Effects of Kadsura coccinea L. Fruit Extract on Growth Performance, Meat Quality, Immunity, Antioxidant, Intestinal Morphology and Flora of White-Feathered Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Kadsura coccinea Fruit Extract
2.2. Determination of the Main Components of K. coccinea Fruit Extracts
2.3. Animals and Experimental Details
2.4. Details Data and Samples Collection
2.4.1. Growth Performance
2.4.2. Serum Index, Immune Organs and Meat Samples
2.4.3. Jejunal and Cecum Chyme Samples
2.5. Collection Index Determination Method
2.5.1. Slaughter Performance
2.5.2. Determination of Meat Quality
2.5.3. Antioxidant Indices and Immune Function
2.5.4. Function Histomorphometry of Jejunum
2.5.5. Serum Mucosa Cytokines Expression
2.5.6. Analysis of Cecum Microbe
2.6. Statistical Analysis
3. Results
3.1. Main Chemical Composition Content
3.2. Growth Performance
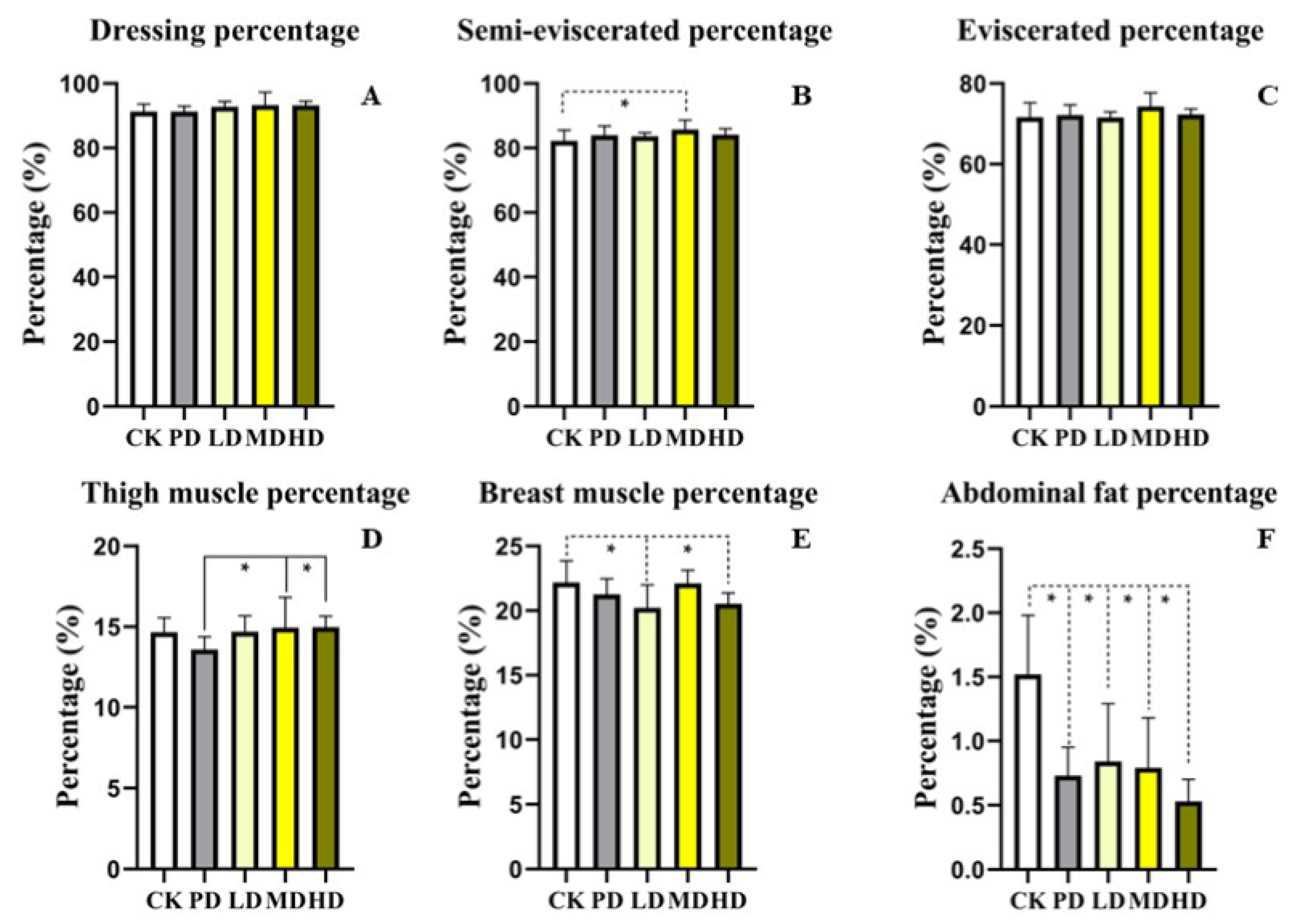
3.3. Slaughter Performance
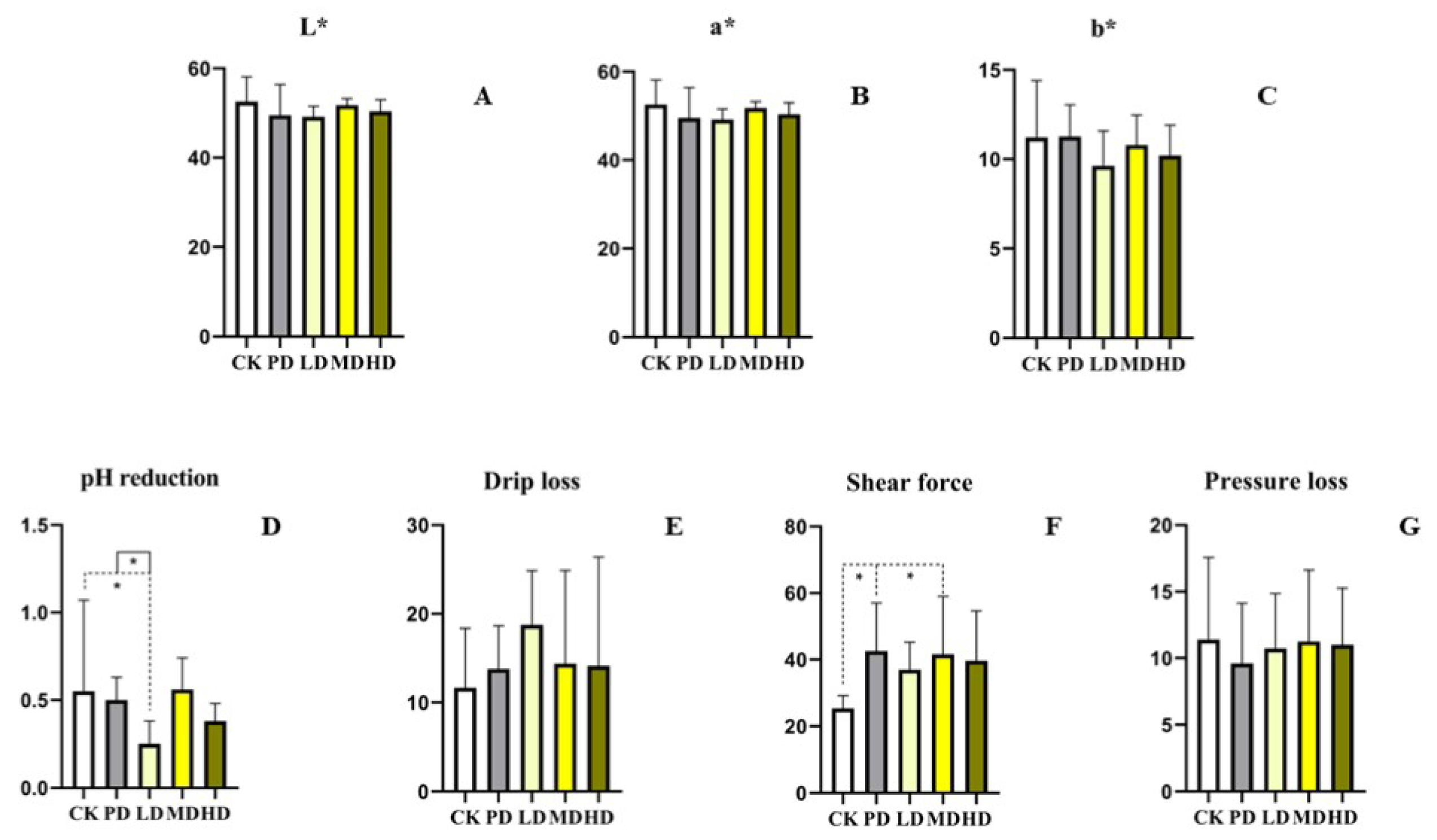
3.4. Meat Quality
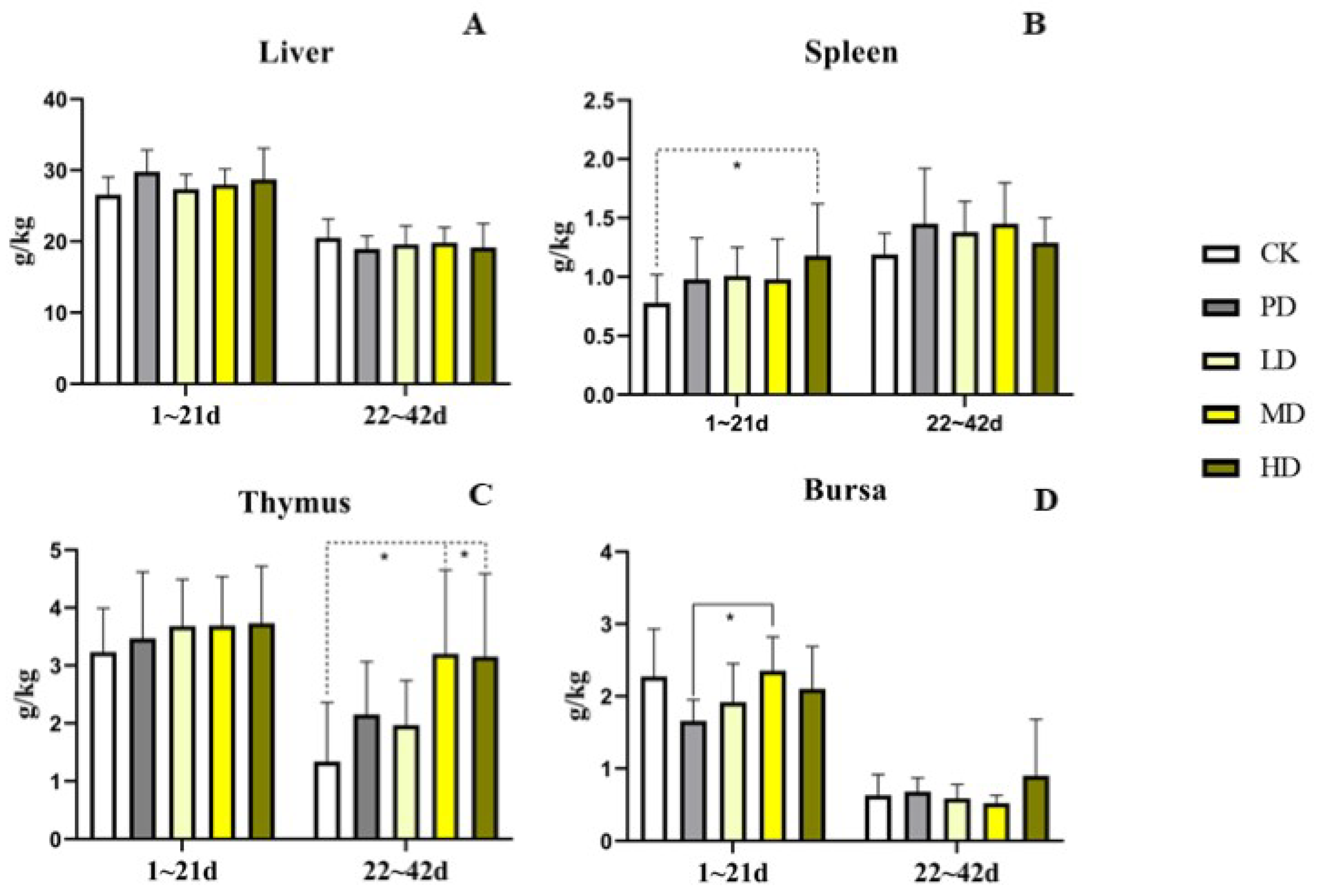
3.5. Immune Organs
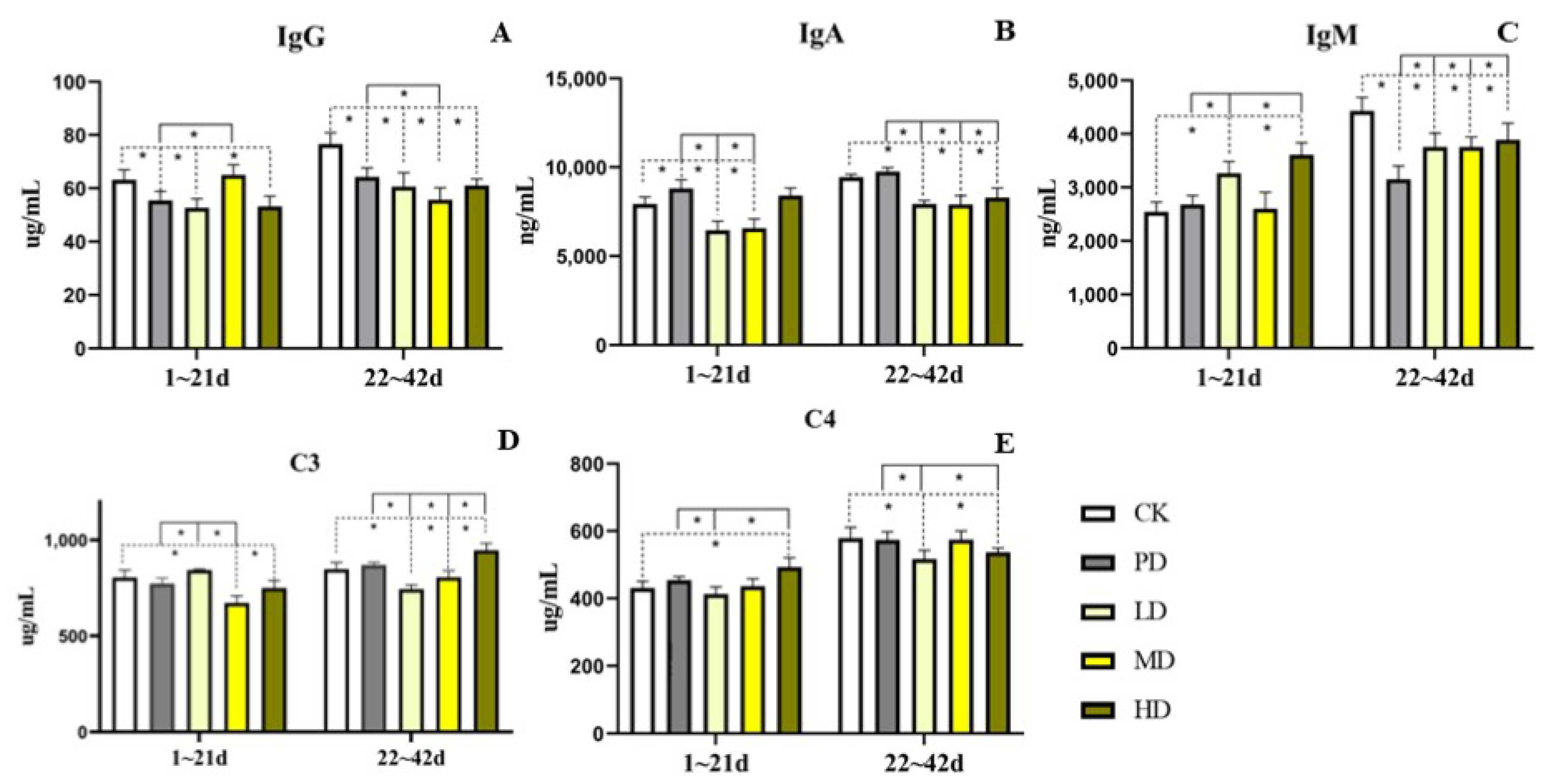
3.6. Serum Immunoglobulin
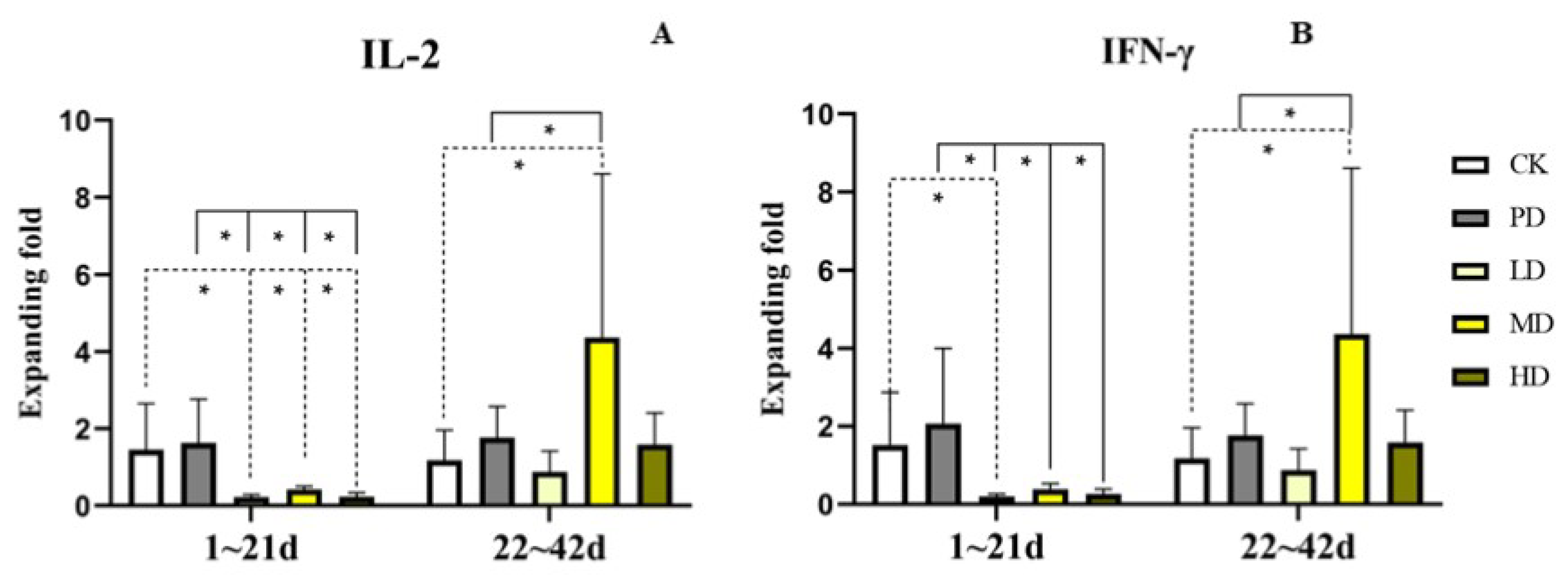
3.7. Cytokine Expression in Serum
3.8. Serum Antioxidant
3.9. Intestinal Morphology
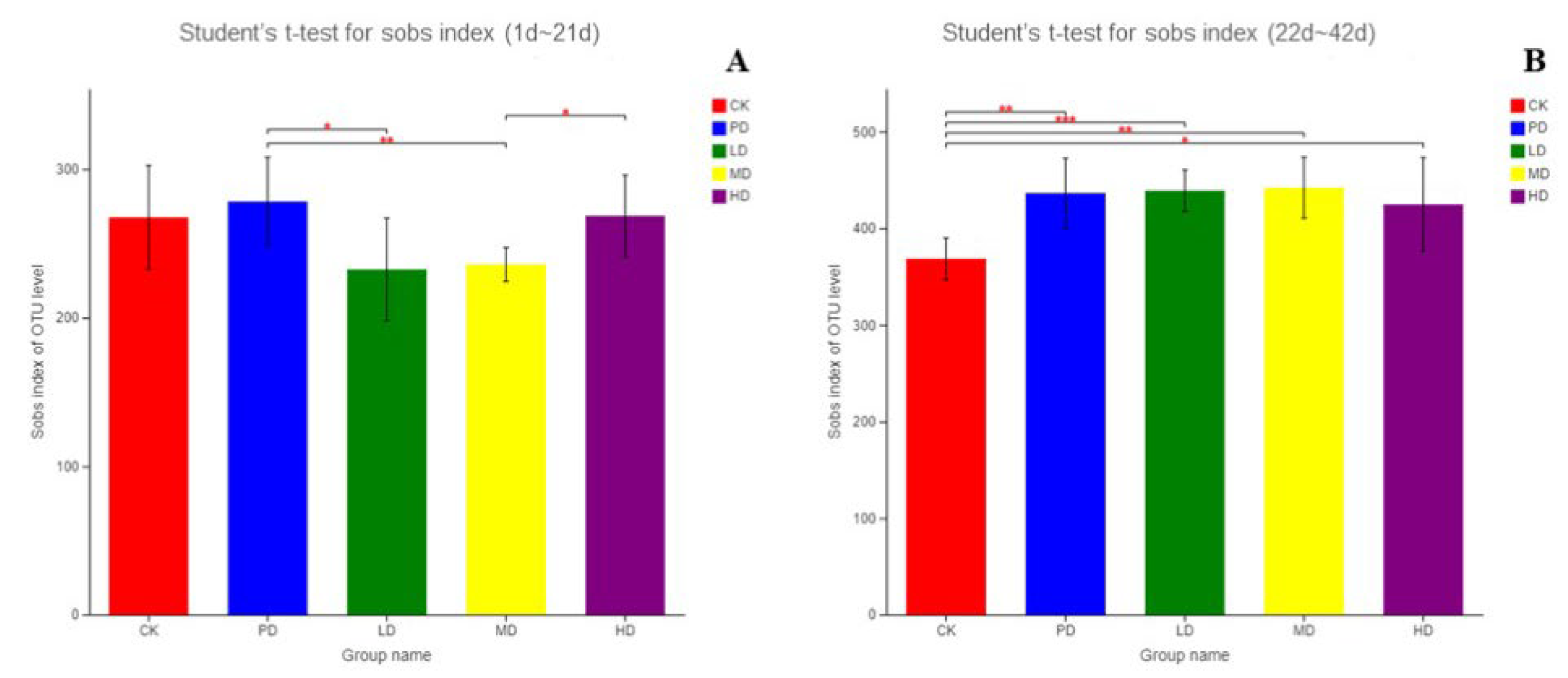
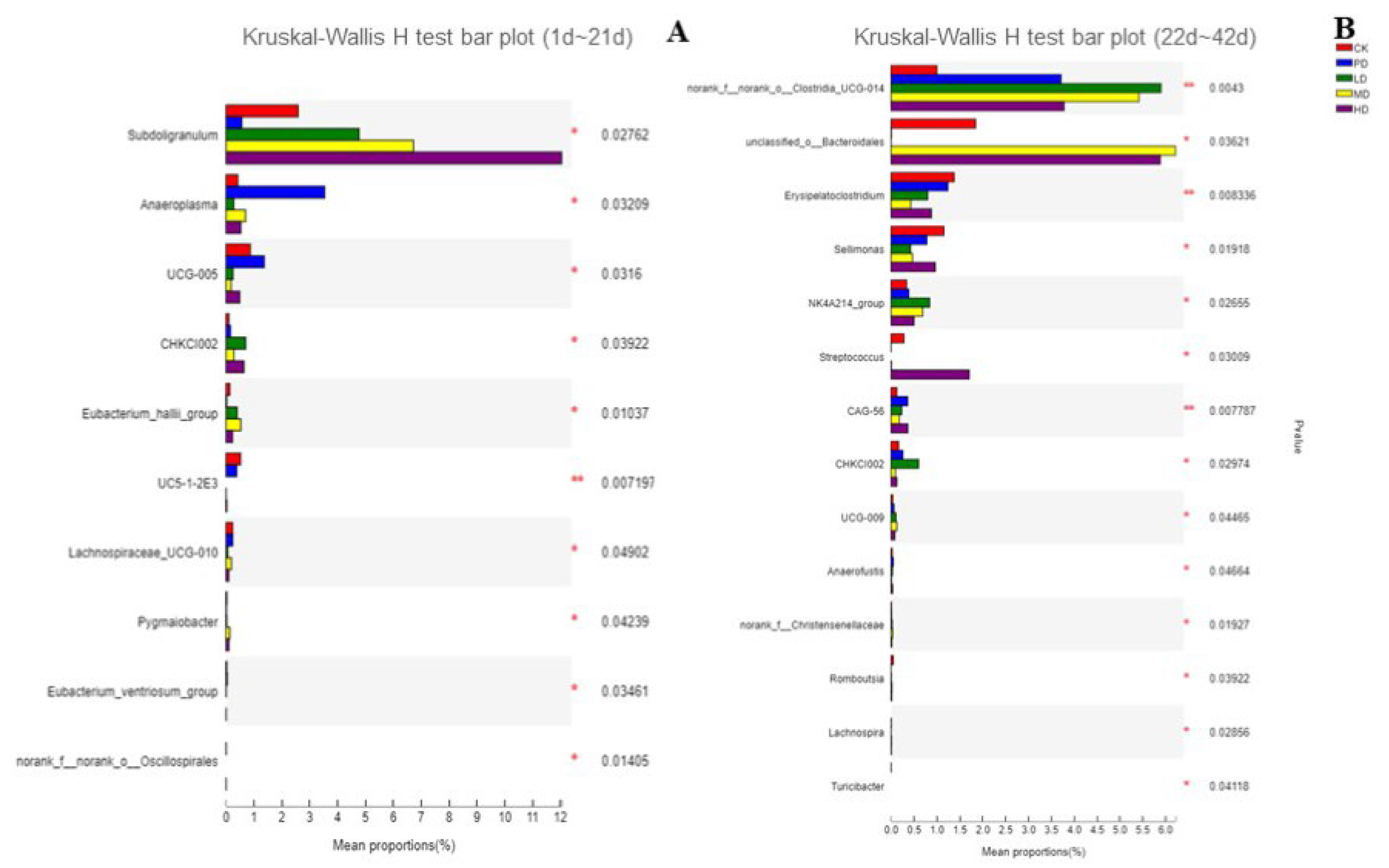
3.10. Intestinal Flora
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.L. Pharmaceutical Chemistry; China Medical Science Press: Beijing, China, 2014; pp. 295–326. [Google Scholar]
