Comparing Ant Assemblages and Functional Groups across Urban Habitats and Seasons in an East Asia Monsoon Climate Area
Abstract
:Simple Summary
Abstract
1. Introduction
- (i.)
- If more complex vegetation and less fragmentation resulted in greater ant diversity.
- (ii.)
- Whether there were seasonal differences in ant species richness and assemblage composition in relation to any effects of vegetation composition and/or habitat fragmentation.
- (iii.)
- Whether and how species richness and assemblage composition sensitivity varied in response to vegetation composition and/or habitat fragmentation between ant functional groups.
2. Materials and Methods
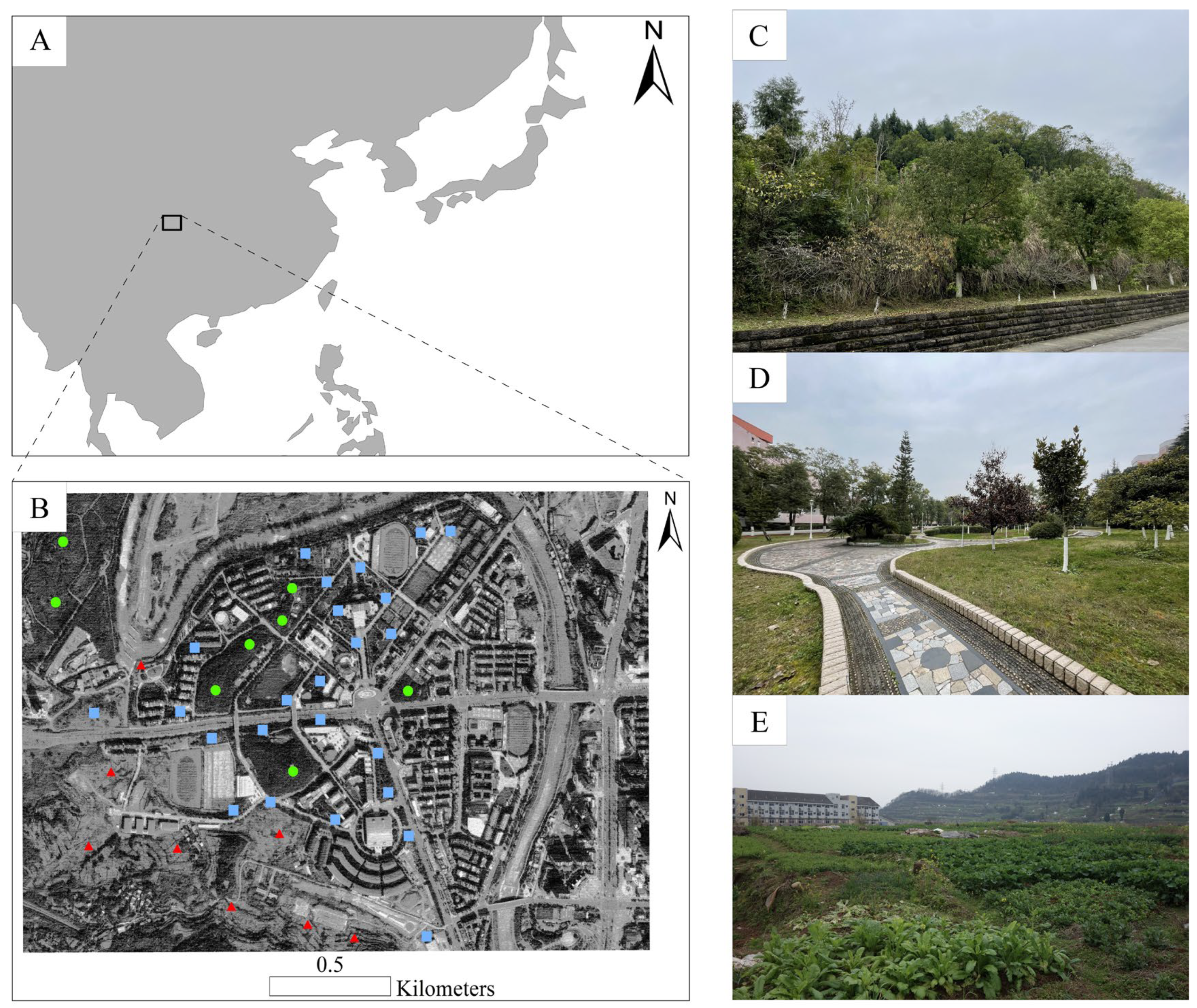
2.1. Study Area
2.2. Ant Sampling and Identification
2.3. Environmental Variables
2.4. Data Analyses
3. Results
3.1. Taxonomic Diversity
3.2. Drivers of Species Richness
3.3. Drivers of Assemblage Composition
4. Discussion
- (i.)
