Life History Traits of Sperm Whales Physeter macrocephalus Linnaeus, 1758 Stranded along Italian Coasts (Cetartiodactyla: Physeteridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Genetic Analysis
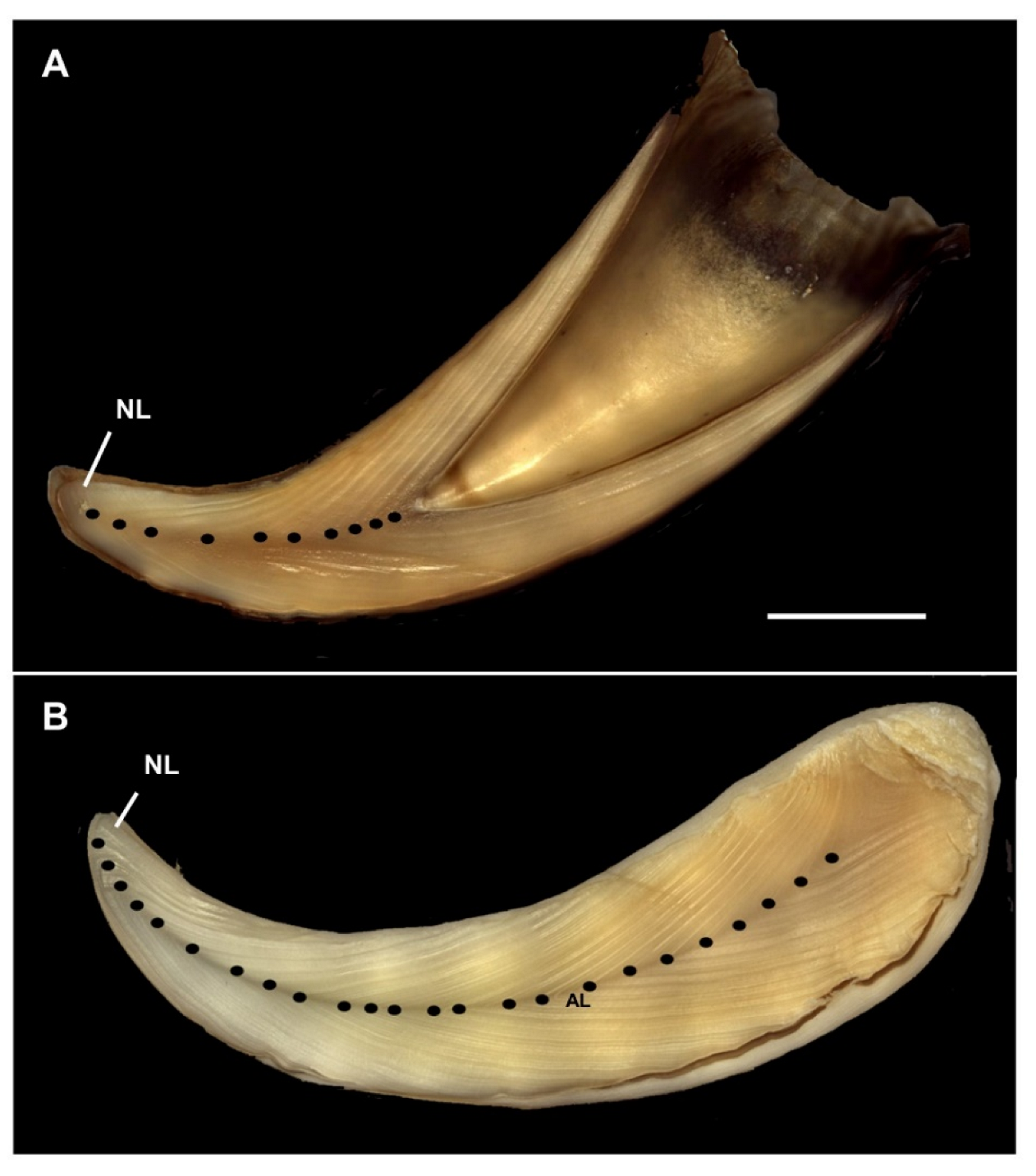
2.3. Age Determination
2.4. Reproductive Status and Sexual Maturity
3. Results
3.1. Genetic Analysis
3.2. Age Estimation and Body Length
3.3. Reproductive Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bearzi, G.; Pierantonio, N.; Affronte, M.; Holcer, D.; Maio, N.; DI Sciara, G.N. Overview of sperm whale Physeter macrocephalus mortality events in the Adriatic Sea, 1555–2009. Mammal Rev. 2011, 41, 276–293. [Google Scholar] [CrossRef]
- Cagnolaro, L.; Cozzi, B.; Notarbartolo di Sciara, G.; Podestà, M. Fauna d’Italia. XLIX–Mammalia IV. Cetacea. Calderini-Edizioni Calderini de il Sole 24 ORE S.p.A.: Bologna, Italy, 2015. [Google Scholar]
- Loy, A.; Aloise, G.; Ancillotto, L.; Angelici, F.M.; Bertolino, S.; Capizzi, D.; Castiglia, R.; Colangelo, P.; Contoli, L.; Scandura, M.; et al. Mammals of Italy: An annotated checklist. Hystrix 2019, 30, 87–106. [Google Scholar] [CrossRef]
- Maio, N.; Giovannotti, M.; Barucchi, V.C.; Petraccioli, A.; Pollaro, F.; Guarino, F.M.; Splendiani, A.; De Stasio, R.; Odierna, G. Haplotype characterization of a young stranded Common Minke Whale (Balaenoptera acutorostrata Lacépède, 1804): Is the MEditerranean Sea a potential calving or nursery ground for the species? Hystrix 2016, 27, 205–208. [Google Scholar] [CrossRef]
- Maio, N.; Pollaro, F.; Gasparro, A.; Petraccioli, A.; Mezzasalma, M.; Guariglia, M.; Galiero, G.; Di Nocera, F.; Iaccarino, D.; Santoro, M.; et al. New record of Dwarf Sperm Whale Kogiasima (Owen, 1866) from the Mediterranean Sea (Cetacea Kogiidae). Biodivers. J. 2017, 8, 947–950. [Google Scholar]
- Maio, N.; Pollaro, F.; Petraccioli, A.; Guarino, F.M. Guida Naturalistica di Campo ai Cetacei delle Acque Costiere del Parco Nazionale del Cilento, Vallo di Diano e Alburni. Biologia, Eco-Logia, Distribuzione e Conservazione. PNCVDA–Quaderni della Biodiversità n; 2022; in press; Volume 5, pp. XXVII + 314. ISBN 9788894503913. [Google Scholar]
- Mazzariol, S.; Di Guardo, G.; Petrella, A.; Marsili, L.; Fossi, C.M.; Leonzio, C.; Zizzo, N.; Vizzini, S.; Gaspari, S.; Pavan, G.; et al. Sometimes sperm whales (Physeter macrocephalus) cannot find their way back to the high seas: A multi-disciplinary study on a mass stranding. PLoS ONE 2011, 6, 19417. [Google Scholar] [CrossRef]
- Tomilin, A.G. 1957. Mammals of the U.S.S.R. and Adjacent Countries. Vol. 9. Cetacea. Izdatel’stvo Akademi Nauk SSSSR, Mosca, 717p; Israel Program for Scientific Translations Ltd.: Jerusalem, Israel, 1967; 756p. [Google Scholar]
- Best, P.B. The sperm whale (Physeter catodon) off the west coast of South Africa. 3. Reproduction in the male. Investig. Rep. Div. Sea Fish. S. Afr. 1969, 72, 1–20. [Google Scholar]
- Best, P.B.; Canham, P.A.S.; Macleod, N. Patterns of reproduction in sperm whales Physeter macrocephalus. Rep. Int. Whal. Comm. 1984, 6, 51–79. [Google Scholar]
