Effect of Qiling Jiaogulan Powder on Pulmonary Fibrosis and Pulmonary Arteriole Remodeling in Low-Temperature-Exposed Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of QLJP
2.2. Analysis of QLJP Using Ultrahigh Phase Liquid Chromatography–Mass Spectrometry (UHPLC–MS)
2.3. Animal Experimental Design
2.4. Sample Collection
2.5. Masson’s Trichrome Staining of the Lung
2.6. Determination of the Hydroxyproline Content in the Lung
2.7. Determination of Pulmonary Arteriole Remodeling
2.8. Immunohistochemical Analysis
2.9. Quantitative Real-Time PCR Detection
2.10. ELISA Detection
2.11. Statistical Analysis
3. Results
3.1. Identification of the Components in QLJP using UHPLC-MS
3.2. The Effect of QLJP on Broiler Pulmonary Fibrosis
3.3. The Effect of QLJP on the HYP Content in Lung
3.4. The Effect of QLJP on the Pathological Changes of Pulmonary Arterioles
3.5. The Effect of QLJP on Pulmonary Arteriole Remodeling
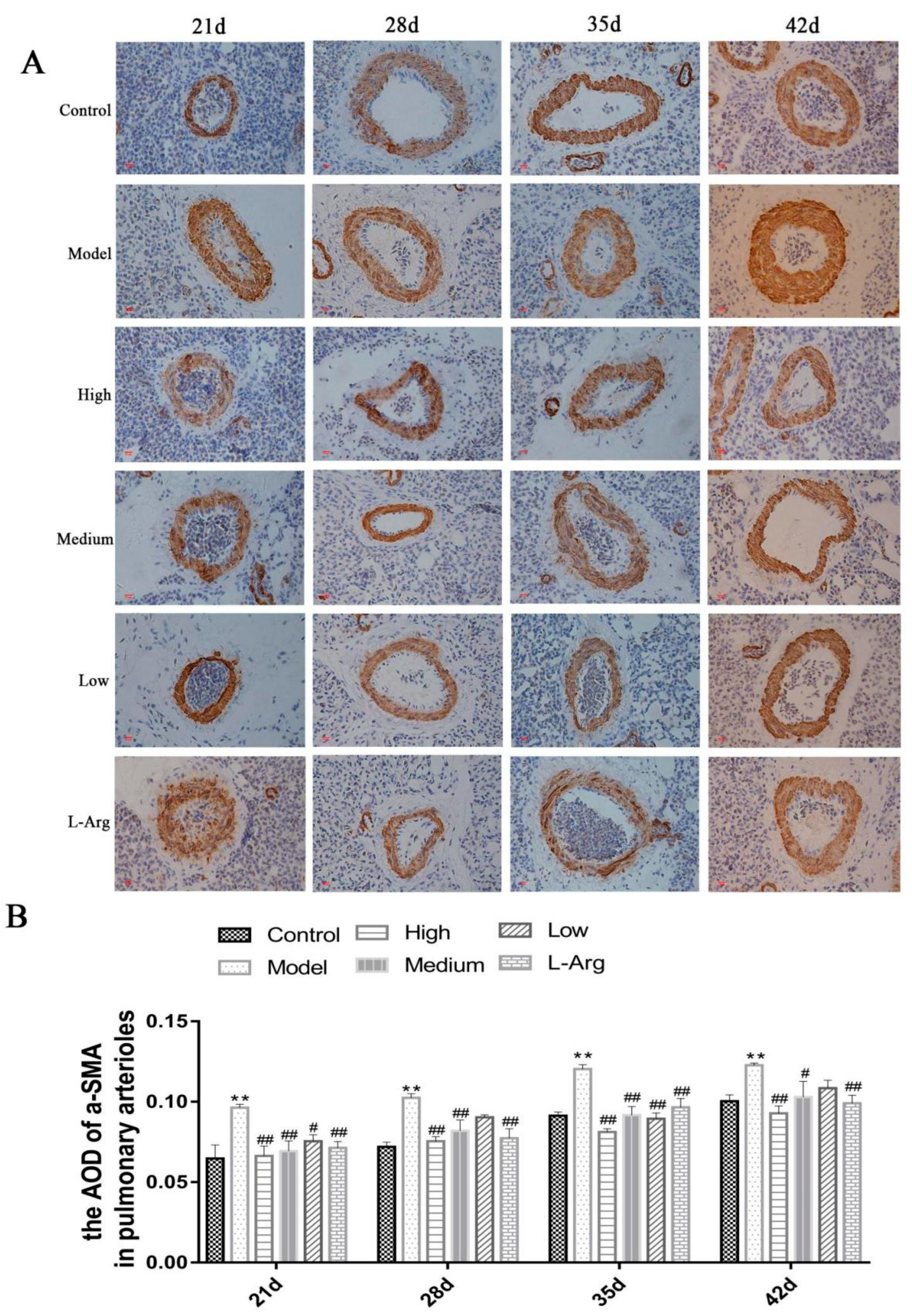
3.6. The Effect of QLJP on the Level of a-SMA Protein in Pulmonary Arterioles by IHC
3.7. QLJP Reduced the Expression Levels of TGF-β1 and Smad2 in the Lung
3.8. QLJP Downregulated the Expression Levels of COL1A1 and MMP2 in the Lung
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wideman, R.F.; Rhoads, D.D.; Erf, G.F.; Anthony, N.B. Pulmonary arterial hypertension (ascites syndrome) in broilers: A review. Poult. Sci. 2013, 92, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Barbera, J.A.; Gaine, S.P.; Harari, S.; Martinez, F.J.; Olschewski, H.; Olsson, K.M.; Peacock, A.J.; Pepke-Zaba, J.; Provencher, S.; et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur. Respir. J. 2019, 53, 1801914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurakula, K.; Smolders, V.F.E.D.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, A.; Decuypere, E. Ascites syndrome in broilers: Physiological and nutritional perspectives. Avian. Pathol. 2008, 37, 117–126. [Google Scholar] [CrossRef]
- Wideman, R.F.; Hamal, K.R.; Bayona, M.T.; Lorenzoni, A.G.; Cross, D.; Khajali, F. Plexiform lesions in the lungs of domestic fowl selected for susceptibility to pulmonary arterial hypertension: Incidence and histology. Anat. Rec. 2011, 294, 739–755. [Google Scholar] [CrossRef]
- Liu, J.H.; Liang, L.C.; Jin, J.S. The Relationship between Pulmonary Vascular Structural Remodeling and the Development of Pulmonary Hypertension of Broilers. Acta Vet. Zootech. Sin. 2003, 34, 515–520. [Google Scholar]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Migura, L.; Hendriksen, R.S.; Fraile, L.; Aarestrup, F.M. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 2014, 170, 1–9. [Google Scholar] [CrossRef]
