Simple Summary
Low-protein diets can effectively alleviate the pressure of protein resource shortage and nitrogen emission from the pig industry. In recent years, the effects of low-protein diet and amino acids or additives on growth performance, meat quality and odor emission of growing finishing pigs have been studied. However, gut health is also an important indicator, as well as whether the changes in intestinal flora caused by the reduction in dietary protein levels are beneficial to the growth of finishing pigs. Additionally, how will the metabolites of the microbes change when the microbes change in the hindgut?
Abstract
This study is aimed at the effects of low-protein diets with four amino acids balanced on serum biochemical parameters and colonic microflora of finishing pigs. Fifty-four healthy (Duroc × Landrace × Yorkshire) hybrid barrows with an average body weight of 70.12 ± 4.03 kg were randomly assigned to one of three dietary treatments with three barrows per pen and six pens per treatment. The barrows were fed a normal protein diet (NP), a low-protein diet (LP), and a very low-protein diet (VLP). Compared with the NP diet, reduced dietary protein did not influence serum biochemical parameters (p > 0.05). The valeric acid was significantly increased with the VLP diet (p < 0.05). Compared with the NP diets, the abundance of Terrisporobacter (13.37%) Clostridium_sensu_stricto_1 (23.37%) and Turicibacter (2.57%) increased to 21.04, 33.42 and 13.68% in LP diets and 16.72, 43.71 and 14.61% in VLP diets, while the abundance of Lactobacillus (9.30%) and Streptococcus (25.26%) decreased to 3.57 and 14.50% in LP diets and 1.86 and 4.07% in VLP diets. Turicibacter and Clostridium_sensu_stricto_6 had a powerful negative correlation with the content of valeric acid (p < 0.01), while Peptococcus and Clostridia_UCG-014 had a very solid positive correlation (p < 0.01). In conclusion, reducing dietary protein level can improve colon microbiota composition, especially reducing the abundance of bacteria related to nitrogen metabolism, but has no significant effect on SCFA except valeric acid. In addition, reduction in the dietary protein level by 5.48% had more different flora than that of 2.74% reduction in dietary CP level.
1. Introduction
Dietary protein is the fundamental source of amino acids for livestock. However, simply increasing the protein content of the diet is not necessarily beneficial to the growth of pigs but will increase the nitrogen emission and waste of protein raw materials in production. The shortage of protein source and the nitrogen excretion environmental pollution is a serious global problem at present. The need of animals for protein is essentially the need for amino acids [1], so we can ensure the supply of amino acids to animals while reducing the level of dietary protein, so as to ensure that the performance of livestock and poultry is not reduced. Previous studies reported that low-protein diets could decrease the concentration of ammonia–nitrogen in feces and urine of finishing pigs [2,3], but the effects on gut health have not been well-documented.
Gut microbiota represents a large and complex microbial community composed of at least 500 to 1000 species in mammals [4]. Gut microbiota communities interact with each other and their host, which plays a vital role in the host physiology and metabolism, including modulation of energy harvest, nutrient metabolism and immune system development [5]. Protein is one of the most common and principle components in diets, the portion of the dietary nitrogenous compounds escape digestion in the small intestine and enter the large intestine to be further utilized by the hindgut microbiota (mainly at the distal colon) [6]. Then, these dietary nutrients are fermented by microorganisms to produce metabolites such as short-chain fatty acids (SCFAs) and biogenic amines [7].
The bacterial community balance in the gut of finishing pigs has been established, and the bacterial composition structure remains relatively stable [8]. However, even after climax communities are already established, microbial composition changes dynamically in response to new microbial colonization, inflammatory stress and diet [9]. Therefore, the current study aimed to evaluate the effect of low-protein diets balanced with amino acid supplemented on gut microbes in pigs and to investigate the association between colonic SCFAs and gut microbes under low-protein diets.
2. Materials and Methods
The study protocol was approved by College of Animal Science and Technology, Hunan Agricultural University, and the treatment and slaughtering conditions were in accordance with the Animal Care and Use Guidelines of China (The ethical code: ACC20220556).
2.1. Animal Treatment and Experimental Design
Fifty-four healthy (Duroc × Landrace × Yorkshire) hybrid barrows with an average body weight of 70.12 ± 4.03 kg were randomly assigned to one of three dietary treatments with three barrows per pen and six pens per treatment. The barrows were fed a normal protein diet (NP), a low-protein diet (LP), and a very low-protein diet (VLP). As shown in Table 1, all the diets were balanced with four essential amino acids (lysine, methionine, threonine and tryptophan) and crude protein contents were 13.50% (NP; CON), 10.76% (LP) and 8.02% (VLP). The experiment lasted 37 days, during which all the pigs were given free access to clean drinking water and the diet assigned to them.

Table 1.
Ingredient Composition and Nutrient Levels of the Experimental Diets. (%, as-fed basis).
The animal feeding experiment was conducted in Liuyang Animal Testing Base in Hunan Province. The experiment period was 37 days, and the pigs were raised in the whole leaky seam floor piggery, free to eat and drink according to the routine procedures of deinsectization and immunization and regular cleaning and disinfection of piggery. The thermostat automatically regulates the ambient temperature in the house and the window is opened for ventilation at regular times. Pigs are not fed on the morning of feeding day 38; one pig was randomly chosen from each pen (n = 6) and selected for slaughter, blood was collected before slaughter and serum was separated by centrifugation and placed at −20 °C. Colonic digesta was collected and stored at −80 °C.
