1. Introduction
In tropical areas, most ruminants are fed poor-quality roughages, especially rice straw [
1,
2]. The inclusion of concentrate, which is high in protein and other nutrients, can greatly enhance the efficiency of ruminant production [
3]. However, the high cost and competitiveness of feedstuffs, especially soybean meal, has led to the search for alternative feeds, especially agricultural and agro-industrial by-products [
4,
5]. The sustainability of using by-products as feed for ruminants is based on their nutritive value, the availability of nutrients, rumen fermentation patterns, production responses and feed cost compared to conventional diets [
6]. Consequently, they recirculate these wastes into the food supply chain [
7]. Researchers have suggested that their waste products can be used as a source of protein in concentrate mixtures for ruminants at a rate of 10–30% [
8,
9,
10]. In addition, the proper utilization of agro-industrial by-products in ruminant nutrition and the finding of new, inexpensive feed resources that promote ecological sustainability in feedstuffs are necessary [
11,
12].
Indigo (
Indigofera tinctoria L.) is a legume plant classified in the family Fabaceae and distributed in Africa, South Asia and South East Asia, especially in Thailand [
13]. Indigo is used in a variety of industries, including coloring food, cosmetics and pharmaceuticals but most commonly in textile products [
14]. The indigo leaf contains 30.5% CP, 2.4% EE, 19.0% crude fiber and 36.6% carbohydrate [
15]. Moreover, the indigo stem contains 5.1% CP, 2.0% EE, 54.5% crude fiber and 30.0% carbohydrate [
15], as well as some phytonutrients, including condensed tannins, saponins and flavonoids [
16,
17]. Muda et al. [
17] reported that supplementation with indigo leaf extract reduced fecal egg count but had no effect on growth performance or hematology in sheep.
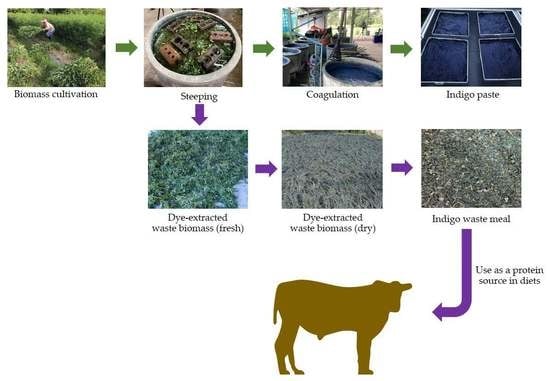
A report from Thailand’s Sakon Nakhon Province’s Community Development Department showed that there were 120 groups of producers and enterprises producing indigo dye in 2015. Natural indigo dye making with indigo plant biomass is claimed to produce the best indigo dye purity, according to the traditional method [
18]. After the dye extraction process, the remaining indigo waste consists of the stem and leaves. Through the recycling of by-products indigo waste can be used as animal feed and low-cost feed opportunities [
19]. Indigo waste is believed to be rich in protein and has the potential to be used as a protein source to replace soybean meal in ruminant diets and reduce the cost of feed. In addition, the indigo plant has successfully served as a source for antibacterial, antioxidant, anti-inflammatory and immunomodulatory effects [
20,
21,
22,
23]. We hypothesize that the use of indigo waste will maintain feed utilization and growth performance while improving rumen fermentation, hematology and immune function in beef cattle. Therefore, the objective of this study is to assess the effects of the inclusion of indigo waste in concentrate on feed intake, nutrient digestibility, rumen fermentation, growth performance, hematological and immunological responses in beef cattle.
4. Discussion
The CP content of the indigo waste was 19.8%. However, Bhatta et al. [
16] indicated that the CP of indigo leaf was 26.0% DM. Because indigo waste is composed of leaves and stems, it has a lower CP than indigo leaf. The inclusion of indigo waste increases the fiber and GE content in the concentrate. The indigo waste contained NDF, ADF and GE at 46.6%, 32.4% DM and 3487.5 kcal/kg DM, respectively. The treatment chemical composition indicates that adding indigo waste increased the fiber concentration and gross energy.
It is generally accepted that the NDF concentrations of the diet, which limit the DM digestibility of the diet, are a major factor limiting the voluntary DM intake of animals [
35]. The inclusion of indigo waste in concentrate diets decreased concentrate intake, total intake and DM digestibility in the present study. Indigo waste (stem and leaf) has a high fiber content. Increased fiber content in concentrate diets with the addition of indigo waste results in reduced feed intake and the digestibility of DM and OM with increasing levels of indigo waste. Moreover, indigo waste has a high GE content, and GE increases when indigo waste levels are increased in concentrate diets. However, DM and OM digestibility declined. This could be due to the high-fiber energy of the by-product with reduced digestible energy when the addition of indigo waste, especially at 30% in concentrate, results in a decrease in beef cattle’s DM and OM digestibility. The greater NDF digestibility of the diet was mainly due to enhanced hemicellulose digestion [
36]. In the current study, increasing levels of indigo waste by 20% in concentrate diets improved digestibility. Similarly, Kongphitee et al. [
37] reported that NDF digestibility increased with increasing levels of by-product in the diets of beef cattle. Lyu et al. [
38] reported that the inclusion of by-products in diets enhanced the digestibility of NDF in dairy cows. However, ADF digestibility was not affected by indigo waste supplementation. This is plausible because a higher fraction of the hemicellulose is present in indigo waste in concentrated diets, and therefore, NDF digestibility is increased but cellulose digestion is not affected.