- Habitat in relation to vegetation differences within the mosaic of croplands, secondary woodlands, and cultivated gardens where this sort of land-use transformation is ongoing in the East Asia monsoon zone. Greater species richness was mainly contributed to by a greater variety of Opportunist species and occurred in a habitat with higher vegetation coverage and less fragmentation.
- (ii.)
- Seasonality. Warm summer conditions were consistently associated with higher ant presence and species richness, which was partly due to higher rates of ant foraging activity [50], and habitat predictors of assemblage composition also varied among seasons.
- (iii.)
- Ant functional groups. They differ in species diversity and assemblage composition mediated by vegetation composition and fragmentation.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, M.; Correa, C.; Marzluff, J.M.; Hendry, A.P.; Palkovacs, E.P.; Gotanda, K.M.; Hunt, V.M.; Apgar, T.M.; Zhou, Y. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl. Acad. Sci. USA 2017, 114, 8951–8956. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.T.J.; Munshi-South, J. Evolution of life in urban environments. Science 2017, 358, eaam8327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.Q.; Isaksson, C.; Schmidt, C.; Hutton, P.; Bonier, F.; Dominoni, D. A new framework for urban ecology: An integration of proximate and ultimate responses to anthropogenic change. Integr. Comp. Biol. 2018, 58, 915–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Des Roches, S.; Brans, K.I.; Lambert, M.R.; Rivkin, L.R.; Savage, A.M.; Schell, C.J.; Correa, C.; De Meester, L.; Diamond, S.E.; Grimm, N.B.; et al. Socio-eco-evolutionary dynamics in cities. Evol. Appl. 2021, 14, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Piano, E.; De Wolf, K.; Bona, F.; Bonte, D.; Bowler, D.E.; Isaia, M.; Lens, L.; Merckx, T.; Mertens, D.; van Kerckvoorde, M.; et al. Urbanization drives community shifts towards thermophilic and dispersive species at local and landscape scales. Glob. Chang. Biol. 2017, 23, 2554–2564. [Google Scholar] [CrossRef] [Green Version]
- Merckx, T.; Van Dyck, H. Urbanization-driven homogenization is more pronounced and happens at wider spatial scales in nocturnal and mobile flying insects. Glob. Ecol. Biogeogr. 2019, 28, 1440–1455. [Google Scholar] [CrossRef]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Davison, C.W.; Rahbek, C.; Morueta-Holme, N. Land-use change and biodiversity: Challenges for assembling evidence on the greatest threat to nature. Glob. Chang. Biol. 2021, 27, 5414–5429. [Google Scholar] [CrossRef]
- Fu, C. Potential impacts of human-induced land cover change on East Asia monsoon. Glob. Planet. Chang. 2003, 37, 219–229. [Google Scholar] [CrossRef]
- Kyuma, K. Nature and agriculture in monsoon Asia. World 2003, 13, 1–5. [Google Scholar]
- Zhang, H.; Gao, X. On the atmospheric dynamical responses to land-use change in East Asian monsoon region. Clim. Dyn. 2009, 33, 409–426. [Google Scholar] [CrossRef]
- Niyogi, D.; Kishtawal, C.; Tripathi, S.; Govindaraju, R.S. Observational evidence that agricultural intensification and land use change may be reducing the Indian summer monsoon rainfall. Water Resour. Res. 2010, 46, W03533. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yang, Y.; Sun, B.; Yuan, J.; Yu, M.; Stenseth, N.C.; Bullock, J.M.; Obersteiner, M. Spatial variation in biodiversity loss across China under multiple environmental stressors. Sci. Adv. 2020, 6, eabd0952. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, Z. Comparison of ant community composition among three urban habitats: A case study in Nanchong, Sichuan. Sichuan J. Zool. 2020, 39, 148–155. [Google Scholar] [CrossRef]