2.2. Direction Indicators
2.2.1. Blood Biochemical Parameters
Blood samples were harvested from anterior vena cava and serum were separated after centrifugation at 300 rpm for 10 min under 4 °C. Cobas c-311 coulter chemistry analyzer (Roche Diagnostics International Ltd., Shanghai, China) was used to test serum biochemical parameters, including total bile acid (TBA), triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL) and glucose (Glu).
2.2.2. Short-Chain Fatty Acids Analysis
To determine short-chain fatty acid content in colonic chyme, refer to Yu et al. [10]. In brief, 1.5 g ileal or colonic digesta was suspended in 1.5 mL of distilled water and centrifuged at 15,000× g at 4 °C for 10 min. One milliliter of supernatant mixed with 200 μL of metaphosphoric acid was put in an ampoule and set in an ice bath for 30 min and centrifuged for 10 min. Samples were inserted into an HP 6890 Series Gas Chromatograph (Hewlett Packard, PA, California, CA, USA) with an HP 19091N-213 column with 30.0 m × 0.32 mm i.d. (Agilent, PA, California, CA, USA). Temperatures for injector and detector were set at 185 °C and 210 °C, respectively. Each sample was measured three times.
2.2.3. Microbiota Analysis by 16S RNA
Total genome DNA was extracted from colon samples from growing pigs using the QIAamp Fast DNA Stool mini kit (Qiagen, Hilden, Germany) and checked with 1% agarose gel. The DNA concentration and purity were determined with Nano Drop 2000 UV-vis spectrophotometer (Thermo Fisher Scientific, Wilmington, NC, USA). The specific primer with the barcode (16S V3-V4) was amplified by an ABI Gene Amp R9700 PCR thermocycler (ABI, Los Angeles, CA, USA). Then, the PCR products were extracted, purified and quantified. Paired-end sequencing was performed on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, CA, USA). The raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered and merged according to previous studies [11,12]. The complexity of species diversity was evaluated with ACE and Chao richness estimators and diversity indices of Shannon and Simpson [13]. β-diversity was evaluated using principal component analysis (PCA) based on the Euclid distance. The significant differences between samples were evaluated by the analysis of similarities (ANOSIM). OTUs representing < 0.005% of the population were removed, and taxonomy was assigned using the RDP classifier. The relative abundance of each OTU was counted at different taxonomic levels. Then, bioinformatics analysis was mainly performed using QIIME (V1.7.0; San Diego, CA, USA) and R packages (Version 3.3.1, R Core Team, Vienna, Austria). The OTU table in QIIME was used to calculate OTU-level, and β-diversity was assessed by principal coordinate analysis (PCoA). The cluster analysis and significant differences between samples were tested by ANOSIM [14].
2.3. Statistical Analysis
Data for blood variables and SCFAs were subjected to analysis of variance (ANOVA) suited for a randomized complete block design using the general linear model (GLM) procedure (version 9.2, SAS Institute, Inc., Cary, NC, USA.). For the growth performance, pen served as the experimental unit. Results are expressed as the mean + standard error of the mean (SEM). Statistical differences among groups were separated by the Bonferroni multiple comparisons test. p values of < 0.05 were significant for all data in this paper.
3. Result
3.1. Serum Biochemical Parameters
The effect of dietary crude protein level on the serum biochemical parameters of finishing pigs is presented in Table 2. Reduced dietary protein did not influence serum biochemical parameters (p > 0.05).

Table 2.
Effect of Low-protein Diet on blood lipid profiles of Finishing Pigs.(mmol/L).
3.2. SCFAs
Table 3 shows that different protein diets can significantly affect the content of short-chain fatty acids in the colon and cecum of pigs. Compared with the NP diet, the valeric acid was significantly increased with the VLP diet (p < 0.05). However, other short-chain fatty acids were not significantly different.

Table 3.
Effect of Low-protein Diet on SCFAs content in colon of Finishing Pigs (mg/kg).