- Nishiwaki, M.; Hibiya, T.; Ohsumi, S.K. Age study of sperm whale based on reading of tooth laminations. Rep. Int. Whal. Comm. Spec. 1961, 6, 135–153. [Google Scholar]
- Ohsumi, S. Sexual segregation of the sperm whale in the North Pacific. Sci. Rep. Whales Res. Inst. 1966, 20, 1–16. [Google Scholar]
- Nishiwaki, M.; Hibiya, T. On the Sexual Maturity of the Sperm Whale (Physeter catodon) found in the Adjacent Waters of Japan (I). Sci. Rep. Whales Res. Inst. 1951, 6, 153–166. [Google Scholar]
- Nishiwaki, M.; Hibiya, T.; Hibiya, T. Sexual maturity of the sperm whale found in the adjacent waters of Japan (II). Sci. Rep. Whales Res. Inst. 1952, 7, 121–124. [Google Scholar]
- Borrell, A.; Vacca, A.V.; Pinela, A.M.; Kinze, C.; Lockyer, C.H.; Vighi, M.; Aguilar, A. Stable Isotopes Provide Insight into Population Structure and Segregation in Eastern North Atlantic Sperm Whales. PLoS ONE 2013, 8, e82398. [Google Scholar] [CrossRef] [PubMed]
- IJsseldijk, L.L.; van Neer, A.; Deaville, R.; Begeman, L.; van de Bildt, M.; van den Brand, J.M.A.; Brownlow, A.; Czeck, R.; Dabin, W.; ten Doeschate, M.; et al. Beached bachelors: An extensive study on the largest recorded sperm whale Physeter macrocepha-lus mortality event in the North Sea. PLoS ONE 2018, 13, e0201221. [Google Scholar] [CrossRef] [PubMed]
- Best, P.B. Food and feeding of sperm whales Physeter macrocephalus off the west coast of South Africa. S. Afr. J. Mar. Sci. 1999, 21, 393–413. [Google Scholar] [CrossRef] [Green Version]
- Caruso, F.; Sciacca, V.; Bellia, G.; De Domenico, E.; Larosa, G.; Papale, E.; Pellegrino, C.; Pulvirenti, S.; Riccobene, G.; Simeone, F.; et al. Size Distribution of Sperm Whales Acoustically Identified during Long Term Deep-Sea Monitoring in the Ionian Sea. PLoS ONE 2015, 10, e0144503. [Google Scholar] [CrossRef] [Green Version]
- Mazzariol, S.; Centelleghe, C.; Cozzi, B.; Povinelli, M.; Marcer, F.; Ferri, N.; Di Francesco, G.; Badagliacca, P.; Profeta, F.; Olivieri, V.; et al. Multidisciplinary studies on a sick-leader syndrome-associated mass stranding of sperm whales (Physeter macrocephalus) along the Adriatic coast of Italy. Sci. Rep. 2018, 8, 11577. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.; Steel, D.; Hoekzema, K.; Mesnick, S.L.; Engelhaupt, D.; Kerr, I.; Payne, R.; Baker, C.S. What influences the worldwide genetic structure of sperm whales (Physeter macrocephalus)? Mol. Ecol. 2016, 25, 2754–2772. [Google Scholar] [CrossRef]
- Morin, P.A.; Foote, A.D.; Scott Baker, C.; Hancock-Hanser, B.L.; Kaschner, K.; Mate, B.R.; Mesnick, S.L.; Pease, V.L.; Rosel, P.E.; Alexander, A. Demography or selection on linked cultural traits or genes? Investigating the driver of low mtDNA di-versity in the sperm whale using complementary mitochondrial and nuclear genome analyses. Mol. Ecol. 2018, 27, 2604–2619. [Google Scholar] [CrossRef] [Green Version]