- Ge, B.; Zhou, J.; Yang, R.; Jiang, S.; Yang, L.; Tang, B. Lower land use intensity promoted soil macrofaunal biodiversity on a reclaimed coast after land use conversion. Agric. Ecosyst. Environ. 2021, 306, 107208. [Google Scholar] [CrossRef]
- Mata, L.; Andersen, A.N.; Morán-Ordóñez, A.; Hahs, A.K.; Backstrom, A.; Ives, C.D.; Bickel, D.; Duncan, D.; Palma, E.; Thomas, F.; et al. Indigenous plants promote insect biodiversity in urban greenspaces. Ecol. Appl. 2021, 31, e02309. [Google Scholar] [CrossRef] [PubMed]
- Jim, C.Y.; Chen, W.Y. Ecosystem services and valuation of urban forests in China. Cities 2009, 26, 187–194. [Google Scholar] [CrossRef]
- Jim, C.Y.; Chen, W.Y. Recreation–amenity use and contingent valuation of urban greenspaces in Guangzhou, China. Landscape Urban Plan. 2006, 75, 81–96. [Google Scholar] [CrossRef]
- Liu, H.; Li, F.; Xu, L.; Han, B. The impact of socio-demographic, environmental, and individual factors on urban park visitation in Beijing, China. J. Clean. Prod. 2017, 163, S181–S188. [Google Scholar] [CrossRef]
- Chen, W.Y.; Li, X. Urban forests’ recreation and habitat potentials in China: A nationwide synthesis. Urban For. Urban Gree. 2021, 66, 127376. [Google Scholar] [CrossRef]
- Burger, J. Bioindicators: Types, development, and use in ecological assessment and research. Environ. Bioindic. 2006, 1, 22–39. [Google Scholar] [CrossRef]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Nilon, C.H.; Lepczyk, C.A.; Parker, T.S.; Warren, P.S.; Cilliers, S.S.; Goddard, M.A.; Hahs, A.K.; Herzog, C.; Katti, M.; et al. Hierarchical filters determine community assembly of urban species pools. Ecology 2016, 97, 2952–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, E.R.; Hastings, A. Seasonality in ecology: Progress and prospects in theory. Ecol. Complex. 2020, 44, 100867. [Google Scholar] [CrossRef]
- Bujan, J.; Roeder, K.A.; Yanoviak, S.P.; Kaspari, M. Seasonal plasticity of thermal tolerance in ants. Ecology 2020, 101, e03051. [Google Scholar] [CrossRef]
- Radnan, G.N.; Gibb, H.; Eldridge, D.J. Soil surface complexity has a larger effect on food exploitation by ants than a change from grassland to shrubland. Ecol. Entomol. 2018, 43, 379–388. [Google Scholar] [CrossRef]
- Martínez-Hernández, J.G.; Corona-López, A.M.; Flores-Palacios, A.; Rös, M.; Toledo-Hernández, V.H. Seasonal diversity of Cerambycidae (Coleoptera) is more complex than thought: Evidence from a tropical dry forest of Mexico. PeerJ 2019, 7, e7866. [Google Scholar] [CrossRef] [Green Version]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Andersen, A.N. Functional groups and patterns of organization in north American ant communities: A comparison with Australia. J. Biogeogr. 1997, 24, 433–460. [Google Scholar] [CrossRef]
- Savage, A.M.; Hackett, B.; Guénard, B.; Youngsteadt, E.K.; Dunn, R.R. Fine-scale heterogeneity across Manhattan’s urban habitat mosaic is associated with variation in ant composition and richness. Insect Conserv. Divers. 2015, 8, 216–228. [Google Scholar] [CrossRef]
- Nooten, S.; Schultheiss, P.; Rowe, R.C.; Facey, S.L.; Cook, J.M. Habitat complexity affects functional traits and diversity of ant assemblages in urban green spaces (Hymenoptera: Formicidae). Myrmecol. News 2019, 29, 67–77. [Google Scholar] [CrossRef]
- Deák, B.; Báthori, F.; Lőrinczi, G.; Végvári, Z.; Nagy, D.D.; Mizser, S.; Torma, A.; Valkó, O.; Tóthmérész, B. Functional composition of ant assemblages in habitat islands is driven by habitat factors and landscape composition. Sci. Rep. 2021, 11, 20962. [Google Scholar] [CrossRef] [PubMed]