3.3. Structural Changes in The Microbial Community
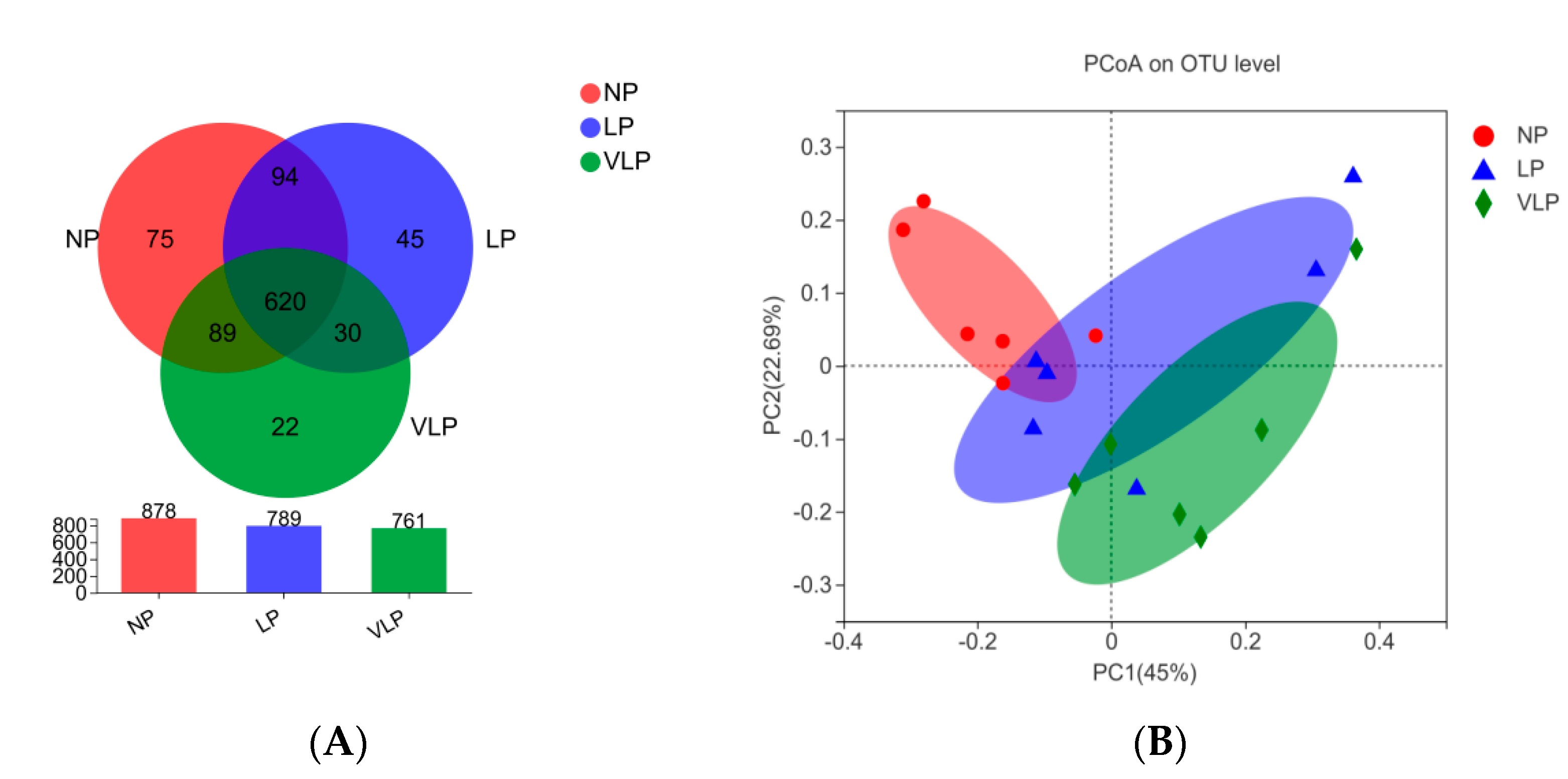
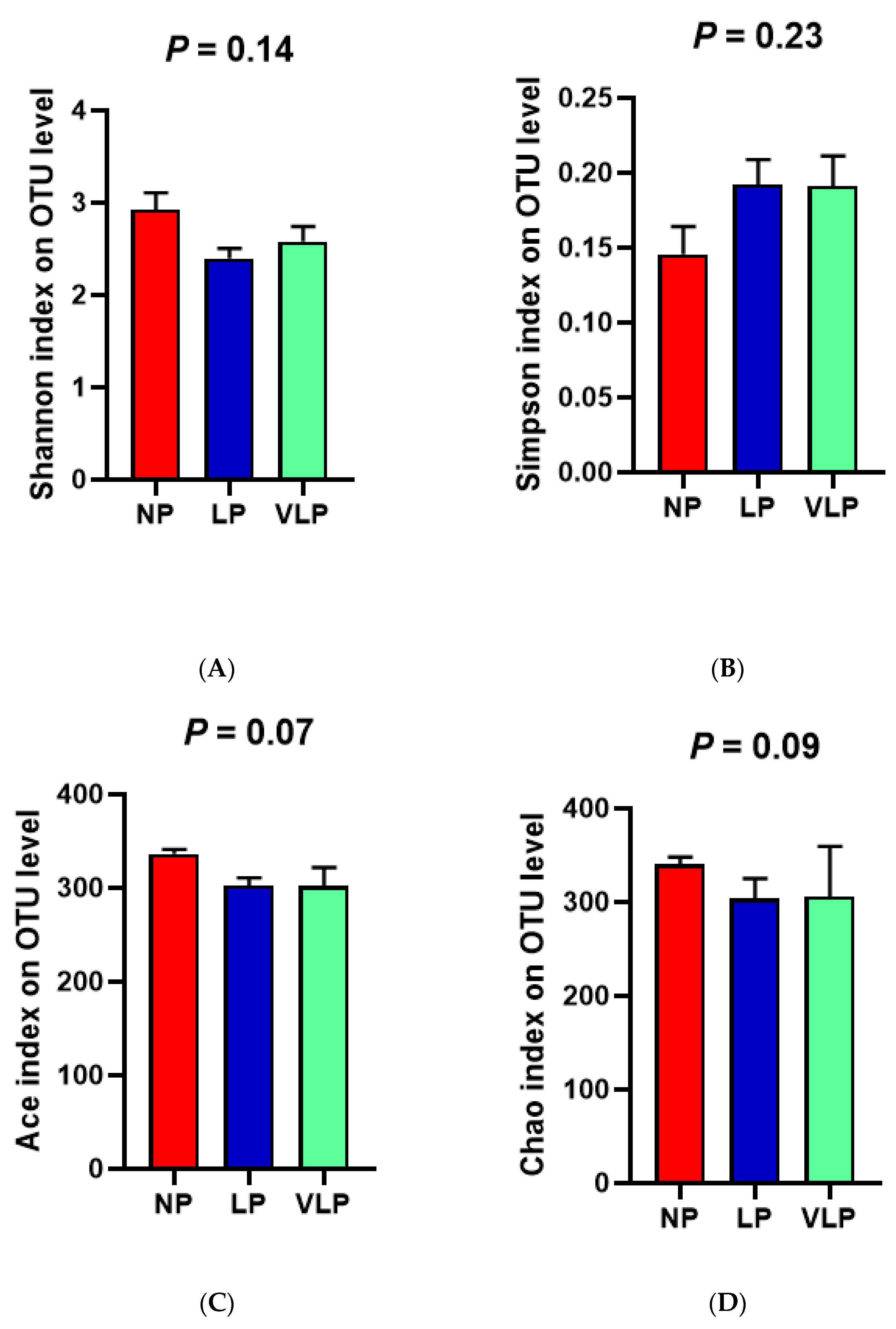
Following Illumina MiSeq sequencing analysis, a Venn diagram of the OTUS exhibited that 878, 789 and 761 OTUs were identified, respectively, in the NP, LP and the VLP diets; 620 OTUs were shared by each of the three groups (Figure 1A). Additionally, no remarkable differences were found in the diversity indices (Shannon and Simpson index) (p > 0.05), same for the richness estimators (ACE and Chao index) of the colon microbiota (Figure 2). The principal coordinate analysis (PCoA) was used to characterize the β diversity of bacterial communities in the fecal samples of growing pigs in the NP, LP and VLP diets. As shown in Figure 1B, PCoA results present that the colon microbial composition of finishing pigs exposed to low-protein-level groups was distinguishable from that of the NP diets.