- Manfrini, V.; Pierantonio, N.; Giuliani, A.; De Pascalis, F.; Maio, N.; Mancia, A. Fin whale (Balaenoptera physalus) mortality along the Italian coast between 1624 and 2021. Animals 2022, 12, 3111. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Di Febbraro, M.; Guarino, F.M.; Odierna, G.; Russo, D. Cold-blooded in the Ice Age: “refugia within refugia”, inter-and intraspecific biogeographic diversification of European whipsnakes (Squamata, Colubridae, Hierophis). Zoology 2018, 127, 84–94. [Google Scholar] [CrossRef]
- Mezzasalma, M.; Visone, V.; Petraccioli, A.; Odierna, G.; Capriglione, T.; Guarino, F.M. Non-random accumulation of LINE1-like sequences on differentiated snake W chromosomes. J. Zool. 2016, 300, 67–75. [Google Scholar] [CrossRef]
- Pallotta, M.M.; Turano, M.; Ronca, R.; Mezzasalma, M.; Petraccioli, A.; Odierna, G.; Capriglione, T.; Capriglione, T. Brain gene expression is influenced by incubation temperature during leopard gecko (Eublepharis macularius) development. J. Experiment. Zool. Mol. Dev. Evol. 2017, 328, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, M.; Said, K.; Chatti, N.; Guarino, F.M.; Odierna, G.; Petraccioli, A.; Picariello, O.; Mezzasalma, M. Karyological characterization of the common chameleon (Chamaeleo chamaeleon) provides insights on the evolution and diversification of sex chromosomes in Chamaeleonidae. Zoology 2020, 141, 125738. [Google Scholar] [CrossRef]
- Unger, C.M.; Devine, J.; Hallgrímsson, B.; Rolian, C. Selection for increased tibia length in mice alters skull shape through parallel changes in developmental mechanisms. eLife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 3. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012, 40, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1990, 41, 95–98. [Google Scholar]
- Drouot, V.; Bérubé, M.; Gannier, A.; Goold, J.C.; Reid, R.J.; Palsboll, P. A note on genetic isolation of Mediterranean Sperm Whales, Physeter macrocephalus, suggested by mitochondrial DNA. J. Cetacean Res. Manag. 2004, 6, 29–32. [Google Scholar]
- Engelhaupt, D.; Rus Hoelzel, A.; Nicholson, C.; Frantzis, A.; Mesnick, S.; Gero, S.; Whitehead, H.; Rendell, L.; Miller, P.; De Stefanis, R.; et al. Female philopatry in coastal basins and male dispersion across the North Atlantic in a highly mobile marine species, the sperm whale (Physeter macrocephalus). Mol. Ecol. 2009, 18, 4193–4205. [Google Scholar] [CrossRef] [PubMed]
- Mesnick, S.L.; Taylor, B.L.; Archer, F.I.; Martien, K.K.; Treviño, S.E.; Hancock-Hanser, B.L.; Medina, S.C.M.; Pease, V.L.; Robertson, K.M.; Straley, J.M.; et al. Sperm whale population structure in the eastern and central North Pacific inferred by the use of single-nucleotide polymorphisms, microsatellites and mitochondrial DNA. Mol. Ecol. Resour. 2011, 11, 278–298. [Google Scholar] [CrossRef]
- Evans, K.; Robertson, K. A note on the preparation of sperm whale teeth (Physeter macrocephalus) for age determination. J. Cetacean Res. Manag. 2001, 3, 101–107. [Google Scholar]