ADG and feed efficiency are essential components of growing beef cattle production efficiency. ADG is an essential component of growing beef cattle production efficiency [
39]. There was a linear decrease in ADG and G:F with an increasing level of indigo waste in growing beef cattle from 0 to 90 days. The ADG as well as the G:F ratio appear to be lower when cattle were fed diets containing 20–30% indigo waste from 0 to 90 days. Kanjanapruthipong et al. [
40] reported that increasing NDF content in the diets decreased the digestibility of nutrients and ADG in dairy cattle. These results may be due to the high NDF content and also the lower DM intake, nutrient availability, and VFA, particularly propionate, when indigo waste was gradually increased in concentrate diets, resulting in decreased growth performance in growing beef cattle. The ADG required to obtain the target body weight is based on the body weight at the beginning of the trial and feed efficiency. The addition of indigo waste reduces the ADG and G:F, thereby decreasing the final BW (90 days of a trial). These results suggest that the use of indigo waste at 10% in concentrate diets is suitable for growth performance in growing beef cattle.
Ruminal pH is among the major fermentation factors that directly affect microbial ecology and, thereby, ruminal fermentation [
41]. In our experiment, the rumen pH range for all diets was 6.8 to 6.9, and the optimum range for microbial activity in the rumen was 6.5–7.0 [
4,
42]. The ruminal pH was similar among treatments, which indicates that the inclusion of indigo waste did not change rumen ecology or fermentation in tropical beef cattle. The main nitrogen source for protein synthesis in the rumen is NH
3-N [
8]. In the present investigation, indigo waste levels had no effect on the ruminal NH
3-N concentration, indicating that indigo waste seemed to have no effect on protein degradation by microorganisms in the rumen. The ruminal NH
3-N concentrations ranged from 16.8 to 21.5 mg/dL, which is closer to the optimum range (15 to 30 mg/dL) [
1,
43].
The pattern of rumen fermentation was changed by the different diets fed to ruminants. As VFA production serves as an energy source for growth performance in ruminants, it is crucial to understand their metabolism [
44]. Acetate, propionate and butyrate are the main VFAs produced in the rumen, and their concentrations vary depending on the feed ingredient, feed intake, digestibility, rumen ecology and the rate of passage [
45]. When indigo waste was added to the diets, the rumen VFA profile changed, with an increase in acetate and a decline in propionate; this also caused the C2:C3 ratio to increase. The association between the C2:C3 ratio and feed has been explained by the metabolic properties of fiber- and starch-degrading bacteria [
46]. Most structural carbohydrate fermentation, which leads to the production of acetate, is caused by cellulolytic bacteria. The major cellulolytic bacteria are thought to be
Ruminococcus albus,
R. flavefaciens and
Fibrobacter succinogenes, which produce more acetate in the rumen [
47]. Several different types of bacteria, including those in the family
Propionibacteriaceae, produce propionate as an end product in the rumen [
48]. A high concentration of starch in the diet is more likely to ferment into propionate production in the rumen, making it advantageous for the production of glucose, which helps the meat animal. [
49]. The addition of a high-fiber by-product feed increased acetate levels while reducing propionate in the rumen [
50]. Wanapat et al. [
46] found that adding high amounts of structural carbohydrates to the diet increased the proportions of acetate in the rumen, which caused the C2:C3 ratio to be higher. This means that indigo waste diets had more fermentable structural carbohydrates, such as hemicellulose, which is thought to increase acetate production and decrease propionate production.
The BUN concentration is often used to assess protein supplies and metabolic concerns related to animal diseases [
51]. The addition of indigo waste to concentrate had no effect on BUN, which ranged from 8.2 to 12.0 mg/dL, which was within the usual range of 8 to 14 mg/dL in tropical beef cattle [
52,
53]. Cattle health and nutrition and the cause of an abnormality or malfunction in cattle are frequently tested using hematological analysis [
42,
54]. The inclusion of indigo waste did not influence all hematological indicators. Similarly, Muda et al. [
17] reported that the hematology of sheep was not affected by indigo leaf extract supplementation. These results indicate that the addition of indigo waste as a feed had no negative effects on the health status of tropical beef cattle. Growing beef cattle had normal concentrations of RBCs, hemoglobin, hematocrit, WBCs, neutrophils, lymphocytes and eosinophils when compared to our previous study [
42,
51,
55]. In addition, previous reports have demonstrated that concentrations of MCV, MCH [
51,
56], monocytes [
57] and platelet count [
57,
58] in ruminant blood are within the accepted range.
Phytonutrients from tropical plants, which have been thought of as possible additions to animal feed, may affect immune and inflammatory responses. There was a high amount of phytonutrients such as total phenolics, total tannins, saponins and flavonoids in the indigo [
22]. Indigo leaf extract has numerous pharmacological effects, including anti-inflammatory, antioxidant, antibacterial, antiviral and other activities [
59]. In addition, this plant improved the immune response and demonstrated its immunostimulating efficacy in vitro and in rats [
20,
21]. In the current study, the addition of indigo waste had no influence on IgA, IgM or IgG immune responses. These results suggest that some phytonutrients may be water-soluble during the dye extraction procedure or that indigo waste contains crude leaf and stem, resulting in a weak effect on the immune response in cattle.