- Xue, Z.; Li, Y.; Zhou, M.; Liu, Z.; Fan, G.; Wang, X.; Zhu, Y.; Yang, J. Traditional Herbal Medicine Discovery for the Treatment and Prevention of Pulmonary Arterial Hypertension. Front. Pharmacol. 2021, 12, 720873. [Google Scholar] [CrossRef]
- Committee of the Veterinary Pharmacopoeia of PR China. Veterinary Drug Quality Standard: 2017 Edition. Chinese Medicine Volume; China Agriculture Press: Beijing, China, 2017; pp. 146–147. [Google Scholar]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Qian, W.; Cai, X.; Qian, Q.; Zhang, W.; Wang, D. Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J. Cell. Mol. Med. 2018, 22, 4354–4365. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiao, Y.; Guo, L.; Ma, Y.; Zhao, R.; Li, X.; Shen, L.; Zhou, Z.; Kim, S.C.; Liu, J. Astragaloside IV blocks monocrotaline-induced pulmonary arterial hypertension by improving inflammation and pulmonary artery remodeling. Int. J. Mol. Med. 2021, 47, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Su, H.F.; Shaker, S.; Kuang, Y.; Zhang, M.; Ye, M.; Qiao, X. Phytochemistry and cardiovascular protective effects of Huang-Qi (Astragali Radix). Med. Res. Rev. 2021, 41, 1999–2038. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta. Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Ze-Xian, D.; Yue, Z.; Yue-Hang, J.; Lei, W.; Jian-Ping, L.; Dai-Yin, P.; Nian-Jun, Y.; Wei-Dong, C. Research progress on chemical structures and pharmacological activities of Poria cocos polysaccharide and its derivatives. Zhongguo Zhong Yao Za Zhi 2020, 45, 4332–4434. [Google Scholar]
- Andújar, I.; Ríos, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Xu, L.; Wang, C.; Chen, K.; Xia, Y.; Li, J.; Li, S.; Wu, L.; Feng, J.; Xu, S.; et al. Alleviation of hepatic fibrosis and autophagy via inhibition of transforming growth factor-β1/Smads pathway through shikonin. J. Gastroenterol. Hepatol. 2019, 34, 263–276. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, J.; Zeng, Y.; Cai, M.; Yao, Y.; Wu, H.; You, L.; Dong, X.; Ni, J. Pharmacology, toxicity and pharmacokinetics of acetylshikonin: A review. Pharm. Biol. 2020, 58, 950–958. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ha, T.K.Q.; Yang, J.L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef]
- Shi, Y.X.; Zhang, Y.Y.; Jia, J. Observation on the regulation effect of Gynostemma pentaphyllum extract on serum superoxide dismutase, nitric oxide and pulmonary arterial pressure in broilers. Chin. J. Vet. Med. 2009, 45, 22–23. [Google Scholar]
