Equine Herpesvirus Type 1 Myeloencephalitis in the Brazilian Amazon
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Blood Sample Collection
2.2. Serological and Molecular Diagnosis
2.3. Animals Clinical Treatment
2.4. Necropsy and Histopathological Examination
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lecollinet, S.; Pronost, S.; Coulpier, M.; Beck, C.; Gonzalez, G.; Leblond, A.; Tritz, P. Viral Equine Encephalitis, a Growing Threat to the Horse Population in Europe? Viruses 2019, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Allkofer, A.; Garvey, M.; Ryan, E.; Lyons, R.; Ryan, M.; Lukaseviciute, G.; Walsh, C.; Venner, M.; Cullinane, A. Primary Vaccination in Foals: A Comparison of the Serological Response to Equine Influenza and Equine Herpesvirus Vaccines Administered Concurrently or 2 Weeks Apart. Arch. Virol. 2021, 166, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Galindo, D.; Rivera, H.; Ramírez, M.; Manchego, A.; Mantilla, J.; Valderrama, W. Seroprevalencia Del Virus de La Rinoneumonitis En Caballos (Equus Caballus) Del Perú. Rev. Investig. Vet. Perú 2015, 26, 342. [Google Scholar] [CrossRef]
- Da Silva, A.A.; Cunha, E.M.S.; Lara, M.D.C.C.D.S.H.; Villalobos, E.M.C.; Nassar, A.F.D.C.; Mori, E.; Zanuzzi, C.N.; Galosi, C.M.; Del Fava, C. Low Occurrence of Equine Herpesvirus 1 (EHV-1) as Cause of Abortion and Perinatal Mortality in Brazil. Arq. Inst. Biol. 2018, 85, 1–7. [Google Scholar] [CrossRef]
- Costa, E.A.; Rosa, R.; Oliveira, T.S.; Assis, A.C.; Paix�o, T.A.; Santos, R.L. Molecular Characterization of Neuropathogenic Equine Herpesvirus 1 Brazilian Isolates. Arq. Bras. Med. Vet. Zootec. 2015, 67, 1183–1187. [Google Scholar] [CrossRef][Green Version]
- Estima-Silva, P.; Riet-Correa, F.; Coelho, A.C.B.; Echenique, J.V.Z.; Marcolongo-Pereira, C.; Lima, M.; Diel, D.G.; Schild, A.L. Identification of Equine Herpesvirus Type 1 as Cause of Abortion in Mares in Southern Brazil. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1421–1424. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Miller, J.; Varnell, S.; Dallap-Schaer, B.; Aceto, H.; Simeone, A. Investigation of an EHV-1 Outbreak in the United States Caused by a New H752 Genotype. Pathogens 2021, 10, 747. [Google Scholar] [CrossRef]
- Cruz, F.; Fores, P.; Mughini-Gras, L.; Ireland, J.; Moreno, M.A.; Newton, J.R. Seroprevalence and Factors Associated with Equine Herpesvirus Type 1 and 4 in Spanish Purebred Horses in Spain. Vet. Rec. 2016, 178, 398. [Google Scholar] [CrossRef]
- Vandenberghe, E.; Boshuizen, B.; Delesalle, C.J.G.; Goehring, L.S.; Groome, K.A.; van Maanen, K.; de Bruijn, C.M. New Insights into the Management of an Ehv-1 (Equine Hospital) Outbreak. Viruses 2021, 13, 1429. [Google Scholar] [CrossRef]