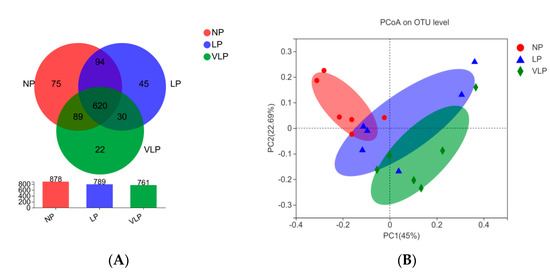
Figure 1.
(A) Effects of different protein diets on colon microflora OTU of finishing pigs. (B) Principal coordinates analysis (PCoA) of microbial composition in the colon of growing pigs (based on the bary_curtis distance). The individual pig was regarded as the experimental unit (n = 6). NP, normal protein; LP, low protein; VLP, very low protein.

Figure 2.
Effects of different protein diets on the alpha diversity of bacterial flora in the colon of finishing pigs. (A) Shannon index, (B) Simpson index, (C) Ace index, (D) Chao index. The individual pig was regarded as the experimental unit (n = 6). NP, normal protein; LP, low protein; VLP, very low protein.
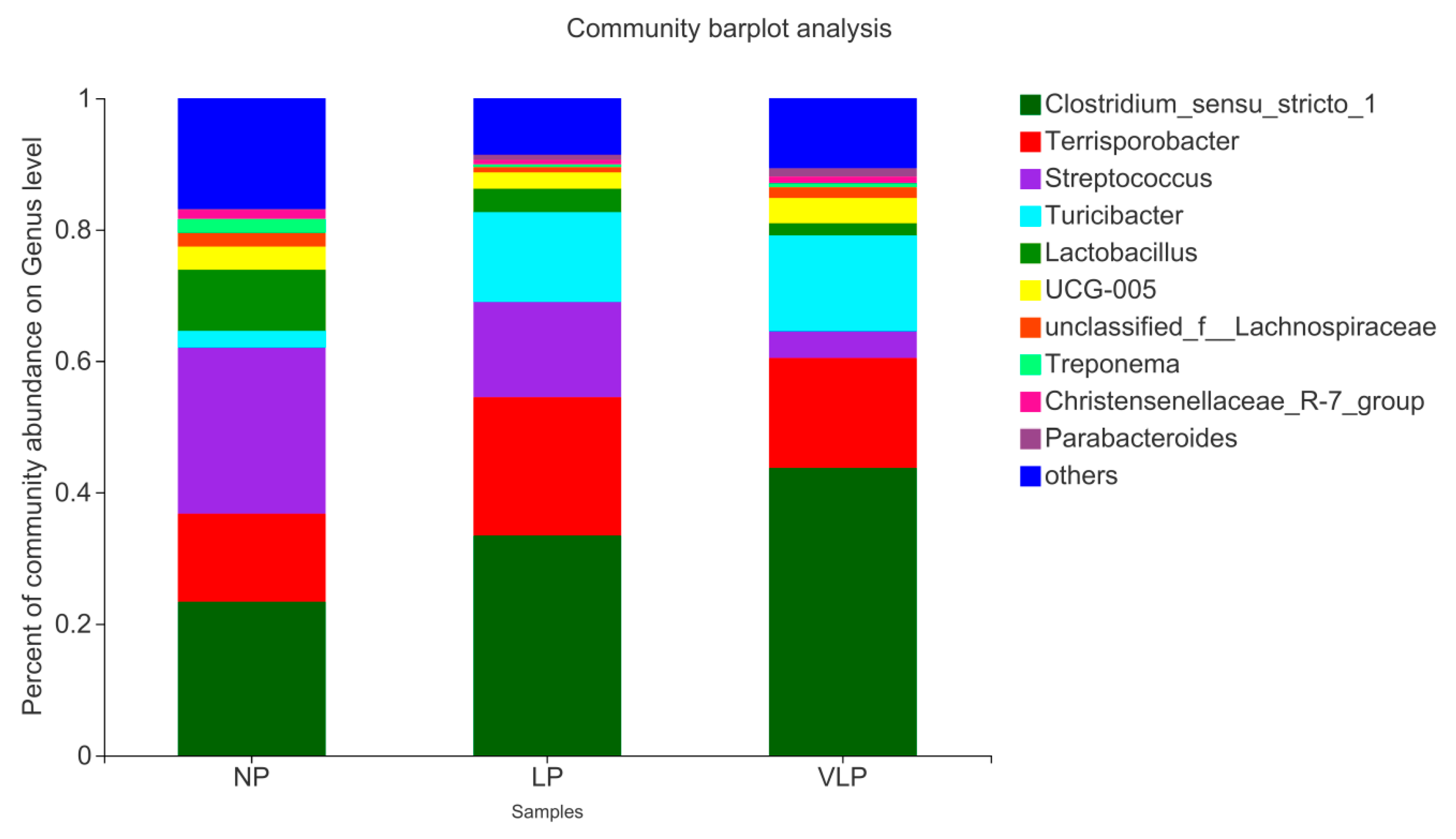
As shown in Figure 3, at the genus level, six phyla were detected in three groups of samples. Different colors in the figure represent different bacterial communities. The higher the column, the larger the proportion of the sample and the higher the relative abundance. The relative abundance and proportion of each group at the genus level could be intuitively seen from the species annotation results. It was uncovered that the dominant strains of colon flora of finishing pigs are