- Castanon, J.I. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Suresh, G.; Das, R.K.; Kaur Brar, S.; Rouissi, T.; Avalos Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to Antibiotics in Poultry Feed: Molecular Perspectives. Crit. Rev. Microbiol. 2018, 44, 318–335. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [Green Version]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as Antibiotic Alternatives to Promote Growth and Enhance Host Health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [Green Version]
- Brüssow, H. Adjuncts and Alternatives in the Time of Antibiotic Resistance and in-Feed Antibiotic Bans. Microb. Biotechnol. 2017, 10, 674–677. [Google Scholar] [CrossRef]
- Chen, Y.J. Effects of Lonicera Flos on Growth, Blood, Biochemistry, Immunity and Organ Histomorphology of Yellow-Feather Broiler; Chongqing Normal University: Chongqing, China, 2020. [Google Scholar]
- Lin, X.L.; LU, W.; Li, S.Z.; Li, X.B.; Guo, X.; Li, H.B. Investigation on the Resources of Medicinal Plant Kadsuracoccinea. Trop. For. 2019, 47, 34–36. [Google Scholar]
- Yang, Y.; Liu, Y.; Daniyal, M.; Yu, H.; Xie, Q.; Li, B.; Jian, Y.; Man, R.; Wang, S.; Zhou, X.; et al. New Lignans from Roots of Kadsura Coccinea. Fitoterapia 2019, 139, 104368. [Google Scholar] [CrossRef]