- Muniz, T.D.P.T.P.; do Carmo Custódio De Souza Hunold Lara, M.; Villalobos, E.M.C.; Pinheiro, J.W.; de Oliveira, E.R.G.; Carneiro, G.F. Sero-Epidemiological and Reproductive Survey for Alphaherpesvirus Infections in Horses in the State of Pernambuco, Brazil. Semin. Cienc. Agrar. 2020, 41, 1079–1086. [Google Scholar] [CrossRef]
- Costa, E.A.; Rosa, R.; Oliveira, T.S.; Furtini, R.; Fonseca Júnior, A.A.; Paixão, T.A.; Santos, R.L. Diagnóstico Etiológico de Enfermidades Do Sistema Nervoso Central de Equinos No Estado de Minas Gerais, Brasil. Arq. Bras. Med. Vet. Zootec. 2015, 67, 391–399. [Google Scholar] [CrossRef][Green Version]
- Da Silva, A.A.; Villalobos, E.M.C.; Cunha, E.M.S.; Lara, M.D.C.C.D.S.H.; Nassar, A.F.D.C.; Piatti, R.M.; Castro, V.; Pinheiro, E.S.; De Carvalho, A.F.; Del Fava, C. Causes of Equine Abortion, Stillbirth, and Perinatal Mortality in Brazil. Arq. Inst. Biol. 2020, 87, 1–9. [Google Scholar] [CrossRef]
- Negussie, H.; Gizaw, D.; Tessema, T.S.; Nauwynck, H.J. Equine Herpesvirus-1 Myeloencephalopathy, an Emerging Threat of Working Equids in Ethiopia. Transbound. Emerg. Dis. 2017, 64, 389–397. [Google Scholar] [CrossRef]
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.B.F.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Barbić, L.; Lojkić, I.; Stevanović, V.; Bedeković, T.; Starešina, V.; Lemo, N.; Lojkić, M.; Madić, J. Papers: Two Outbreaks of Neuropathogenic Equine Herpesvirus Type 1 with Breed-Dependent Clinical Signs. Vet. Rec. 2012, 170, 227. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, M.B.; Lara, M.C.C.S.H. Herpesvírus Equino 1 e 4. In Doenças Infecciosas em Animais de Produção e de Companhia; Megrid, J., Ribeiro, M.G., Paes, A.C., Eds.; Roca: Rio de Janeiro, Brazil, 2016; pp. 723–729. [Google Scholar]
- Lara, M.C.C.S.H.; Cunha, E.M.S.; Villalobos, E.M.C.; Nassar, A.F.C.; Asano, K.M.; Fernandes, W.R.; Richtzenhain, L.J.; Brandão, P.E.; Mori, E. First Isolation of Equine Herpesvirus Type 1 From a Horse With Neurological Disease in Brazil. Arq. Inst. Biol. 2008, 75, 221–224. [Google Scholar] [CrossRef]
- Costa, E.A.; Lima, G.B.L.; Castro, R.T.; Furtini, R.; Portilho, R.V.; Resende, M. Meningoencephalitis in a Horse Associated with Equine Herpesvirus 1. Arq. Bras. Med. Vet. Zootec. 2008, 60, 1580–1583. [Google Scholar] [CrossRef]
- Costa, É.A.; Vasconcelos, A.C.; Bomfim, M.R.Q.; Silva, M.X.; Haddad, J.P.A.; Amorim, H.B.; Lima, G.B.L.; Furtini, R.; Resende, M. Epidemiological and Clinical Aspects of Equine Herpesvirus Encephalitis Infection in Horses That Died with Neurological Signs from Minas Gerais State, Brazil. Braz. J. Vet. Res. Anim. Sci. 2009, 46, 262–272. [Google Scholar] [CrossRef][Green Version]