- Su, C.; Li, N.; Ren, R.; Wang, Y.; Su, X.; Lu, F.; Zong, R.; Yang, L.; Ma, X. Progress in the Medicinal Value, Bioactive Compounds, and Pharmacological Activities of Gynostemma pentaphyllum. Molecules 2021, 26, 6249. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Song, H.H.; Ahn, K.S.; Oh, S.R.; Sadikot, R.T.; Joo, M. Ethanol extract of the tuber of Alisma orientale reduces the pathologic features in a chronic obstructive pulmonary disease mouse model. J. Ethnopharmacol. 2016, 188, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Guo, J.; Li Zong-ai Tian, S.S.; Zhu, J.J.; Yan, L.H.; Wang, Z.M.; Gao, L. Advances in studies on chemical compositions of Alismatis Rhizoma and their biological activities. China J. Chin. Mater. Med. 2020, 45, 1578–1595. [Google Scholar]
- Xue, X.H.; Zhou, X.M.; Wei, W.; Chen, T.; Su, Q.P.; Tao, J.; Chen, L.D. Alisol A 24-Acetate, a Triterpenoid Derived from Alisma orientale, Inhibits Ox-LDL-Induced Phenotypic Transformation and Migration of Rat Vascular Smooth Muscle Cells through Suppressing ERK1/2 Signaling. J. Vasc. Res. 2016, 53, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect Biol. 2018, 10, a022293. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Zhang, D.; Qian, F.; Wu, Y. Baccatin III ameliorates bleomycin-induced pulmonary fibrosis via suppression of TGF-β1 production and TGF-β1-induced fibroblast differentiation. Int. Immunopharmacol. 2019, 74, 105696. [Google Scholar] [CrossRef]
- Upton, P.D.; Davies, R.J.; Tajsic, T.; Morrell, N.W. Transforming growth factor-β(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1135–1145. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.J.; Holmes, A.M.; Deighton, J.; Long, L.; Yang, X.; Barker, L.; Walker, C.; Budd, D.C.; Upton, P.D.; Morrell, N.W. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-β in pulmonary artery smooth muscle cells: Role of proinflammatory cytokines. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L604–L615. [Google Scholar] [CrossRef]
- Tozzi, C.A.; Christiansen, D.L.; Poiani, G.J.; Riley, D.J. Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am. J. Respir. Crit. Care Med. 1994, 149, 1317–1326. [Google Scholar] [CrossRef]
- Talele, N.P.; Fradette, J.; Davies, J.E.; Kapus, A.; Hinz, B. Expression of alpha-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Rep. 2015, 4, 1016–1030. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B.; Dugina, V.; Ballestrem, C.; Wehrle-Haller, B.; Chaponnier, C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell. 2003, 14, 2508–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Han, X.; Fan, F.; Liu, Y.; Wang, T.; Wang, J.; Hu, P.; Ma, A.; Tian, H. Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF-β1/Smad3-mediated fibrotic responses through 5-HT(2A) receptors. Mol. Cell Biochem. 2014, 397, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, P.; Seeger, W.; Pullamsetti, S.S. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur. Respir. J. 2012, 40, 766–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepetit, H.; Eddahibi, S.; Fadel, E.; Frisdal, E.; Munaut, C.; Noel, A.; Humbert, M.; Adnot, S.; D’Ortho, M.P.; Lafuma, C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2005, 25, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Monticone, R.E.; McGraw, K.R. Proinflammation, profibrosis, and arterial aging. Aging Med. 2020, 3, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Feng, J.A.; Li, P.; Xing, D.; Zhang, Y.; Serra, R.; Ambalavanan, N.; Majid-Hassan, E.; Oparil, S. Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J. Appl. Physiol. 2006, 100, 564–571. [Google Scholar] [CrossRef]
- Li, N.; Wu, K.; Feng, F.; Wang, L.; Zhou, X.; Wang, W. Astragaloside IV alleviates silica-induced pulmonary fibrosis via inactivation of the TGF-β1/Smad2/3 signaling pathway. Int. J. Mol. Med. 2021, 47, 16. [Google Scholar] [CrossRef]
- An LKhajali, F.; Moghaddam, M.H.; Hassanpour, H. Arginine supplement improves broiler hypertensive response and gut function in broiler chickens reared at high altitude. Int. J. Biometeorol. 2014, 58, 1175–1179. [Google Scholar] [CrossRef]
- Barth, P.J.; Kimpel, C.; Roy, S.; Wagner, U. An improved mathematical approach for the assessment of the medial thickness of pulmonary arteries. Pathol. Res. Pract. 1993, 189, 567–576. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, J.; Wang, H.; Gao, M.; Ou, D.; Zhang, J.; Sun, M.; Yang, X.; Zhang, X.; Guo, Y. Calcium antagonist verapamil prevented pulmonary arterial hypertension in broilers with ascites by arresting pulmonary vascular remodeling. Eur. J. Pharmacol. 2007, 561, 137–143. [Google Scholar] [CrossRef]
- Wideman, R.F., Jr.; Hamal, K.R. Idiopathic pulmonary arterial hypertension: An avian model for plexogenic arteriopathy and serotonergic vasoconstriction. J. Pharmacol. Toxicol. Methods 2011, 63, 283–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wideman, R.F.; Tackett, C.D. Cardio-pulmonary function in broilers reared at warm or cool temperatures: Effect of acute inhalation of 100% oxygen. Poult. Sci. 2000, 79, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Shimoda, L.A. Lung Circulation. Compr. Physiol. 2016, 6, 897–943. [Google Scholar] [PubMed]
- King, C.S.; Nathan, S.D. Pulmonary hypertension due to interstitial lung disease. Curr. Opin. Pulm. Med. 2019, 25, 459–467. [Google Scholar] [CrossRef]
- Cuttica, M.J. Pulmonary hypertension associated with lung diseases and hypoxemia. Heart Fail Rev. 2016, 21, 299–308. [Google Scholar] [CrossRef]