- Yang, Y.; Jian, Y.; Cheng, S.; Jia, Y.; Liu, Y.; Yu, H.; Cao, L.; Li, B.; Peng, C.; Iqbal Choudhary, M.; et al. Dibenzocyclooctadiene Lignans from Kadsura Coccinea Alleviate APAP-Induced Hepatotoxicity via Oxidative Stress Inhibition and Activating the Nrf2 Pathway In Vitro. Bioorg. Chem. 2021, 115, 105277. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wei, Z.J.; Qiao, W.L.; Sun, X.M.; Jin, Z.L.; Gong, W.; Jia, B.X. Discovery of Cyclooxygenase-2 Inhibitors from Kadsura Coccinea by Affinity Ultrafiltration Mass Spectrometry and the Anti-Inflammatory Activity. Fitoterapia 2021, 151, 104872. [Google Scholar] [CrossRef]
- Lin, Z.H.; Zhang, T.L.; Zeng, J.G.; Tang, Q.; Wu, L.X.; Lu, Y. Study on Anti-Diarrhea Effect of Extract from Kadsura coccinea Fruit. Chin. J. Vet. Drug 2021, 55, 42–48. [Google Scholar]
- Tang, C.L.; Chen, J.L.; Zhang, R.F.; Xu, X.J.; Huang, W.H.; Yang, D.P.; Zhao, Z.M. Determination of Total Phenolic and Flavonoid Content and Study on Antioxidant Activity of Amomum Villosum from Various Producing Areas. China J. Tradit. Chin. Med. Pharm. 2021, 36, 122–126. [Google Scholar]
- Ge, Y.Z.; Ma, Q.Y.; Cai, C.H.; Kong, F.D.; Xie, Q.Y.; Yu, Z.F.; Zhao, Y.X. Analysis on activity of different Ganoderma lucidum and content of total triterpenoids produced in Hainan. Lishizhen Med. Mater. Med. Res. 2020, 31, 556–559. [Google Scholar]
- Lu, X.; Li, J.; Jiang, X.J.; Zhang, K.F.; Duan, X.Q. Content Determination of Lignans in Kadsura coccinea (Lem.) A.C.Smith. J. Anhui Agric. Sci. 2011, 39, 12152–12153. [Google Scholar]