- Mori, E.; Borges, A.S.; Delfiol, D.J.Z.; Oliveira Filho, J.P.; Gonçalves, R.C.; Cagnini, D.Q.; Lara, M.C.C.S.H.; Cunha, E.M.S.; Villalobos, E.M.C.; Nassar, A.F.C.; et al. First Defection of the Equine Herpesvirus 1 Neuropathogenic Variant in Brazil. OIE Rev. Sci. Tech. 2011, 30, 949–954. [Google Scholar] [CrossRef]
- Cunha, E.M.S.; Lara, M.C.C.S.H.; Villalobos, E.M.C.; Nassar, A.F.C.; Del Fava, C.; Scannapieco, E.M.; Cunha, M.S.; Mori, E.; Gomes, M.N. Investigation of Neurological Diseases in Equine Tested Negative for Rabies. Rev. Educ. Contin. CRMV-SP 2011, 9, 43. [Google Scholar]
- Boom, R.; Sol, C.J.A.; Salimans, M.M.M.; Jansen, C.L.; Wertheim-Van Dillen, P.M.E.; van der Noordaa, J. Rapid and Simple Method for Purification of Nucleic Acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Borchers, K.; Slater, J. A Nested PCR for the Detection and Differentiation of EHV-1 and EHV-4. J. Virol. Methods 1993, 45, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.; Eshetu, A.; Gizaw, D. Equine Herpesvirus 1 and/or 4 in Working Equids: Seroprevalence and Risk Factors in North Shewa Zone, Ethiopia. Ethiop. Vet. J. 2017, 21, 28. [Google Scholar] [CrossRef][Green Version]
- Traub-Dargatz, J.L.; Pelzel-Mccluskey, A.M.; Creekmore, L.H.; Geiser-Novotny, S.; Kasari, T.R.; Wiedenheft, A.M.; Bush, E.J.; Bjork, K.E. Case-Control Study of a Multistate Equine Herpesvirus Myeloencephalopathy Outbreak. J. Vet. Intern. Med. 2013, 27, 339–346. [Google Scholar] [CrossRef]
- Henninger, R.W.; Reed, S.M.; Saville, W.J.; Allen, G.P.; Hass, G.F.; Kohn, C.W.; Sofaly, C. Outbreak of Neurologic Disease Caused by Equine Herpesvirus-1 at a University Equestrian Center. J. Vet. Intern. Med. 2007, 21, 157–165. [Google Scholar] [CrossRef]
- Allen, G.P. Risk Factors for Development of Neurologic Disease after Experimental Exposure to Equine Herpesvirus-1 in Horses. Am. J. Vet. Res. 2008, 69, 1595–1600. [Google Scholar] [CrossRef]
- Sebastiano, M.; Chastel, O.; de Thoisy, B.; Eens, M.; Costantini, D. Oxidative Stress Favours Herpes Virus Infection in Vertebrates: A Meta-Analysis. Curr. Zool. 2016, 62, 325–332. [Google Scholar] [CrossRef]
- Ata, E.B.; Zaghawa, A.; Ghazy, A.A.; Elsify, A.; Shaapan, R.M. Equine Herpes Virus Type-1 Infection: Etiology, Epidemiology, Pathogenesis, Identification and Recent Diagnosis. Asian J. Epidemiol. 2018, 11, 34–45. [Google Scholar] [CrossRef]