- Ossola, A.; Nash, M.A.; Christie, F.J.; Hahs, A.K.; Livesley, S.J. Urban habitat complexity affects species richness but not environmental filtering of morphologically-diverse ants. PeerJ 2015, 3, e1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, M.H.; Hung, Y.C.; Liu, K.L.; Neoh, K.B. Landscape configuration and habitat complexity shape arthropod assemblage in urban parks. Sci. Rep. 2020, 10, 16043. [Google Scholar] [CrossRef]
- Schultz, T.R. In search of ant ancestors. Proc. Natl. Acad. Sci. USA 2000, 97, 14028–14029. [Google Scholar] [CrossRef] [Green Version]
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The little things that run the world revisited: A review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146. [Google Scholar]
- Hevia, V.; Ortega, J.; Azcárate, F.M.; López, C.A.; González, J.A. Exploring the effect of soil management intensity on taxonomic and functional diversity of ants in Mediterranean olive groves. Agric. Forest Entomol. 2019, 21, 109–118. [Google Scholar] [CrossRef]
- Sheikh, A.H.; Ganaie, G.A.; Thomas, M.; Bhandari, R.; Rather, Y.A. Ant pitfall trap sampling: An overview. J. Entomol. Res. Soc. 2018, 42, 421–436. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.L. The Ants of China; China Forestry Publishing House: Beijing, China, 1995. [Google Scholar]
- Feng, L.A. Taxonomy study on genera Crematogaster and Myrmica from China (Hymenoptera: Formicida: Myrmicinae). Master’s Thesis, Guangxi Normal University, Guilin, China, 2007. [Google Scholar]
- Pan, Y.S. Systematic study on the ant genera Pheidole Westwood and Aphaenogaster Mayr (Hymenoptera: Formincidae: Myrmicinae) in China. Master’s Thesis, Guangxi Normal University, Guilin, China, 2007. [Google Scholar]
- Qian, F. Systematic study on the ant genera Tetramorium Mayr and Myrmica Latreille (Hymenoptera: Formicidae: Myrmicinae) from China. Master’s Thesis, Guangxi Normal University, Guilin, China, 2008. [Google Scholar]
- Bolton, B. A New General Catalogue of the Ants of the World; Harvard University Press: Cambridge, MA, USA, 1995. [Google Scholar]
- McIntyre, N.E.; Rango, J.; Fagan, W.F.; Faeth, S.H. Ground arthropod community structure in a heterogeneous urban environment. Landsc. Urban Plan. 2001, 52, 257–274. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Leite, M.F.; Vilhena, J.M.S.; Lima, A.P.; Magnusson, W.E. Ant diversity in an Amazonian savanna: Relationship with vegetation structure, disturbance by fire, and dominant ants. Austral. Ecol. 2008, 33, 221–231. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps; Computer Software Program; University of Massachusetts: Amherst, MA, USA, 2012; Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 10 March 2021).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Hough-Snee, N.; Roper, B.B.; Wheaton, J.M.; Budy, P.; Lokteff, R.L. Riparian vegetation communities change rapidly following passive restoration at a northern Utah stream. Ecol. Eng. 2013, 58, 371–377. [Google Scholar] [CrossRef]
- Zwiener, V.P.; Cardoso, F.C.G.; Padial, A.A.; Marques, M.C.M. Disentangling the effects of facilitation on restoration of the Atlantic Forest. Basic Appl. Ecol. 2014, 15, 34–41. [Google Scholar] [CrossRef]