- Guarino, F.M.; Di Nocera, F.; Pollaro, F.; Galiero, G.; Iaccarino, D.; Iovino, D.; Mezzasalma, M.; Petraccioli, A.; Odierna, G.; Maio, N. Skeletochronology, age at maturity and cause of mortality of loggerhead sea turtles Caretta caretta stranded along the beaches of Campania (south-western Italy, western Mediterranean Sea). Herpetozoa 2020, 33, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Guarino, F.M.; Di Nocera, F.; Galiero, G.; Iaccarino, D.; Giglio, S.; Madeo, E.; Pollaro, F.; Mezzasalma, M.; Iavarone, I.; Odierna, G.; et al. Age estimation and growth of striped dolphins Stenella coeruleoalba stranded along the coasts of south-western Italy. Eur. Zool J. 2021, 88, 417–424. [Google Scholar] [CrossRef]
- Hohn, A.A.; Chivers, S.J.; Barlow, J. Reproductive maturity and seasonality of male spotted dolphins, Stenella attenuata, in the Eastern Tropical Pacific. Marine Mamm. Sci. 1985, 1, 273–293. [Google Scholar] [CrossRef]
- Read, F.L.; Hohn, A.; Lockyer, C.H. A review of age estimation methods in marine mammals with special reference to monodontid. NAMMCO Sci. Publ. 2018, 10. [Google Scholar] [CrossRef]
- Buddhachat, K.; Brown, J.L.; Kaewkool, M.; Poommouang, A.; Kaewmong, P.; Kittiwattanawong, K.; Nganvongpanit, K. Life Expectancy in Marine Mammals Is Unrelated to Telomere Length but Is Associated with Body Size. Front. Genet. 2021, 12, 1792. [Google Scholar] [CrossRef]
- Chivers, S.J. Cetacean life history. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: SanDiego, CA, USA, 2009; pp. 215–220. [Google Scholar]
- Betty, E.; A Stockin, K.; Smith, A.N.H.; Bollard, B.; Orams, M.B.; Murphy, S. Sexual maturation in male long-finned pilot whales (Globicephala melas edwardii): Defining indicators of sexual maturity. J. Mammal. 2019, 100, 1387–1402. [Google Scholar] [CrossRef]
- Best, P.B.; Tormosov, D.; Brandao, A.; Mikhalev, H. Geographical variation in the body size of adult female sperm whales (Physeter macrocephalus)—An example of McNab’s resource rule? Mammalia 2016, 81, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D. Why squid, though not fish, may be better understoodby pretending they are. S. Afr. J. mar. Sci. 1998, 20, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Rosa, R.; Gonzalez, L.; Dierssen, H.M.; Seibel, B.A. Environmental determinants of latitudinal size-trends in cephalopods. Mar. Ecol. Progr. Ser. 2012, 464, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Paliza, O.; Aguayo Al, A. Sperm whales of the southeast Pacific. 4: Fatness, food and feeding. Invest. Cetacea. 1988, 21, 153–195. [Google Scholar]
- Huertas, I.E.; Ríos, A.F.; García-Lafuente, J.; Navarro, G.; Makaoui, A.; Sánchez-Román, A.; Rodriguez-Galvez, S.; Orbi, A.; Ruíz, J.; Pérez, F.F. Atlantic forcing of the Mediterranean oligotrophy. Global Biogeochem. Cycles 2012, 26, GB2022. [Google Scholar] [CrossRef]
- Garibaldi, F.; Podestà, M. Stomach contents of a sperm whale (Physeter macrocephalus) stranded in Italy (Ligurian Sea, north-western Mediterranean. J. Marine Biol. Assoc. 2014, 94, 1087–1091. [Google Scholar] [CrossRef]
- González, M.; Fernández-Casado, M.; Rodríguez, M.D.P.; Segura, A.; Martín, J.J. First record of the giant squid Architeuthis sp. (Architeuthidae) in the Mediterranean Sea. J. Mar. Biol. Assoc. 2000, 80, 745–746. [Google Scholar] [CrossRef]