- Li, L.C.; Kan, L.D. Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J. Ethnopharmacol. 2017, 198, 45–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, P.; Qin, H.; Zhang, Y.; Sun, X.; Song, X.; Liu, J.; Peng, H.; Liu, Y.; Nwafor, E.O.; et al. Traditional Chinese medicine combined with pulmonary drug delivery system and idiopathic pulmonary fibrosis: Rationale and therapeutic potential. Biomed. Pharmacother. 2021, 133, 111072. [Google Scholar] [CrossRef]
- Enkvetchakul, B.; Beasley, J.; Bottje, W. Pulmonary arteriole hypertrophy in broilers with pulmonary hypertension syndrome (ascites). Poult. Sci. 1995, 74, 1677–1682. [Google Scholar] [CrossRef]
- Balog, J.M.; Anthony, N.B.; Cooper, M.A.; Kidd, B.D.; Huff, G.R.; Huff, W.E.; Rath, N.C. Ascites syndrome and related pathologies in feed restricted broilers raised in a hypobaric chamber. Poult. Sci. 2000, 79, 318–323. [Google Scholar] [CrossRef]
- Maxwell, M.H. The histology and ultrastructure of ectopic cartilaginous and osseous nodules in the lungs of young broilers with an ascitic syndrome. Avian Pathol. 1988, 17, 201–219. [Google Scholar] [CrossRef]
- Bordenave, J.; Tu, L.; Savale, L.; Huertas, A.; Humbert, M.; Guignabert, C. New insights in the pathogenesis of pulmonary arterial hypertension. Rev. Mal. Respir. 2019, 36, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, T.T.; Gao, N.N.; Zhou, X.L.; Jiang, H.; Guo, R.; Jia, L.N.; Chang, H.; Gao, Y.; Gao, Z.M.; et al. Effect of Tongxinluo on pulmonary hypertension and pulmonary vascular remodeling in rats exposed to a low pressure hypoxic environment. J. Ethnopharmacol. 2016, 194, 668–673. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Nan, X.; Li, S.; Su, S.; Ma, K.; Li, Z.; Lu, D.; Ge, R. Tsantan Sumtang Alleviates Chronic Hypoxia-Induced Pulmonary Hypertension by Inhibiting Proliferation of Pulmonary Vascular Cells. Biomed. Res. Int. 2018, 2018, 9504158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, Y.; Huang, Y.; Peng, L. Effect of total flavonoids from astragalus complanatus on paraquat poisoning-induced pulmonary fibrosis in rats and its mechanisms. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2015, 33, 838–840. [Google Scholar] [PubMed]
- Xu, J.; Tan, Y.L.; Liu, Q.Y.; Huang, Z.C.; Qiao, Z.H.; Li, T.; Hu, Z.Q.; Lei, L. Quercetin regulates fibrogenic responses of endometrial stromal cell by upregulating miR-145 and inhibiting the TGF-β1/Smad2/Smad3 pathway. Acta Histochem. 2020, 122, 151600. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.Q.; Wang, M.C.; Chen, H.; Chen, L.; Liu, D.; Zhao, H.; Zhao, Y.Y. Poricoic acid ZA, a novel RAS inhibitor, attenuates tubulo-interstitial fibrosis and podocyte injury by inhibiting TGF-β/Smad signaling pathway. Phytomedicine 2017, 36, 243–253. [Google Scholar] [CrossRef]
- Deng, H.Y.; Lu, M.L.; Wang, H.X. Astragalus polysaccharide protects mice against pulmonary vascular remodeling after hypoxia-induced pulmonary hypertension. J. Army Med. Univ. 2022, 44, 40–46. [Google Scholar]