- Shen, X.F.; Huang, J.T.; Zhang, L.J. Determination of Optimal Conditions of Polysaccharide Content of Hazel’s Flower by Anthrone Sulfuric Acid Method. Food Res. Dev. 2017, 18, 160–164. [Google Scholar]
- Liao, S.; Liao, L.; Huang, P.; Wang, Y.; Zhu, S.; Wang, X.; Lv, T.; Li, Y.; Fan, Z.; Liu, T.; et al. Effects of Different Levels of Garlic Straw Powder on Growth Performance, Meat Quality, Antioxidant and Intestinal Mucosal Morphology of Yellow-Feathered Broilers. Front. Physiol. 2022, 13, 902995. [Google Scholar] [CrossRef]
- Ban, N.K.; Thanh, B.V.; Kiem, P.V.; Minh, C.V.; Cuong, N.X.; Nhiem, N.X.; Huong, H.T.; Anh, H.T.; Park, E.J.; Sohn, D.H.; et al. Dibenzocyclooctadiene lignans and lanostane derivatives from the roots of Kadsura coccinea and their protective effects on primary rat hepatocyte injury induced by t-butyl hydroperoxide. Planta Med. 2009, 75, 1253–1257. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Y.; Tasneem, S.; Hussain, N.; Daniyal, M.; Yuan, H.; Xie, Q.; Liu, B.; Sun, J.; Jian, Y.; et al. Lignans from Tujia Ethnomedicine Heilaohu: Chemical Characterization and Evaluation of Their Cytotoxicity and Antioxidant Activities. Molecules 2018, 23, 2147. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.M.; Pu, J.X.; Huang, S.X.; Lu, Y.; Lou, L.G.; Li, R.T.; Xiao, W.L.; Chang, Y.; Sun, H.D. Kadcoccilactones A-J, Triterpenoids from Kadsura Coccinea. J. Nat. Prod. 2008, 71, 1182–1188. [Google Scholar] [CrossRef]
