A.; Janmohammadi, H.; Moghaddam, G.A.; Khani, A.H.; Alijani, S. Effects of Microwave Irradiation on In Vitro Ruminal Fermentation and Ruminal and Post-Ruminal Disappearance of Safflower Seed. J. Biodivers. Environ. Sci. 2014, 5, 349–356. [Google Scholar]
- Xiang, S.; Zou, H.; Liu, Y.; Ruan, R. Effects of Microwave Heating on the Protein Structure, Digestion Properties and Maillard Products of Gluten. J. Food Sci. Technol. 2020, 57, 2139–2149. [Google Scholar] [CrossRef]
- Jahani-Azizabadi, H.; Mesgaran, M.D.; Vakili, A.R.; Vatandoost, M.; Ghezeljeh, E.A.; Mojtahedi, M. The Effect of Heat or Heat-Xylose Processing on Nitrogen Fractions and in Situ/in Vitro Ruminal and Post-Ruminal Protein Disappearance of Guar Meal. Am. J. Anim. Vet. Sci. 2010, 5, 266–273. [Google Scholar] [CrossRef][Green Version]
- Ceresnakova, Z.; Sommer, A.; Chrenková, M.; Dolešová, P. Amino Acid Profile of Escaped Feed Protein after Rumen Incubation and Their Intestinal Digestibility. Arch. Anim. Nutr. 2002, 56, 409–418. [Google Scholar] [CrossRef]
- Pinto, A.C.; Millen, D.D. Nutritional Recommendations and Management Practices Adopted By Feedlot Cattle Nutritionists: The 2016 Brazilian. Can. J. Anim. Sci. 2018, 99, 392–407. [Google Scholar] [CrossRef]
- Silva, L.F.C.E.; Filho, S.d.V.; Benedeti, P.d.; Detmann, E.; Menezes, A.C.B.; Silva, T.E.; Silva, F.A.d. Development of an Equation to Predict Net Protein Requirements for the Growth of Zebu Beef Cattle. Animal 2019, 14, 963–972. [Google Scholar] [CrossRef]
- Hill, G.M. Peanut By-Products Fed to Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 295–315. [Google Scholar] [CrossRef]
- Valadares Filho, S.C.; Lopes, S.A. Tabelas Brasileiras de Composição de Alimentos Para Ruminantes. Available online: www.cqbal.com.br (accessed on 28 November 2022).
- Molosse, V.L.; Pereira, D.A.B.; Rigon, F.; Rigon, F.; Magnani, E.; Marcondes, M.I.; Arnandes, R.H.B.; Benedeti, P.D.B.; Paula, E.M. Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 2) Cotton-Seed Meal. 2022; unpublished. [Google Scholar]