primarily composed of Turicibacter, Terrisporobacter, Clostridium_sensu_stricto_1, Lactobacillus, Streptococcus, UCG-005 and so on. Compared with the NP diets, the abundance of Terrisporobacter (13.37%), Clostridium_sensu_stricto_1 (23.37%) and Turicibacter (2.57%) increased to 21.04, 33.42 and 13.68% in the LP diets and 16.72, 43.71 and 14.61% in the VLP diets, while the abundance of Lactobacillus (9.30%) and Streptococcus (25.26%) decreased to 3.57 and 14.50% in the LP diets and 1.86 and 4.07% in the VLP diets.

Figure 3.
Relative abundance of colonic bacteria based on the genus level. The individual pig was regarded as the experimental unit (n = 6).NP, normal protein; LP, low protein; VLP, very low protein.
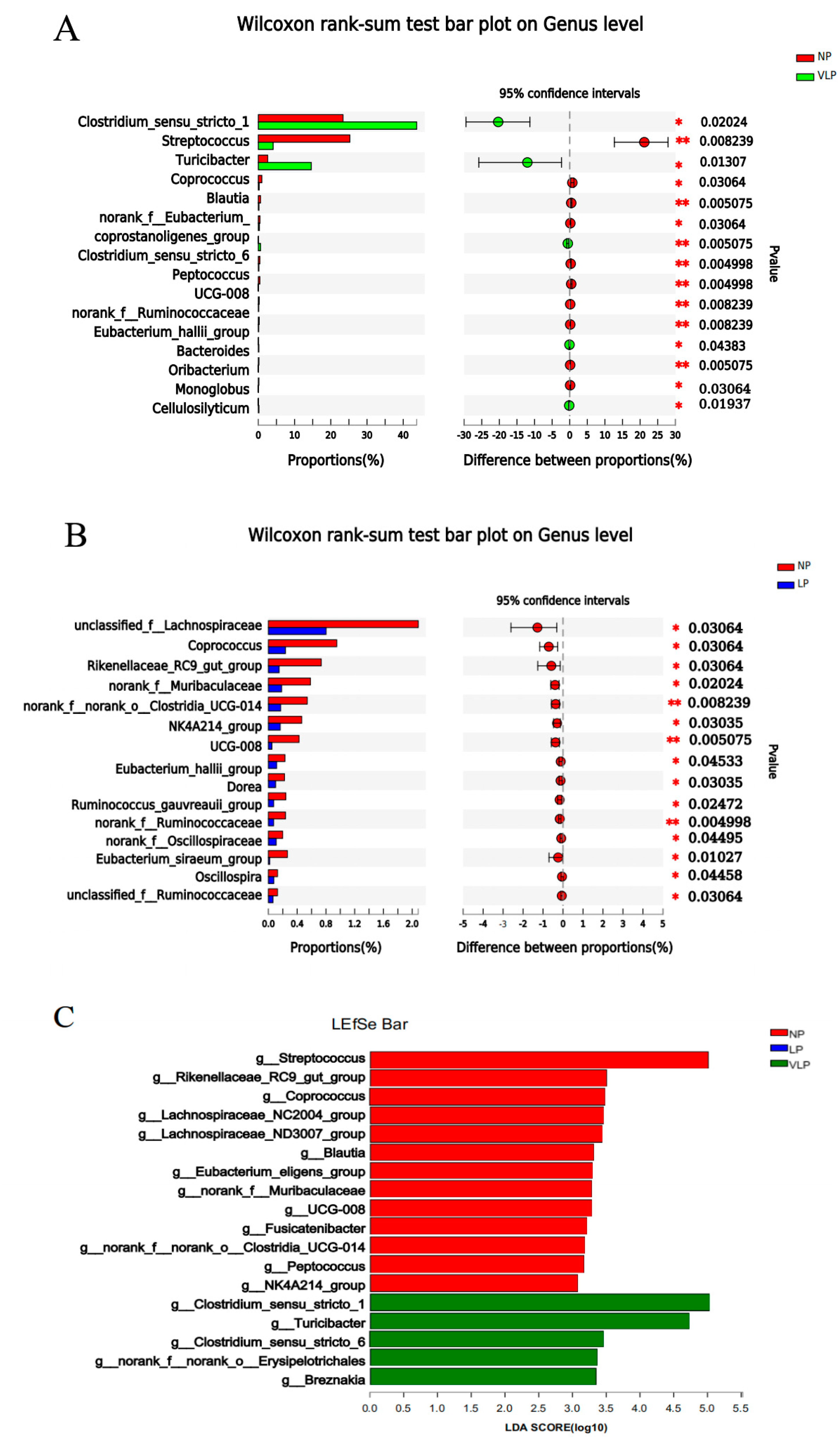
As shown in Figure 4, in addition, it was revealed, through LEfSe analysis (LDA threshold: 3.0), that the LP diet was not found to be significant; the VLP diet induced a significant enrichment of Clostridium_sensu_stricto_1, Turicibacter and Clostridium_sensu_stricto_6. By contrast, Streptococcus, Coprococcus, Blautia, Rikenellaceae_RC9_gut_group, UCG-008, Muribaculaceae, Clostridia_UCG-014, NK4A214_group and Peptococcuswere were the predominant species in the NP diet.