- Sharir, Y.; Kerem, D.; Goldin, P.; Spanier, E. Small size in the common bottlenose dolphin Tursiops truncatus in the eastern Mediterranean: A possible case of Levantine nanism. Mar. Ecol. Progr. Ser. 2011, 438, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Pitman, R.L.; Perryman, W.L.; LeRoi, D.; Eilers, E. A dwarf form of killer whale in Antarctica. J. Mammal. 2007, 88, 43–48. [Google Scholar] [CrossRef]
- Rendell, L.; Frantzis, A. Mediterranean Sperm Whales, Physeter macrocephalus: The Precarious State of a Lost Tribe. In: Notarbartolo Di Sciara G, Podestà M and Curry BE, editors. Mediterranean Marine Mammals Ecology and Conservation. Adv. Mar. Biol. 2016, 75, 37–74. [Google Scholar]
- Violi, B. Population Dynamics and Structure of Sperm Whale (Physeter macrocephalus) in Mediterranean Sea. Ph.D. Dissertation, Università degli Studi di Genova, Genova, Italy, 2020. [Google Scholar]
- Kardos, M.; Taylor, H.R.; Ellegren, H.; Luikart, G.; Allendorf, F.W. Genomics advances the study of inbreeding depression in the wild. Evol. Appl. 2016, 9, 1205–1218. [Google Scholar] [CrossRef]
- Attard, C.R.M.; Beheregaray, L.B.; Möller, L.M. Towards population-level conservation in the critically endangered Antarctic blue whale: The number and distribution of their populations. Sci. Rep. 2016, 6, 22291. [Google Scholar] [CrossRef] [Green Version]
- Fioravanti, T.; Maio, N.; Latini, L.; Splendiani, A.; Guarino, F.M.; Mezzasalma, M.; Petraccioli, A.; Cozzi, B.; Mazzariol, S.; Centelleghe, C.; et al. Nothing is as it seems: Genetic analyses on stranded fin whales unveil the presence of a fin-blue whale hybrid in the Mediterranean Sea (Balaenopteridae). Eur. Zool. J. 2022, 89, 590–600. [Google Scholar] [CrossRef]
- Gilpin, M.E.; Soulé, M.E. Minimum Viable Populations: Processes of Species Extinction. In Conservation Biology: The Science of Scarcity and Diversity; Soulé, M.E., Ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 1986; pp. 19–34. [Google Scholar]


| ID | Stranding Place | Stranding Date | Sex | TBL (m) |
|---|---|---|---|---|
| Ph1 | Forio (Napoli) | 23 April 1770 | M | 10 |
| GP-1 | Castellabate (Salerno) | 1980 | M juv | 7.2 |
| 172 | Cagnano Varano (Foggia) | 10 December 2009 | M | 11.2 |
| 173 | Cagnano Varano (Foggia) | 10 December 2009 | M | 12.14 |
| 174 | Cagnano Varano (Foggia) | 10 December 2009 | M | 10.50 |
| 2330A | Polignano a Mare (Bari) | 29 September 2014 | juv | 8 |
| 549 | Acquedolci (Messina) | 3 June 2015 | F | 6.50 |
| 400 | Bagheria (Palermo) | 12 October 2016 | F | 8.4 |
| 154159 | Parghelia (Vibo Valentia) | 26 December 2017 | juv | 6.10 |
| 456 | Forio (Napoli) | 26 December 2018 | M juv | 8.6 |
| 45486 | San Lucido (Cosenza) | 3 April 2018 | M | 12.16 |
| 463 | Porto Cervo, Arzachena (Sassari) | 28 March 2019 | F | 8 |
| 465 | Capo Plaia, Cefalù (Palermo) | 16 May 2019 | F | 6.26 |
| 466 | Gioiosa Marea (Messina) | 21 May 2019 | M | 5.35 |
| 467 | Acqua dei Corsari (Palermo) | 19 May 2019 | M | 8.5 |
| 12988 | Near to Palmarola (Latina) | 19 June 2019 | F | 10 |