- Detmann, E.; Valadares Filho, S.C.; Berchielli, T.T.; Saliba, E.O.; Cabral, L.S.; Pina, D.S.; Ladeira, M.; Azevedo, J. Métodos Para Análise de Alimentos; Suprema: Visconde do Rio Branco, MG, Brazil, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Orskov, E.R.; Mcdonald, I. The Estimation of Protein Degradability in the Rumen from Incubation Measurements Weighted According to Rate of Passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Denham, S.C.; Morantes, G.A.; Bates, D.B.; Moore, J.E. Comparison of Two Models Used to Estimate In Situ Nitrogen Disappearance. J. Dairy Sci. 1989, 72, 708–714. [Google Scholar] [CrossRef]
- NRC. Requirements of Dairy Cattle Seventh Revised Edition; NRC: Washington, DC, USA, 2001; ISBN 0309069971. [Google Scholar]
- Calsamiglia, S.; Stern, M.D. A Three-Step in Vitro Procedure for Estimating Intestinal Digestion of Protein in Ruminants. J. Anim. Sci. 1995, 73, 1459–1465. [Google Scholar] [CrossRef]
- Gargallo, S.; Calsamiglia, S.; Ferret, A. Technical Note: A Modified Three-Step in Vitro Procedure to Determine Intestinal Digestion of Proteins. J. Anim. Sci. 2006, 84, 2163–2167. [Google Scholar] [CrossRef]
- Doiron, K.; Yu, P.; McKinnon, J.J.; Christensen, D.A. Heat-Induced Protein Structure and Subfractions in Relation to Protein Degradation Kinetics and Intestinal Availability in Dairy Cattle. J. Dairy Sci. 2009, 92, 3319–3330. [Google Scholar] [CrossRef]
- Peng, Q.; Khan, N.A.; Wang, Z.; Yu, P. Moist and Dry Heating-Induced Changes in Protein Molecular Structure, Protein Subfractions, and Nutrient Profiles in Camelina Seeds. J. Dairy Sci. 2014, 97, 446–457. [Google Scholar] [CrossRef]
- Broderick, G.A.; Wallace, R.J.; Ørskov, E.R. Control of Rate and Extent of Protein Degradation. In Physiological Aspects of Digestion and Metabolism in Ruminants; Academic Press: Cambridge, MA, USA, 1991; pp. 541–592. [Google Scholar] [CrossRef]
- Haryanto, B. Manipulating Protein Degradability in the Rumen to Support Higher Ruminant Production. Indones. Bull. Anim. Vet. Sci. 2014, 24, 131–138. [Google Scholar] [CrossRef][Green Version]