- McFadden, A.M.J.; Hanlon, D.; McKenzie, R.K.; Gibson, I.; Bueno, I.M.; Pulford, D.J.; Orr, D.; Dunowska, M.; Stanislawek, W.L.; Spence, R.P.; et al. The First Reported Outbreak of Equine Herpesvirus Myeloencephalopathy in New Zealand. N. Z. Vet. J. 2016, 64, 125–134. [Google Scholar] [CrossRef]
- Maxwell, L.K.; Bentz, B.G.; Gilliam, L.L.; Ritchey, J.W.; Pusterla, N.; Eberle, R.; Holbrook, T.C.; McFarlane, D.; Rezabek, G.B.; Meinkoth, J.; et al. Efficacy of the Early Administration of Valacyclovir Hydrochloride for the Treatment of Neuropathogenic Equine Herpesvirus Type-I Infection in Horses. Am. J. Vet. Res. 2017, 78, 1126–1139. [Google Scholar] [CrossRef]
- Pusterla, N.; Hatch, K.; Crossley, B.; Wademan, C.; Barnum, S.; Flynn, K. Equine Herpesvirus-1 Genotype Did Not Significantly Affect Clinical Signs and Disease Outcome in 65 Horses Diagnosed with Equine Herpesvirus-1 Myeloencephalopathy. Vet. J. 2020, 255, 105407. [Google Scholar] [CrossRef] [PubMed]
- van Galen, G.; Leblond, A.; Tritz, P.; Martinelle, L.; Pronost, S.; Saegerman, C. A Retrospective Study on Equine Herpesvirus Type-1 Associated Myeloencephalopathy in France (2008–2011). Vet. Microbiol. 2015, 179, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Mapes, S.; Wademan, C.; White, A.; Estell, K.; Swain, E. Investigation of the Role of Mules as Silent Shedders of EHV-1 during an Outbreak of EHV-1 Myeloencephalopathy in California. Vet. Rec. 2012, 170, 465. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Wilkie, G.S.; Russell, C.A.; Compston, L.; Grafham, D.; Clissold, L.; McLay, K.; Medcalf, L.; Newton, R.; Davison, A.J.; et al. Genetic Diversity of Equine Herpesvirus 1 Isolated from Neurological, Abortigenic and Respiratory Disease Outbreaks. Transbound. Emerg. Dis. 2018, 65, 817–832. [Google Scholar] [CrossRef]
- Hafshejani, T.T.; Nekoei, S.; Vazirian, B.; Doosti, A.; Khamesipour, F.; Anyanwu, M.U. Molecular Detection of Equine Herpesvirus Types 1 and 4 Infection in Healthy Horses in Isfahan Central and Shahrekord Southwest Regions, Iran. BioMed Res. Int. 2015, 2015, 917854. [Google Scholar] [CrossRef] [PubMed]
- Goehring, L.S.; Brandes, K.; Ashton, L.V.; Wittenburg, L.A.; Olea-Popelka, F.J.; Lunn, D.P.; Hussey, G.S. Anti-Inflammatory Drugs Decrease Infection of Brain Endothelial Cells with EHV-1 in Vitro. Equine Vet. J. 2017, 49, 629–636. [Google Scholar] [CrossRef]
- Mesquita, L.P.; Costa, R.C.; Zanatto, D.A.; Bruhn, F.R.P.; Mesquita, L.L.R.; Lara, M.C.C.S.H.; Villalobos, E.M.C.; Massoco, C.O.; Mori, C.M.C.; Mori, E.; et al. Equine Herpesvirus 1 Elicits a Strong Pro-Inflammatory Response in the Brain of Mice. J. Gen. Virol. 2021, 102, 001556. [Google Scholar] [CrossRef] [PubMed]
- Baltrusch, S. The Role of Neurotropic B Vitamins in Nerve Regeneration. BioMed Res. Int. 2021, 2021, 9968228. [Google Scholar] [CrossRef]