Figure 4.
Different microbiota comparison by the student t-test on the genus of the colon is shown in (A,B). p-values (* p < 0.05, ** p < 0.01) are shown on the right. Identification of the most differentially abundant genera in the colon. The plot (C) is generated from Linear Discriminant Analysis Effect Size (LEfSe) analysis with CSS-normalized OTU table and displays taxa with LDA scores above 3.0 and p-values below 0.05. Genera enriched in the samples with the NP diet are indicated with red bars, genera enriched in the samples with the LP diet are indicated with blue bars and genera enriched in the samples with the VLP diet are indicated with green bars.
3.4. Correlation of Bacteria and Colonic SCFAs
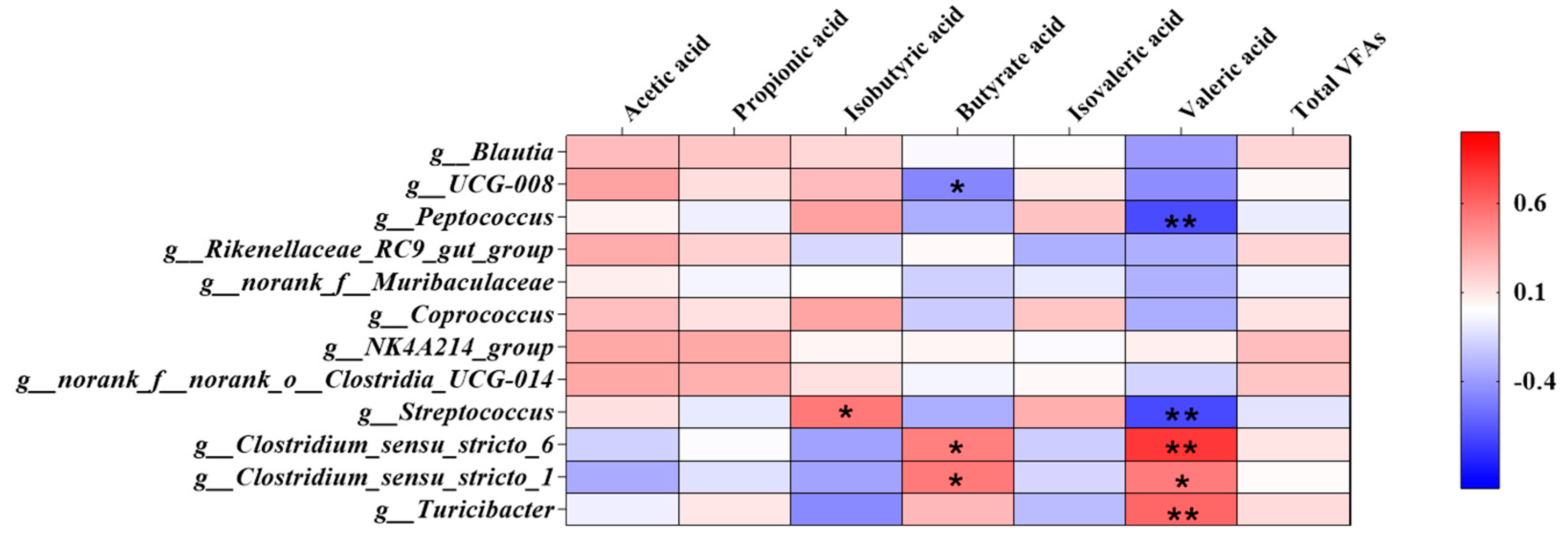
The correlation between the top 12 genera and the concentration of SCFAs content in colon is shown in Figure 5, wherein the bacteria including Turicibacter, Clostridium_sensu_stricto_6, Blautia, UCG-008 Clostridium_sensu_stricto_1, Peptococcus, Coprococcus, NK4A214_group, Streptococcus, Muribaculaceae, Rikenellaceae_RC9_gut_group and Clostridia_UCG-014. Turicibacter and Clostridium_sensu_stricto_6 had a very significant negative correlation with the content of valeric acid (p < 0.01), while Peptococcus and Clostridia_UCG-014 had a very solid positive correlation (p < 0.01).