- Higgs, R.J.; Chase, L.E.; Ross, D.A.; van Amburgh, M.E. Updating the Cornell Net Carbohydrate and Protein System Feed Library and Analyzing Model Sensitivity to Feed Inputs. J. Dairy Sci. 2015, 98, 6340–6360. [Google Scholar] [CrossRef]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; van Soest, P.J.; Sniffen, C.J. A Net Carbohydrate and Protein System for Evaluating Cattle Diets: I. Ruminal Fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Parnian Khajehdizaj, F.; Taghizadeh, A.; Baghbanzadeh Nobari, B. Effect of Feeding Microwave Irradiated Sorghum Grain on Nutrient Utilization, Rumen Fermentation and Serum Metabolites in Sheep. Livest. Sci. 2014, 167, 161–170. [Google Scholar] [CrossRef]
- Broderick, G.A. Review: Optimizing Ruminant Conversion of Feed Protein to Human Food Protein. Animal 2018, 12, 1722–1734. [Google Scholar] [CrossRef]
- Ahmad Khan, N.; Booker, H.; Yu, P. Effect of Heating Method on Alteration of Protein Molecular Structure in Flaxseed: Relationship with Changes in Protein Subfraction Profile and Digestion in Dairy Cows. J. Agric. Food Chem. 2015, 63, 1057–1066. [Google Scholar] [CrossRef]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of Microwave—A Brief Review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef]
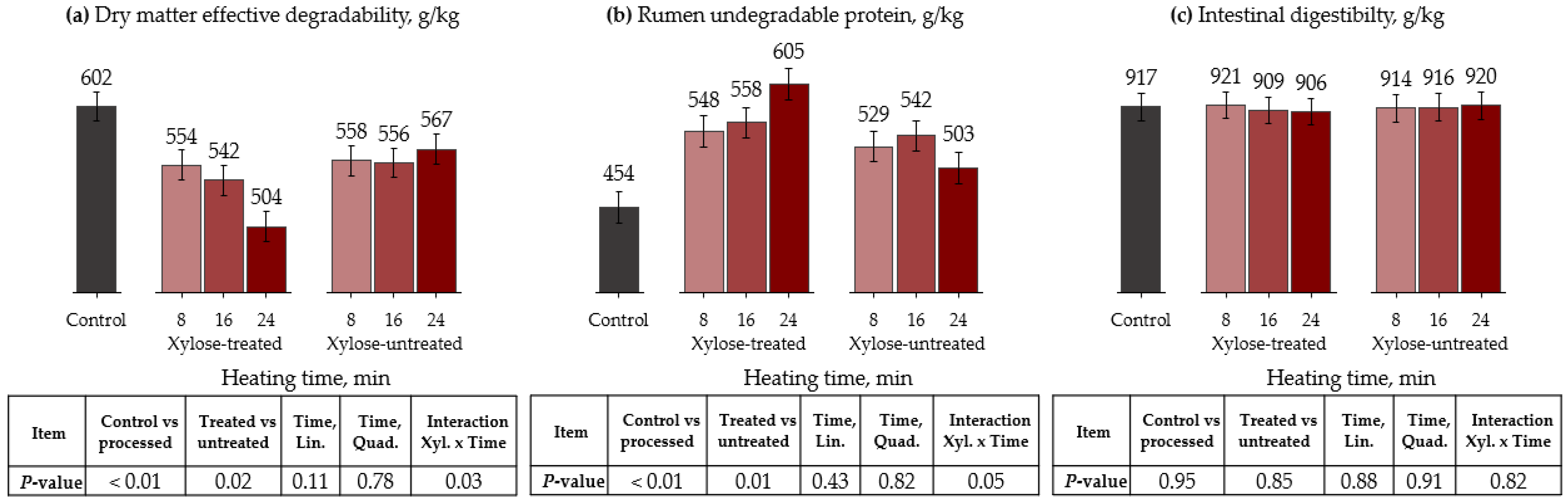

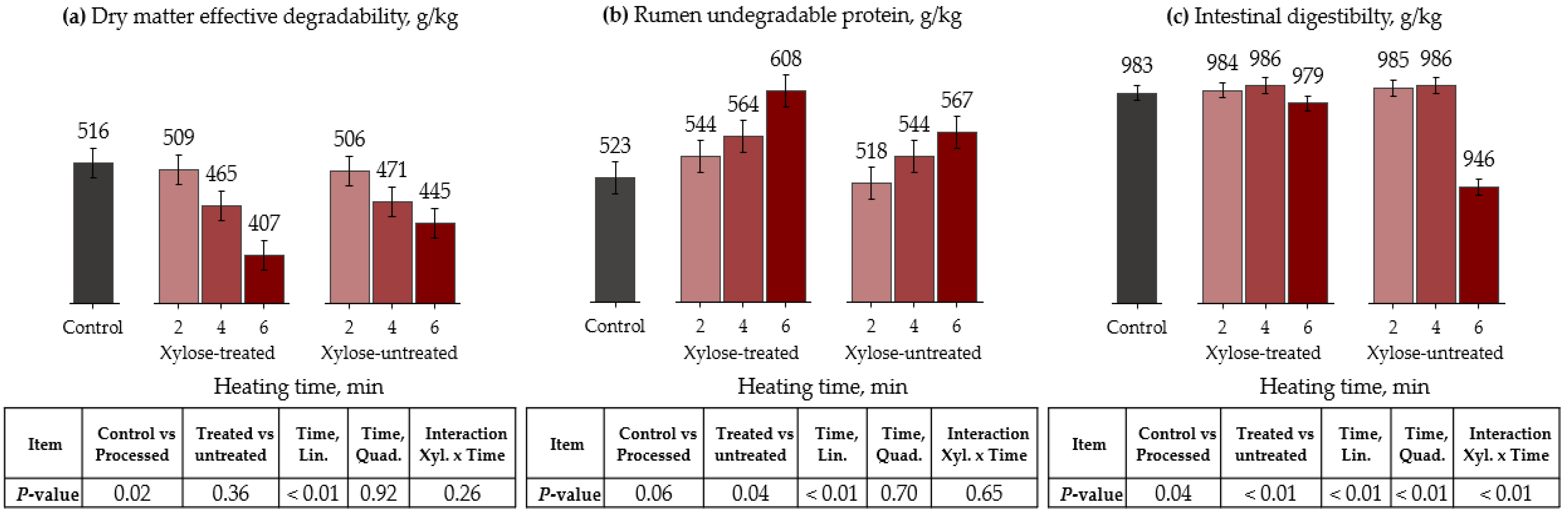
| Item | Control | Xylose-Treated | Xylose-Untreated | ||||
|---|---|---|---|---|---|---|---|
| 8 | 16 | 24 | 8 | 16 | 24 | ||
| Dry matter, g/kg | 910 | 908 | 911 | 910 | 916 | 912 | 914 |
| Organic matter, g/kg DM | 946 | 948 | 951 | 947 | 946 | 949 | 947 |
| Crude protein, g/kg DM | 628 | 645 | 639 | 643 | 647 | 655 | 650 |
| Neutral detergent fiber, g/kg DM | 164 | 285 | 300 | 315 | 259 | 254 | 260 |
| Item | Control | Xylose-Treated | Xylose-Untreated | ||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 30 | 60 | 90 | ||
| Dry matter, g/kg | 910 | 904 | 936 | 930 | 926 | 921 | 920 |
| Organic matter, g/kg DM | 946 | 970 | 948 | 947 | 947 | 946 | 946 |
| Crude protein, g/kg DM | 628 | 645 | 639 | 643 | 647 | 655 | 650 |
| Neutral detergent fiber, g/kg DM | 164 | 310 | 456 | 449 | 263 | 455 | 368 |