- Studdert, M.J.; Hartley, C.A.; Dynon, K.; Sandy, J.R.; Slocombe, R.R.; Charles, J.A.; Milne, M.E.; Clarke, A.F.; El-Hage, C. Outbreak of Equine Herpesvirus Type 1 Myeloencephalitis: New Insights from Virus Identification by PCR and the Application of an EHV-1 -Specific Antibody Detection ELISA. Vet. Rec. 2003, 153, 417–423. [Google Scholar] [CrossRef]
- Hussey, G.S. Key Determinants in the Pathogenesis of Equine Herpesvirus 1 and 4 Infections. Vet. Pathol. 2019, 56, 656. [Google Scholar] [CrossRef] [PubMed]
- Holz, C.L.; Sledge, D.G.; Kiupel, M.; Nelli, R.K.; Goehring, L.S.; Soboll Hussey, G. Histopathologic Findings Following Experimental Equine Herpesvirus 1 Infection of Horses. Front. Vet. Sci. 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed]
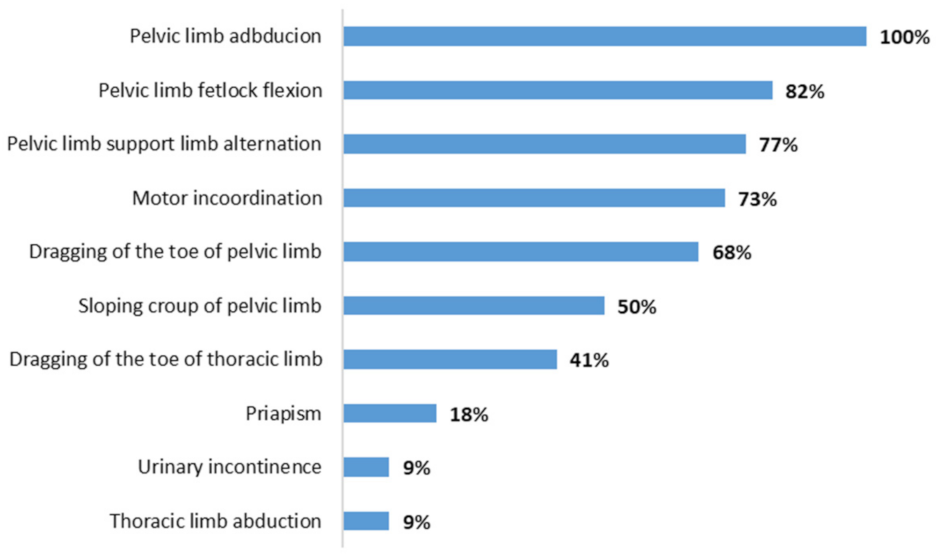


| Horses | Outbreak | Sex | Age * | Signs ** | Serology | PCR | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | F | 2 | Present | P | P | NT | Death |
| 2 | M | 9 | Present | P | N | T1 | Recovery | |
| 3 | F | 7 | Present | N | P | T1 | Recovery | |
| 4 | 2 | F | 4 | Present | P | P | T1 | Recovery |
| 5 | F | 3 | Present | P | N | NT | Recovery | |
| 6 | F | 5 | Present | P | P | T1 | Death | |
| 7 | 3 | M | 6 | Present | N | P | TIR | Recovery |
| 8 | F | 8 | Present | P | P | TIR | Recovery | |
| 9 | F | 2 | Present | N | P | NT | Recovery | |
| 10 | 4 | M | 9 | Present | P | P | TIR | Recovery |
| 11 | F | 9 | Present | P | P | TIR | Recovery | |
| 12 | 5 | M | 1 | Present | P | P | NT | Recovery |
| 13 | F | 3 | Present | P | P | T1 | Death | |
| 14 | 6 | F | 14 | Present | P | P | T1 | Recovery |
| 15 | F | 12 | Absence | N | P | NT | NA | |
| 16 | F | 8 | Absence | N | P | NT | NA | |
| 17 | 7 | F | 12 | Present | P | P | T2 | Recovery |
| 18 | F | 12 | Present | N | P | T2 | Recovery | |
| 19 | 8 | F | 2.5 | Present | P | P | T2 R | Death |
| 20 | F | 13 | Present | P | P | T2 R | Death | |
| 21 | 9 | M | 7 | Present | P | P | T1 | Recovery |
| 22 | M | 4 | Present | P | P | T1 | Recovery | |
| 23 | 10 | F | 8 | Present | N | P | T2 | Recovery |
| 24 | F | 12 | Absence | P | N | NT | NA | |
| 25 | F | 7 | Present | P | P | T1 R | Death |
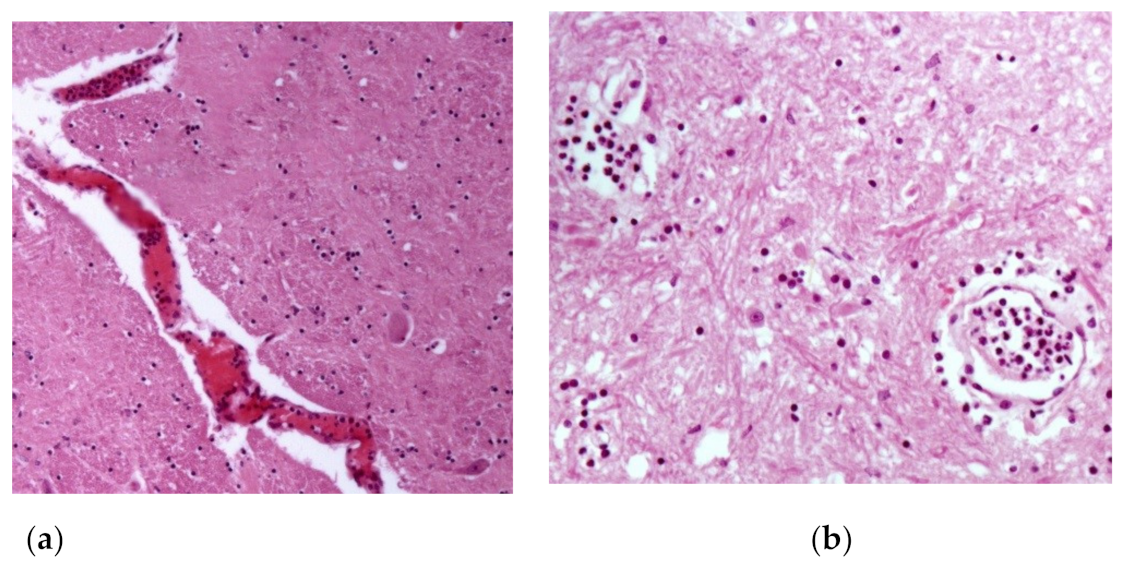
| Horse | Macroscopic Changes | Microscopic Changes |
|---|---|---|
| 1 | Brownish focal areas (suggestive of hemorrhage) in the gray matter of the spinal cord. | Spinal cord: congestion +(+) and neutrophilic leukocytosis inside and around meningeal and gray matter vessels, sometimes accompanied by fibrin inside vessels; presence of rare axonal spheroids; focal gliosis +(+). |