- Jayatilaka, P.; Narendra, A.; Reid, S.F.; Cooper, P.; Zeil, J. Different effects of temperature on foraging activity schedules in sympatric Myrmecia ants. J. Exp. Biol. 2011, 214, 2730–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, E.A.; Dainese, M.; Clough, Y.; Báldi, A.; Bommarco, R.; Gagic, V.; Garratt, M.P.D.; Holzschuh, A.; Kleijin, D.; Kovács-Hostyánszki, A.; et al. The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 2019, 22, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Cheng, Q.; Zhuang, Z.; Tang, H.; Li, M.; Cheng, L.; Jin, X. The dynamic mechanism of landscape structure change of arable landscape system in China. Agric. Ecosyst. Environ. 2018, 251, 26–36. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- McIntyre, B.D.; Herren, H.R.; Wakhungu, J.; Watson, R.T. Agriculture at a Crossroads: Synthesis Report of the International Assessment of Agricultural Knowledge, Science, and Technology for Development (IAASTD); Island Press: Washington, DC, USA, 2009. [Google Scholar]
- Queiroz, C.; Beilin, R.; Folke, C.; Lindborg, R. Farmland abandonment: Threat or opportunity for biodiversity conservation? A global review. Front. Ecol. Environ. 2014, 12, 288–296. [Google Scholar] [CrossRef]
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Bai, H.; Ma, H.; Feng, G. Bird diversity in Chinese urban parks was more associated with natural factors than anthropogenic factors. Urban For. Urban Gree. 2019, 43, 126358. [Google Scholar] [CrossRef]
- Cui, W.; Liu, J.; Jia, J.; Wang, P. Terrestrial ecological restoration in China: Identifying advances and gaps. Environ. Sci. Eur. 2021, 33, 123. [Google Scholar] [CrossRef]
- Lu, Z.; Hoffmann, B.D.; Chen, Y. Can reforested and plantation habitats effectively conserve SW China’s ant biodiversity? Biodivers. Conserv. 2016, 25, 753–770. [Google Scholar] [CrossRef]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, Z.B.; Chen, L.F.; Du, J.; Yang, J.J.; Lin, P.F.; Li, J. Changes in species diversity, aboveground biomass, and distribution characteristics along an afforestation successional gradient in semiarid Picea crassifolia Plantations of Northwestern China. Forest. Sci. 2017, 63, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Hoffmann, B.D.; Lu, Z.; Chen, Y. Ants show that the conservation potential of afforestation efforts in Chinese valley-type savanna is dependent upon the afforestation method. J. Insect. Conserv. 2017, 21, 621–631. [Google Scholar] [CrossRef]
- Dalle Laste, K.C.; Durigan, G.; Andersen, A.N. Biodiversity responses to land-use and restoration in a global biodiversity hotspot: Ant communities in Brazilian Cerrado. Austral. Ecol. 2019, 44, 313–326. [Google Scholar] [CrossRef]
- de Queiroz, A.C.M.; Rabello, A.M.; Braga, D.L.; Santiago, G.S.; Zurlo, L.F.; Philpott, S.M.; Ribas, C.R. Cerrado vegetation types determine how land use impacts ant biodiversity. Biodivers. Conserv. 2020, 29, 2017–2034. [Google Scholar] [CrossRef]
- Wen, C.; Graf, H.F.; Ronghui, H. The interannual variability of East Asian Winter Monsoon and its relation to the summer monsoon. Adv. Atmos. Sci. 2000, 17, 48–60. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Li, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.C.; Eubanks, M.D.; Gold, R.E.; Behmer, S.T. Seasonality directs contrasting food collection behavior and nutrient regulation strategies in ants. PLoS ONE 2011, 6, e25407. [Google Scholar] [CrossRef] [Green Version]
- Kaspari, M.; Alonso, L.; O’Donnell, S. Three energy variables predict ant abundance at a geographical scale. Proc. Biol. Sci. 2000, 267, 485–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.; Pan, X.; Luo, X.; Wenda, C.; Zhao, Y.; Hu, Y.; Robinson, S.K.; Liu, Y. Seasonal variation in community composition and distributional ranges of birds along a subtropical elevation gradient in China. Divers. Distrib. 2021, 27, 2527–2541. [Google Scholar] [CrossRef]