| Item | Control | Xylose-Treated | Xylose-Untreated | ||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 2 | 4 | 6 | ||
| Dry matter, g/kg | 910 | 919 | 929 | 928 | 918 | 921 | 931 |
| Organic matter, g/kg DM | 946 | 946 | 948 | 947 | 945 | 933 | 945 |
| Crude protein, g/kg DM | 628 | 610 | 617 | 639 | 630 | 639 | 638 |
| Neutral detergent fiber, g/kg DM | 164 | 264 | 322 | 240 | 249 | 268 | 278 |
| Item 1 | Control | Xylose-Treated 2 | Xylose-Untreated 2 | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 16 | 24 | 8 | 16 | 24 | Control × Processed | Xyl.-Treated × -Untreated | Time, Lin. | Time, Quad. | Interaction Xyl. × Time | |||
| Dry matter | |||||||||||||
| A, g/kg | 355 | 300 | 299 | 258 | 287 | 286 | 308 | 12.5 | <0.01 | 0.47 | 0.41 | 0.68 | 0.03 |
| B, g/kg | 582 | 642 | 586 | 607 | 560 | 610 | 578 | 27.6 | 0.61 | 0.22 | 0.76 | 0.95 | 0.08 |
| kd, h−1 | 0.054 | 0.048 | 0.053 | 0.050 | 0.069 | 0.058 | 0.061 | 0.007 | 0.75 | 0.05 | 0.62 | 0.80 | 0.30 |
| Crude protein | |||||||||||||
| A, g/kg | 299 | 158 | 170 | 119 | 191 | 177 | 184 | 15.1 | <0.01 | 0.01 | 0.15 | 0.46 | 0.41 |
| B, g/kg | 717 | 870 | 807 | 784 | 660 | 770 | 767 | 48.4 | 0.28 | 0.04 | 0.83 | 0.68 | 0.07 |
| kd, h−1 | 0.038 | 0.037 | 0.040 | 0.041 | 0.056 | 0.044 | 0.051 | 0.007 | 0.40 | 0.08 | 0.95 | 0.49 | 0.30 |
| Item 1 | Control | Xylose-Treated 2 | Xylose-Untreated 2 | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 30 | 60 | 90 | Control × Processed | Xyl.-Treated × -Untreated | Time, Lin. | Time, Quad. | Interaction Xyl. × Time | |||
| Dry matter | |||||||||||||
| A, g/kg | 358 | 262 | 247 | 279 | 255 | 225 | 267 | 23.2 | <0.01 | 0.49 | 0.55 | 0.19 | 0.78 |
| B, g/kg | 653 | 743 | 528 | 466 | 716 | 648 | 756 | 140 | 0.95 | 0.30 | 0.43 | 0.52 | 0.62 |
| kd, h−1 | 0.039 | 0.033 | 0.025 | 0.036 | 0.034 | 0.026 | 0.018 | 0.007 | 0.19 | 0.40 | 0.36 | 0.43 | 0.98 |
| Crude protein | |||||||||||||
| A, g/kg | 345 | 242 | 235 | 257 | 244 | 244 | 245 | 18.7 | <0.01 | 0.97 | 0.70 | 0.67 | 0.86 |
| B, g/kg | 871 | 937 | 447 | 501 | 748 | 498 | 662 | 189 | 0.28 | 0.96 | 0.21 | 0.19 | 0.55 |