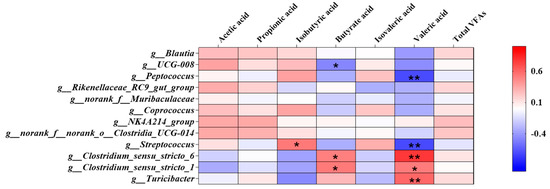
Figure 5.
The correlation between the top 12 genera and the concentration of Colon SCFAs content. The individual pig was regarded as the experimental unit (n = 6). NP, normal protein; LP, low protein; VLP, very low protein. “*” means there is a significant difference, “**” means a very significant difference.
4. Discussion
Many experimental studies have shown that the reduction in dietary protein level may affect the lipid metabolism status of animals [15,16] because the use of low-protein diets can reduce animal energy loss, protein turnover and animal heat production, so that energy use efficiency in the body increases [17,18]. However, we did not observe significant changes in lipid levels. It is worth noting that the contents of TG, TC and HDL-C in the VLP diet tend to increase compared with the control group. According to Madeira et al.’s [19] study, the restriction of dietary protein increased total lipids, total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. Additionally, the reason we did not see a significant change probably has to do with how long the pigs were fasting.
Intestinal microbiota and its fermentative metabolites have potential effects on host health. Chao estimate indicated the bacterial richness and Shannon index reflected the bacterial diversity [20]. In our experiment, with four balanced essential amino acids, reducing the dietary CP level had no significant effect on the α diversity index of colonic microbiota in finishing pigs, which was consistent with Zhou et al. [21]. Meanwhile, Fan et al. [22] found that the Chao and Shannon index of the bacterial community in the colon was not significantly different. Though bacterial diversity was slightly affected, the samples of the three groups were clustered separately. Furthermore, a separation between colon samples of the NP and VLP diets could be best observed from PCoA results. Therefore, a reduction in dietary protein concentration was indicated to affect the colonic microbiota.
Species richness of colonic microbiota revealed that the abundance values of Terrisporobacter, Clostridium_sensu_stricto_1 and Turicibacter were increased with reduction in dietary protein. Turicibacter is positively correlated with colitis [23]. Clostridium_sensu_stricto_1 is an opportunistic pathogen [22,24], which can cause intestinal inflammation and decrease the content of SCFAs [25]. However, we did not observe significant reduction in SCFAs in colonic contents in our study. On the contrary, the content of valeric acid was significantly increased with the VLP diet. Fan et al. [15] also found the same result in the colon, but no significant damage on colonic morphology and expression of tight junction proteins were observed in low-protein diet groups. It may be partly due to the mild change in the colonic bacteria community. The literature also reported that Terrisporobacter and Clostridium_sensu_stricto_1 were fiber-degrading bacteria at each growing stage [26,27,28]. In our study, soybean meal was partially replaced by corn and bran in the preparation of the low-protein diet. Therefore, there were more fibers and starches in the VLP diets, which could increase the abundance of Terrisporobacter and Clostridium_sensu_stricto_1. The carbohydrates which are available to microorganisms are rich in variety and complex in structure, including resistant starch, non-starch polysaccharide and indigestible monosaccharides [29]. Reduced dietary protein levels relatively reduce abnormal protein fermentation in the hindgut, while short-chain fatty acids produced by carbohydrate fermentation are rapidly absorbed in the hindgut, providing approximately 30% of the energy of pigs [30].
Gut microbiota can synthesize abundant proteases and peptidases to hydrolyzed proteins and peptides and catabolize almost all kinds of amino acids (AA) [31]. Clostridia, streptococci, Fusobacterium and Lactobacillus are also frequently seen proteolytic bacteria [32,33]. At the same time, Lactobacillus and Streptococcus are capable of producing amines through the decarboxylation of AA. In this study, we observed a decrease in the abundance of Streptococcus in the colon of VLP diets. It is worth noting that the proportion of RC9_gut_group belongings to Rikenellaceae, Lachnospiraceae belonging to Firmicutes, Coprococcus and 12 other bacterial genera were decreased in the LP diet. The decrease in abundance of RC9_gut_group may be related to the decrease in dietary nitrogen sources, or the influence of dietary amino acid imbalance on lipid utilization [34]. Interestingly, we found the abundance of Coprococcus was significantly decreased in both the NP and VLP diets, which suggested that Coprococcus was sensitive to dietary protein changes.
5. Conclusions
Reducing dietary protein level can improve colon microbiota composition, especially reducing the abundance of bacteria related to nitrogen metabolism, but it has no significant effect on SCFA except valeric acid. In addition, reduction in the dietary protein level by 5.48% had more different flora than that of a 2.74% reduction in dietary CP level.
Author Contributions
S.L. and Z.F. designed the study. S.L. and Z.F. conducted the study. S.L. and Z.F. participated in samples collection and data analysis. S.L. drafted the manuscript. Z.F. gave suggestions for the study. S.L. and Z.F. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the Hunan Co-Innovation Center of Animal Production Safety, College of Animal Science and Technology, Hunan Agriculture University for technical support. This study was supported by Hunan Provincial Natural Science Foundation of China 2021JJ30318.