- Zhu, W.B.; Zhao, C.L.; Liao, C.L.; Zou, B.; Xu, D.; Zhu, W.; Zhao, T.; Jiang, J.P. Spatial and temporal patterns of amphibian species richness on Tianping Mountain, Hunan Province, China. Zool. Res. 2020, 41, 182–187. [Google Scholar] [CrossRef]
- Ren, Y.; Yin, J.; Tan, Y.; Liu, H.; Yu, L.; Li, K. Monsoon-driven seasonal and spatial distribution of the copepod community along the northwest continental shelf of the South China Sea. J. Marine Syst. 2021, 218, 103529. [Google Scholar] [CrossRef]
- He, D.; Shen, W.; Eberwein, J.; Zhao, Q.; Ren, L.; Wu, Q.L. Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest. Soil Biol. Biochem. 2017, 115, 499–510. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.Y.; Yang, X.R.; Zhu, Y.G.; Hong, Y.W.; Su, J.Q. Spatial and seasonal variation of the airborne microbiome in a rapidly developing city of China. Sci. Total Environ. 2019, 665, 61–68. [Google Scholar] [CrossRef]
- Luo, S.; He, B.; Zeng, Q.; Li, N.; Yang, L. Effects of seasonal variation on soil microbial community structure and enzyme activity in a Masson pine forest in Southwest China. J. Mt. Sci. Engl. 2020, 17, 1398–1409. [Google Scholar] [CrossRef]
- Crist, T.O. Biodiversity, species interactions, and functional roles of ants (Hymenoptera: Formicidae) in fragmented landscapes: A review. Myrmecol. News 2009, 12, 3–13. [Google Scholar]
- Zhao, Y.; Dunn, R.R.; Zhou, H.; Si, X.; Ding, P. Island area, not isolation, drives taxonomic, phylogenetic and functional diversity of ants on land-bridge islands. J. Biogeogr. 2020, 47, 1627–1637. [Google Scholar] [CrossRef]
- Zhao, Y.; Sanders, N.J.; Liu, J.; Jin, T.; Zhou, H.; Lu, R.; Ding, P.; Si, X. β diversity among ant communities on fragmented habitat islands: The roles of species trait, phylogeny and abundance. Ecography 2021, 44, 1568–1578. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Andersen, A.N. Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral. Ecol. 2003, 28, 444–464. [Google Scholar] [CrossRef]
- Fotso Kuate, A.; Hanna, R.; Tindo, M.; Nanga, S.; Nagel, P. Ant diversity in dominant vegetation types of southern cameroon. Biotropica 2015, 47, 94–100. [Google Scholar] [CrossRef]
- Andersen, A.N. Responses of ant communities to disturbance: Five principles for understanding the disturbance dynamics of a globally dominant faunal group. J. Anim. Ecol. 2019, 88, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Aguillon, S.M.; Fitzpatrick, J.W.; Bowman, R.; Schoech, S.J.; Clark, A.G.; Coop, G.; Chen, N. Deconstructing isolation-by-distance: The genomic consequences of limited dispersal. PLoS Genet. 2017, 13, e1006911. [Google Scholar] [CrossRef] [Green Version]
- Paolucci, L.N.; Schoereder, J.H.; Brando, P.M.; Andersen, A.N. Fire-induced forest transition to derived savannas: Cascading effects on ant communities. Biol. Conserv. 2017, 214, 295–302. [Google Scholar] [CrossRef]
- Leal, I.R.; Filgueiras, B.K.C.; Gomes, J.P.; Iannuzzi, L.; Andersen, A.N. Effects of habitat fragmentation on ant richness and functional composition in Brazilian Atlantic forest. Biodivers. Conserv. 2012, 21, 1687–1701. [Google Scholar] [CrossRef]
- Solar, R.R.d.C.; Barlow, J.; Andersen, A.N.; Schoereder, J.H.; Berenguer, E.; Ferreira, J.N.; Gardner, T.A. Biodiversity consequences of land-use change and forest disturbance in the Amazon: A multi-scale assessment using ant communities. Biol. Conserv. 2016, 197, 98–107. [Google Scholar] [CrossRef]
- Mauda, E.V.; Joseph, G.S.; Seymour, C.L.; Munyai, T.C.; Foord, S.H. Changes in landuse alter ant diversity, assemblage composition and dominant functional groups in African savannas. Biodivers. Conserv. 2018, 27, 947–965. [Google Scholar] [CrossRef]