- Sritalahareuthai, V.; Temviriyanukul, P.; On-Nom, N.; Charoenkiatkul, S.; Suttisansanee, U. Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura Coccinea (Lem.) A.C. Sm. Foods 2020, 9, 1222. [Google Scholar] [CrossRef]
- Long, H.; Xia, X.; Liao, S.; Wu, T.; Wang, L.; Chen, Q.; Wei, S.; Gu, X.; Zhu, Z. Physicochemical Characterization and Antioxidant and Hypolipidaemic Activities of a Polysaccharide From the Fruit of Kadsura coccinea (Lem.) A. C. Smith. Front. Nutr. 2022, 9, 903218. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.; Abdallah, F.; Abdel-Hamid, T.; Hernandez-Santana, A. Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Br. Poult. Sci. 2016, 57, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.S.; Sallam, E.A.; Edris, S.N.; Khalifa, O.A.; Soliman, M.M.; Shehata, S.F. Growth Performance, Economic Efficiency, Meat Quality, and Gene Expression in Two Broiler Breeds Fed Different Levels of Tomato Pomace. Vet. Res. Commun. 2021, 45, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, R.; Liu, J.; Wang, W.; Chen, Y.; Cai, W. Effects of Novel Microecologics Combined with Traditional Chinese Medicine and Probiotics on Growth Performance and Health of Broilers. Poult. Sci. 2022, 101, 101412. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Pratama, A.R.; Yudiarti, T.; Ayaşan, T. Effect of novel natural feed additive containing Averrhoa bilimbi L. fruit filtrate, wheat bran, and Saccharomyces cerevisiae on growth performance and meat characteristics of broilers. Vet. World 2021, 14, 3007–3014. [Google Scholar] [CrossRef]