Institutional Review Board Statement
The experiment was approved by the Animal Care Committee of College of Animal Science and Technology, Hunan Agricultural University. (The ethical code: ACC20220556).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Wu, G.; Bazer, F.W.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Leek, A.B.; Hayes, E.T.; Curran, T.P.; Callan, J.J.; Beattie, V.E.; Dodd, V.A.; O’Doherty, J.V. The influence of manure composition on emissions of odour and ammonia from finishing pigs fed different concentrations of dietary crude protein. Bioresour. Technol. 2007, 98, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- PPrandini, A.L.D.O.; Sigolo, S.; Morlacchini, M.; Grilli, E.; Fiorentini, L. Microencapsulated lysine and low-protein diets: Effects on performance, carcass characteristics and nitrogen excretion in heavy growing–finishing pigs. J. Anim. Sci. 2013, 91, 4226–4234. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; De Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Van Kessel, A.G. Host pathways for recognition: Establishing gastrointestinal microbiota as relevant in animal health and nutrition. Livest. Sci. 2010, 133, 82–91. [Google Scholar] [CrossRef]
- Gibson, J.A.; Sladen, G.E.; Dawson, A.M. Protein absorption and ammonia production: The effects of dietary protein and removal of the colon. Br. J. Nutr. 1976, 35, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Jin, C.; Flavell, R.A. Innate sensors of pathogen and stress: Linking inflammation to obesity. J. Allergy Clin. Immunol. 2013, 132, 287–294. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, C.; Yang, Y.; Mu, C.; Su, Y.; Yu, K.; Zhu, W. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. J. Anim. Sci. Biotechnol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, L.; Liu, N.; Zhang, F.; Liu, X.; Jiang, Q.; Chen, J.; Ma, X. Comparative Effects of Compound Enzyme and Antibiotics on Growth Performance, Nutrient Digestibility, Blood Biochemical Index, and Intestinal Health in Weaned Pigs. Front. Microbiol. 2021, 12, 768767. [Google Scholar] [CrossRef]
- Figueroa, J.L.; Lewis, A.J.; Miller, P.S.; Fischer, R.L.; Gómez, R.S.; Diedrichsen, R.M. Nitrogen metabolism and growth performance of gilts fed standard corn-soybean meal diets or low-crude protein, amino acid-supplemented diets. J. Anim. Sci. 2002, 80, 2911–2919. [Google Scholar] [CrossRef]
- Zervas, S.; Zijlstra, R.T. Effects of dietary protein and fermentable fiber on nitrogen excretion patterns and plasma urea in grower pigs. J. Anim. Sci. 2002, 80, 3247–3256. [Google Scholar] [CrossRef]
- Kerr, B.J.; McKeith, F.K.; Easter, R.A. Effect on performance and carcass characteristics of nursery to finisher pigs fed reduced crude protein, amino acid-supplemented diets. J. Anim. Sci. 1995, 73, 433–440. [Google Scholar] [CrossRef]
- Figueroa, J.L.; Lewis, A.J.; Miller, P.S.; Fischer, R.L.; Diedrichsen, R.M. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J. Anim. Sci. 2003, 81, 1529–1537. [Google Scholar] [CrossRef]
- Madeira, M.S.; Rolo, E.A.; Lopes, P.A.; Ramos, D.A.; Alfaia, C.M.; Pires, V.M.; Prates, J.A. Betaine and arginine supplementation of low protein diets improves plasma lipids but does not affect hepatic fatty acid composition and related gene expression profiling in pigs. J. Sci. Food Agric. 2018, 98, 598–608. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Xu, Z. Analysis of Blueberry Plant Rhizosphere Bacterial Diversity and Selection of Plant Growth Promoting Rhizobacteria. Curr. Microbiol. 2022, 79, 331. [Google Scholar] [CrossRef]
- Zhou, L.; Fang, L.; Sun, Y.; Su, Y.; Zhu, W. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe 2016, 38, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [PubMed]
- Kujawa-Szewieczek, A.; Adamczak, M.; Kwiecień, K.; Dudzicz, S.; Gazda, M.; Więcek, A. The effect of Lactobacillus plantarum 299v on the incidence of Clostridium difficile infection in high risk patients treated with antibiotics. Nutrients 2015, 7, 10179–10188. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; Lee, Y.; Lu, H.; Chou, C.H.; Wang, C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 2019, 14, e0205784. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef]
- Kllingray, L.; Le Gall, G.; Defernez, M.; Beales, I.L.; Franslem-Elumogo, N.; Narbad, A. Microbial taxonomic and metabolic alterations during faecal microbiota transplantation to treat Clostridium difficile infection. J. Infect. 2018, 77, 107–118. [Google Scholar] [CrossRef]
- Navarro, D.M.; Abelilla, J.J.; Stein, H.H. Structures and characteristics of carbohydrates in diets fed to pigs: A review. J. Anim. Sci. Biotechnol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Morita, T.; Kasaoka, S.; Kiriyama, S. Physiological functions of resistant proteins: Proteins and peptides regulating large bowel fermentation of indigestible polysaccharide. J. AOAC Int. 2004, 87, 792–796. [Google Scholar] [CrossRef]
- Han, P.; Ma, X.; Yin, J. The effects of lipoic acid on soybean β-conglycinin-induced anaphylactic reactions in a rat model. Arch. Anim. Nutr. 2010, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Cummings, J.H.; Allison, C. Protein degradation by human intestinal bacteria. Microbiology 1986, 132, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Meerveld, G.V.; Johnson, A.C.; Grundy, D. Gastrointestinal physiology and function. In Gastrointestinal Pharmacology; Springer: Cham, Switzerland, 2017; pp. 1–16. [Google Scholar]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Yu, B. Dietary protein levels and amino acid supplementation patterns alter the composition and functions of colonic microbiota in pigs. Anim. Nutr. 2020, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).