| 19 | Petechiae and ecchymoses +(+) in bladder; bladder ulcer with 4 cm of diameter with raised and irregular edges and surface covered by fibrin. | Spinal cord: activation of endothelial cells in meningeal vessels; rare lymphocytes and eosinophils inside meningeal vessels, sometimes leukocytostatis of neutrophils and fibrin; gliosis focuses (+); few axonal spheroids. |
| Brain cortex: leukocytostasis of neutrophils in meningeal vessels; endothelial cell activation in meningeal vessels; | ||
| Lung: diffuse edema +++; diffuse congestion ++; presence of shock bodies. | ||
| 25 | Brownish focal areas (suggestive of hemorrhage) in the gray matter ++ and white matter + of the spinal cord with meningeal hyperemia. | Spinal cord: endothelial cell activation in meningeal vessels; leukocytostatis neutrophils focuses on the meninges and white matter; rare axonal spheroids in white and gray matter. |
| Hippocampus: gliosis focuses +. | ||
| Thalamus: endothelial cell activation and leukocytostatis of neutrophils; areas of perivascular edema; neutrophilic vasculitis. | ||
| Brain cortex: vasculitis focuses composed by neutrophils and macrophages; endothelial cell activation in gray matter vessels. | ||
| Cerebellum: areas of perivascular edema. | ||
| Lung: diffuse congestion ++; neutrophil leukocytoesterase in interstitial and alveolar vessels. | ||
| Liver: circulating neutrophils ++, especially in centriolobular veins; pigment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, J.D.; Lins, A.d.M.C.; Bomjardim, H.d.A.; Silveira, N.d.S.e.S.; Barbosa, C.C.; Beuttemmuller, E.A.; Brito, M.F.; Salvarani, F.M. Equine Herpesvirus Type 1 Myeloencephalitis in the Brazilian Amazon. Animals 2023, 13, 59. https://doi.org/10.3390/ani13010059
Barbosa JD, Lins AdMC, Bomjardim HdA, Silveira NdSeS, Barbosa CC, Beuttemmuller EA, Brito MF, Salvarani FM. Equine Herpesvirus Type 1 Myeloencephalitis in the Brazilian Amazon. Animals. 2023; 13(1):59. https://doi.org/10.3390/ani13010059
Chicago/Turabian StyleBarbosa, José Diomedes, André de Medeiros Costa Lins, Henrique dos Anjos Bomjardim, Natália da Silva e Silva Silveira, Camila Cordeiro Barbosa, Edsel Alves Beuttemmuller, Marilene Farias Brito, and Felipe Masiero Salvarani. 2023. "Equine Herpesvirus Type 1 Myeloencephalitis in the Brazilian Amazon" Animals 13, no. 1: 59. https://doi.org/10.3390/ani13010059
APA StyleBarbosa, J. D., Lins, A. d. M. C., Bomjardim, H. d. A., Silveira, N. d. S. e. S., Barbosa, C. C., Beuttemmuller, E. A., Brito, M. F., & Salvarani, F. M. (2023). Equine Herpesvirus Type 1 Myeloencephalitis in the Brazilian Amazon. Animals, 13(1), 59. https://doi.org/10.3390/ani13010059