- Greene, E.S.; Cauble, R.; Kadhim, H.; de Almeida Mallmann, B.; Gu, I.; Lee, S.O.; Orlowski, S.; Dridi, S. Protective Effects of the Phytogenic Feed Additive “Comfort” on Growth Performance via Modulation of Hypothalamic Feeding- and Drinking-Related Neuropeptides in Cyclic Heat-Stressed Broilers. Domest. Anim. Endocrinol. 2021, 74, 106487. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, X.; Yao, X.; Zhang, H.; Song, Z.; He, X.; Cao, R. Effects of Feeding Fermented Mulberry Leaf Powder on Growth Performance, Slaughter Performance, and Meat Quality in Chicken Broilers. Animals 2021, 11, 3294. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q. Genome-Wide Analysis of lncRNA and mRNA Expression During Differentiation of Abdominal Preadipocytes in the Chicken. G3 2017, 7, 953–966. [Google Scholar] [CrossRef] [Green Version]
- Galli, G.M.; Griss, L.G.; Boiago, M.M.; Petrolli, T.G.; Glombowsky, P.; Bissacotti, B.F.; Copetti, P.M.; da Silva, A.D.; Schetinger, M.R.; Sareta, L.; et al. Effects of Curcumin and Yucca Extract Addition in Feed of Broilers on Microorganism Control (Anticoccidial and Antibacterial), Health, Performance and Meat Quality. Res. Vet. Sci. 2020, 132, 156–166. [Google Scholar] [CrossRef]
- Sun, H.; Chen, D.; Cai, H.; Chang, W.; Wang, Z.; Liu, G.; Deng, X.; Chen, Z. Effects of Fermenting the Plant Fraction of a Complete Feed on the Growth Performance, Nutrient Utilization, Antioxidant Functions, Meat Quality, and Intestinal Microbiota of Broilers. Animals 2022, 12, 2870. [Google Scholar] [CrossRef]