- Pereira, S.C.; Parente, J.M.; Belo, V.A.; Mendes, A.S.; Gonzaga, N.A.; do Vale, G.T.; Ceron, C.S.; Tanus-Santos, J.E.; Tirapelli, C.R.; Castro, M.M. Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 2018, 270, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Z.; Chen, K.F.; Wei, W. Effect of compound fulinggancao decoction on the expression of Transforming growth factor β1 in pulmonary artery of rats with pulmonary hypertension. J. Guangzhou Univ. Tradit. Chin. Med. 2009, 26, 50–53. [Google Scholar]
- Peng, J.H.; Li, X.M.; Feng, Q.; Hu, Y.Y. Effect of Gypenosides on TGF-β1/ Smad Signal Pathway in rats with liver fibrosis induced by carbon tetrachloride. J. Shanghai Univ. Tradit. Chin. Med. 2012, 26, 86–90. [Google Scholar]
- Wu, Y.; Cai, C.; Yang, L.; Xiang, Y.; Zhao, H.; Zeng, C. Inhibitory effects of formononetin on the monocrotaline-induced pulmonary arterial hypertension in rats. Mol. Med. Rep. 2020, 21, 1192–1200. [Google Scholar] [CrossRef] [PubMed]







| English Name | Latin Scientific Name | Parts Used | Origin | Amount (g) |
|---|---|---|---|---|
| Radix Astragalus | Astragalus membranaceus (Fisch.) Bunge | Radix | Inner Mongolia | 100 |
| Poria | Poria cocos (Schw.) Wolf | Peeled sclerotia | Yun Nan | 75 |
| Radix Arnebiae | Arnebia euchroma (Royle) Johnst | Radix | Yili, Xinjiang | 75 |
| Herba Gynostemmae | Gynostemma pentaphyllum (Thunb.) Makino | Herba | Shiyan, Hubei | 175 |
| Rhizoma Alismatis | Alisma orientale (Sam.) Juzep | Rhizoma | Si Chuan | 75 |
| Gene Name | Primer Sequences |
|---|---|
| TGF-β1 | F 5′-CCGATGAGTATTGGGCCAAAGAGC-3′ R 5′-GACACGTTGAACACGAAGAAGATGC-3′ |
| Smad2 | F 5′-TGTCATCCATTCTGCCATTCACTCC-3′ R 5′-CACCACTTCTCCTCTTGCCCATTC-3′ |
| COL1A1 | F 5′-TGGATTCTCGGTTACTGCTGTTGATAG-3′ R 5′-TTCGGGTTTCCACACATCCTTATCG-3′ |
| MMP2 | F 5′-CAACAGAAGGCAGGACAGATGGATAC-3′ R 5′-GGAAGATGAAGGGGAATACACAAGGAG-3′ |
| β-actin | F 5′-CATCTATGAAGGCTACGC-3′ R 5′-GGCTGTGGTGGTGAAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Li, P.; Duan, Z.; Liu, X. Effect of Qiling Jiaogulan Powder on Pulmonary Fibrosis and Pulmonary Arteriole Remodeling in Low-Temperature-Exposed Broilers. Animals 2023, 13, 5. https://doi.org/10.3390/ani13010005
Yu J, Li P, Duan Z, Liu X. Effect of Qiling Jiaogulan Powder on Pulmonary Fibrosis and Pulmonary Arteriole Remodeling in Low-Temperature-Exposed Broilers. Animals. 2023; 13(1):5. https://doi.org/10.3390/ani13010005
Chicago/Turabian StyleYu, Juan, Peng Li, Zhibian Duan, and Xingyou Liu. 2023. "Effect of Qiling Jiaogulan Powder on Pulmonary Fibrosis and Pulmonary Arteriole Remodeling in Low-Temperature-Exposed Broilers" Animals 13, no. 1: 5. https://doi.org/10.3390/ani13010005