- Clarke, K.M.; Fisher, B.L.; LeBuhn, G. The influence of urban park characteristics on ant (Hymenoptera, Formicidae) communities. Urban Ecosyst. 2008, 11, 317–334. [Google Scholar] [CrossRef]
- Yamaguchi, T. Influence of urbanization on ant distribution in parks of Tokyo and Chiba City, Japan I. Analysis of ant species richness. Ecol. Res. 2004, 19, 209–216. [Google Scholar] [CrossRef]
- Yamaguchi, T. Influence of urbanization on ant distribution in parks of Tokyo and Chiba City, Japan II. Analysis of species. Entomol. Sci. 2005, 8, 17–25. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Rutkowski, T.; Witek, M.; Ślipiński, P.; Babik, H.; Czechowski, W. Three categories of urban green areas and the effect of their different management on the communities of ants, spiders and harvestmen. Urban Ecosyst. 2020, 23, 803–818. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, A.V.; Sousa-Souto, L.; Klein, A.M.; Tscharntke, T. Seasonal contrasts in the response of coffee ants to agroforestry shade-tree management. Environ. Entomol. 2010, 39, 1744–1750. [Google Scholar] [CrossRef]
- Cuautle, M.; Vergara, C.H.; Badano, E.I. Comparison of ant community diversity and functional group composition associated to land use change in a seasonally dry oak forest. Neotrop. Entomol. 2016, 45, 170–179. [Google Scholar] [CrossRef]
- Zumeaga, H.; Azcárate, F.M.; Concepción, E.D.; Hevia, V.; Díaz, M. Landscape and agri-environmental scheme effects on ant communities in cereal croplands of central Spain. Agric. Ecosyst. Environ. 2021, 312, 107345. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, Z.; Song, J.; Dai, A.; Yang, X.; Huo, F. Effects of urban land-use change in East China on the East Asian summer monsoon based on the CAM5.1 model. Clim. Dyn. 2016, 46, 2977–2989. [Google Scholar] [CrossRef]
- Caddy-Retalic, S.; Hoffmann, B.D.; Guerin, G.R.; Andersen, A.N.; Wardle, G.M.; McInerney, F.A.; Lowe, A.J. Plant and ant assemblages predicted to decouple under climate change. Divers. Distrib. 2019, 25, 551–567. [Google Scholar] [CrossRef] [Green Version]
- Boyle, M.J.W.; Bishop, T.R.; Luke, S.H.; van Breugel, M.; Evans, T.A.; Pfeifer, M.; Fayle, T.M.; Hardwick, S.R.; Lane-Shaw, R.I.; Yusah, K.M.; et al. Localised climate change defines ant communities in human-modified tropical landscapes. Funct. Ecol. 2021, 35, 1094–1108. [Google Scholar] [CrossRef]
- Roeder, K.A.; Bujan, J.; de Beurs, K.M.; Weiser, M.D.; Kaspari, M. Thermal traits predict the winners and losers under climate change: An example from North American ant communities. Ecosphere 2021, 12, e03645. [Google Scholar] [CrossRef]

| Subfamily | Species * | The Number of Individuals | |||
|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | ||
| Dolichoderinae | Ochetellus glaber O | 9 | 92 | 86 | 0 |
| Formicinae | Camponotus japonicus SC | 23 | 90 | 30 | 3 |
| Nylanderia bourbonica O | 176 | 394 | 269 | 29 | |
| Nylanderia flavipes O | 77 | 189 | 269 | 12 | |
| Plagiolepis manczshurica CS | 53 | 91 | 72 | 7 | |
| Myrmicinae | Aphaenogaster japonica O | 168 | 386 | 321 | 28 |
| Crematogaster rogenhoferi GM | 48 | 41 | 84 | 2 | |
| Crematogaster vagula GM | 3 | 11 | 5 | 0 | |
| Monomorium pharaonis GM | 227 | 808 | 154 | 9 | |
| Monomorium chinense GM | 914 | 1877 | 343 | 1 | |
| Pheidole capellinii GM | 210 | 454 | 69 | 1 | |
| Pheidole nodus GM | 589 | 1289 | 741 | 55 | |
| Strumigenys formosa CS | 2 | 1 | 4 | 0 | |
| Strumigenys hispida CS | 0 | 8 | 7 | 0 | |
| Tetramorium shensiense O | 124 | 1029 | 1407 | 4 | |
| Tetramorium tonganum O | 29 | 135 | 164 | 14 | |