- Rivas, A.L.; Fabricant, J. Indications of Immunodepression in Chickens Infected with Various Strains of Marek’s Disease Virus. Avian Dis. 1988, 32, 8. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhang, J.L.; Gao, Y.H.; Wang, Q.H.; Li, S.; Wang, X.L.; Xu, S.W. Effect of Oxygen Free Radicals and Nitric Oxide on Apoptosis of Immune Organ Induced by Selenium Deficiency in Chickens. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2013, 26, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, K.; Chen, J.; Fang, J.; Cui, H.; Zuo, Z.; Deng, J.; Chen, Z.; Geng, Y.; Lai, W. Aflatoxin B1 Affects Apoptosis and Expression of Bax, Bcl-2, and Caspase-3 in Thymus and Bursa of Fabricius in Broiler Chickens. Environ. Toxicol. 2016, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.; Zhou, H.; Wang, L.; Ding, S.; Wang, Y.; Song, D.; Li, A. Effects of Microencapsulated Lactobacillus Plantarum and Fructooligosaccharide on Growth Performance, Blood Immune Parameters, and Intestinal Morphology in Weaned Piglets. Food Agric. Immunol. 2018, 29, 84–94. [Google Scholar] [CrossRef] [Green Version]
- LI, Y.Q.; Li, Y.; Huang, X.; Zhao, X.B.; Gao, X.P.; Wang, H.; Jiang, L.S. Effects of Bacillus Amyloliquefaciens on Lactation Performance, Milk Composition and Serum Biochemical, Immune and Antioxidant Indexes of Dairy Cows. Chin. J. Anim. Nutr. 2022, 34, 979–988. [Google Scholar]