| Diagnostic Sites | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haplotypes | 38 | 53 | 57 | 100 | 102 | 104 | 116 | 145 | 179 | 195 | 202 | 203 | 206 | 230 | 233 | 238 | 255 | 267 | 268 | 278 | 281 | 282 | 283 | 284 | 286 | 290 | 300 | 303 | 314 | 319 | 345 | 569 | 603 | 619 | ||
| C | 001 | 001 | T | T | T | C | A | G | C | C | T | T | A | A | C | A | T | G | A | A | C | C | A | A | G | T | A | G | C | A | G | C | C | G | A | A |
| C | 001 | 002 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G |
| 2330A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 549 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 400 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 154159 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 456 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 45486 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 463 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 465 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 466 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| 467 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | ||
| C | 002 | 001 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . | G |
| ID | Sex | TBL (m) | Age (ys) |
|---|---|---|---|
| GP-1 | M juv | 7.2 | 8 |
| 549 | F | 6.50 | 24–26 |
| 400 | F | 8.40 | 21–22 |
| 154159 | ND juv | 6.10 | 3 |
| 456 | M juv | 8.6 | 10 |
| 45486 | M | 12.16 | 40–42 |
| 465 | F | 6.26 | 5 |
| 12988 | F | 10 | 38–40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maio, N.; Fioravanti, T.; Latini, L.; Petraccioli, A.; Mezzasalma, M.; Cozzi, B.; Mazzariol, S.; Podestà, M.; Insacco, G.; Pollaro, F.; et al. Life History Traits of Sperm Whales Physeter macrocephalus Linnaeus, 1758 Stranded along Italian Coasts (Cetartiodactyla: Physeteridae). Animals 2023, 13, 79. https://doi.org/10.3390/ani13010079
Maio N, Fioravanti T, Latini L, Petraccioli A, Mezzasalma M, Cozzi B, Mazzariol S, Podestà M, Insacco G, Pollaro F, et al. Life History Traits of Sperm Whales Physeter macrocephalus Linnaeus, 1758 Stranded along Italian Coasts (Cetartiodactyla: Physeteridae). Animals. 2023; 13(1):79. https://doi.org/10.3390/ani13010079
Chicago/Turabian StyleMaio, Nicola, Tatiana Fioravanti, Lucrezia Latini, Agnese Petraccioli, Marcello Mezzasalma, Bruno Cozzi, Sandro Mazzariol, Michela Podestà, Gianni Insacco, Francesco Pollaro, and et al. 2023. "Life History Traits of Sperm Whales Physeter macrocephalus Linnaeus, 1758 Stranded along Italian Coasts (Cetartiodactyla: Physeteridae)" Animals 13, no. 1: 79. https://doi.org/10.3390/ani13010079
APA StyleMaio, N., Fioravanti, T., Latini, L., Petraccioli, A., Mezzasalma, M., Cozzi, B., Mazzariol, S., Podestà, M., Insacco, G., Pollaro, F., Lucifora, G., Ferrandino, I., Zizzo, N., Spadola, F., Garibaldi, F., Guarino, F. M., Splendiani, A., & Caputo Barucchi, V. (2023). Life History Traits of Sperm Whales Physeter macrocephalus Linnaeus, 1758 Stranded along Italian Coasts (Cetartiodactyla: Physeteridae). Animals, 13(1), 79. https://doi.org/10.3390/ani13010079