| Tetramorium tsushimae O | 1736 | 4161 | 1483 | 48 | |
| Ponerinae | Leptogenys chinensis SP | 27 | 111 | 63 | 2 |
| Leptogenys diminuta SP | 13 | 6 | 45 | 0 | |
| Odontomachus monticola O | 9 | 43 | 29 | 0 | |
| Season | Opportunists | Generalized Myrmicinae | Subordinate Camponotini | Specialist Predators | Cryptic Species |
|---|---|---|---|---|---|
| Spring | 1.00 (8) | 0.98 (6) | 0.33 (1) | 0.20 (2) | 0.43 (2) |
| Summer | 1.00 (8) | 1.00 (6) | 0.45 (1) | 0.43 (2) | 0.43 (3) |
| Autumn | 1.00 (8) | 1.00 (6) | 0.33 (1) | 0.53 (2) | 0.40 (3) |
| Winter | 0.70 (6) | 0.58 (5) | 0.05 (1) | 0.03 (1) | 0.10 (1) |
| Assemblage | Marginal R2 | Conditional R2 | Effect * | Estimate | S.E. | p | |
|---|---|---|---|---|---|---|---|
| Ant community (n = 40) | 0.61 | 0.63 | Fixed | SVOC | 5.00 | 1.52 | 0.00 |
| PD | 0.10 | 0.04 | 0.03 | ||||
| SPLIT | −0.66 | 0.24 | 0.01 | ||||
| Random | Sampling plot | S.D. = 0.09 | |||||
| Opportunists (n = 40) | 0.53 | 0.55 | Fixed | SVOC | 0.03 | 1.33 | 0.01 |
| PD | 0.08 | 0.04 | 0.04 | ||||
| SPLIT | −0.47 | 0.21 | 0.03 | ||||
| Random | Sampling plot | S.D. = 0.08 | |||||
| Generalized Myrmicinae (n = 40) | 0.52 | 0.59 | Fixed | SVOC | 3.36 | 1.73 | 0.06 |
| PD | 0.04 | 0.05 | 0.42 | ||||
| SPLIT | −0.29 | 0.27 | 0.29 | ||||
| Random | Sampling plot | S.D. = 0.16 | |||||
| Assemblage | Sampling Time | Best Explanatory Set of Environmental Variables * | Correlation (Mantel Statistic r) | Index with the Greatest Explanatory Power * (Correlation) | ||
|---|---|---|---|---|---|---|
| Vegetation Indices | Fragmentation Indices | Vegetation Indices | Fragmentation Indices | |||
| Ant community | Spring | HVUC, BGUC | 0.12 (0.12) | HVUC (0.11) | ||
| Summer | HVUC, HVOC, BGUC | 0.30 (0.30) | HVUC (0.25) | |||
| Autumn | HVOC, BGUC | 0.31 (0.30) | BGUC (0.29) | |||
| Winter | HVOC, BGUC | 0.16 (0.16) | HVOC (0.15) | |||
| 12 months | HVUC, HVOC, BGUC | 0.17 (0.17) | HVOC (0.14) | |||
| Opportunists | Spring | HVUC, BGUC | 0.14 (0.14) | HVUC (0.13) | ||
| Summer | HVUC, HVOC | 0.32 (0.31) | HVUC (0.28) | |||
| Autumn | HVUC, HVOC, SVUC, BGUC | 0.34 (0.32) | BGUC (0.30) | |||
| Winter | HVOC, BGUC | 0.29 (0.24) | BGUC (0.28) | |||
| 12 months | HVUC, HVOC, BGUC | 0.20 (0.20) | HVUC (0.17) | |||
| Generalized Myrmicinae | Spring | HVUC, Simpson’s diversity index | 0.13 (0.11) | Simpson’s diversity index (0.11) | ||
| Summer | HVUC, BGUC | MESH | 0.16 (0.15) | BGUC (0.14) | ||
| Autumn | PD, DIVISION, MESH, SPLIT | 0.20 (0.16) | MESH (0.20) | |||
| Winter | HVOC | 0.15 (0.15) | HVOC (0.15) | |||
| 12 months | HVUC | SPLIT | 0.08 (0.07) | SPLIT (0.07) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.-Y.; Newman, C.; Luo, Y.; Zhou, Z.-M. Comparing Ant Assemblages and Functional Groups across Urban Habitats and Seasons in an East Asia Monsoon Climate Area. Animals 2023, 13, 40. https://doi.org/10.3390/ani13010040
Luo X-Y, Newman C, Luo Y, Zhou Z-M. Comparing Ant Assemblages and Functional Groups across Urban Habitats and Seasons in an East Asia Monsoon Climate Area. Animals. 2023; 13(1):40. https://doi.org/10.3390/ani13010040
Chicago/Turabian StyleLuo, Xin-Yu, Chris Newman, Yi Luo, and Zhao-Min Zhou. 2023. "Comparing Ant Assemblages and Functional Groups across Urban Habitats and Seasons in an East Asia Monsoon Climate Area" Animals 13, no. 1: 40. https://doi.org/10.3390/ani13010040
APA StyleLuo, X.-Y., Newman, C., Luo, Y., & Zhou, Z.-M. (2023). Comparing Ant Assemblages and Functional Groups across Urban Habitats and Seasons in an East Asia Monsoon Climate Area. Animals, 13(1), 40. https://doi.org/10.3390/ani13010040