| kd, h−1 | 0.020 | 0.022 | 0.034 | 0.065 | 0.028 | 0.019 | 0.029 | 0.017 | 0.50 | 0.31 | 0.24 | 0.54 | 0.53 |
| Item 1 | Control | Xylose-Treated 2 | Xylose-Untreated 2 | SEM | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 2 | 4 | 6 | Control × Processed | Xyl.-Treated × -Untreated | Time, Lin. | Time, Quad. | Interaction Xyl. × Time | |||
| Dry matter | |||||||||||||
| A, g/kg | 247 | 290 | 238 | 202 | 252 | 206 | 204 | 18.4 | 0.46 | 0.15 | <0.01 | 0.38 | 0.29 |
| B, g/kg | 778 | 811 | 850 | 822 | 816 | 851 | 890 | 37.6 | 0.14 | 0.43 | 0.28 | 0.65 | 0.41 |
| kd, h−1 | 0.041 | 0.028 | 0.027 | 0.025 | 0.035 | 0.034 | 0.028 | 0.007 | 0.15 | 0.34 | 0.47 | 0.82 | 0.79 |
| Crude protein | |||||||||||||
| A, g/kg | 175 | 172 | 168 | 179 | 199 | 155 | 162 | 13.5 | 0.88 | 0.95 | 0.27 | 0.18 | 0.12 |
| B, g/kg | 905 | 941 | 964 | 905 | 918 | 923 | 948 | 38.7 | 0.52 | 0.83 | 0.93 | 0.65 | 0.41 |
| kd, h−1 | 0.038 | 0.031 | 0.028 | 0.022 | 0.033 | 0.035 | 0.029 | 0.003 | 0.03 | 0.07 | 0.06 | 0.34 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigon, F.; Pereira, D.A.B.; Loregian, K.E.; Magnani, E.; Marcondes, M.I.; Branco, R.H.; Benedeti, P.D.B.; Paula, E.M. Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 1) Peanut Meal. Animals 2023, 13, 23. https://doi.org/10.3390/ani13010023
Rigon F, Pereira DAB, Loregian KE, Magnani E, Marcondes MI, Branco RH, Benedeti PDB, Paula EM. Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 1) Peanut Meal. Animals. 2023; 13(1):23. https://doi.org/10.3390/ani13010023
Chicago/Turabian StyleRigon, Fernanda, David A. B. Pereira, Kalista E. Loregian, Elaine Magnani, Marcos I. Marcondes, Renata H. Branco, Pedro D. B. Benedeti, and Eduardo M. Paula. 2023. "Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 1) Peanut Meal" Animals 13, no. 1: 23. https://doi.org/10.3390/ani13010023
APA StyleRigon, F., Pereira, D. A. B., Loregian, K. E., Magnani, E., Marcondes, M. I., Branco, R. H., Benedeti, P. D. B., & Paula, E. M. (2023). Use of Heating Methods and Xylose to Increase Rumen Undegradable Protein of Alternative Protein Sources: 1) Peanut Meal. Animals, 13(1), 23. https://doi.org/10.3390/ani13010023