- Movahhedkhah, S.; Rasouli, B.; Seidavi, A.; Mazzei, D.; Laudadio, V.; Tufarelli, V. Summer Savory (Satureja hortensis L.) Extract as Natural Feed Additive in Broilers: Effects on Growth, Plasma Constituents, Immune Response, and Ileal Microflora. Animals 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.J.; Wang, J.; He, T.F.; Liu, H.S.; Piao, X.S. Effects of natural capsicum extract on growth performance, nutrient utilization, antioxidant status, immune function, and meat quality in broilers. Poult. Sci. 2021, 100, 101301. [Google Scholar] [CrossRef]
- Loman, S.; Jansen, H.M.; Out, T.A.; Lutter, R. Interleukin-4 and Interferon-Gamma Synergistically Increase Secretory Component Gene Expression, But Are Additive in Stimulating Secretory Immunoglobulin A Release by Calu-3 Airway Epithelial Cells. Immunology 1999, 96, 537–543. [Google Scholar] [CrossRef]
- Song, B.; Tang, D.; Yan, S.; Fan, H.; Li, G.; Shahid, M.S.; Mahmood, T.; Guo, Y. Effects of Age on Immune Function in Broiler Chickens. J. Anim. Sci. Biotechnol. 2021, 12, 42. [Google Scholar] [CrossRef]
- Kelly, N.; Friend, K.; Boyle, P.; Zhang, X.R.; Wong, C.; Hackam, D.J.; Zamora, R.; Ford, H.R.; Upperman, J.S. The Role of the Glutathione Antioxidant System in Gut Barrier Failure in a Rodent Model of Experimental Necrotizing Enterocolitis. Surgery 2004, 136, 557–566. [Google Scholar] [CrossRef]
- Wang, Y.; Heng, C.; Zhou, X.; Cao, G.; Jiang, L.; Wang, J.; Li, K.; Wang, D.; Zhan, X. Supplemental Bacillus Subtilis DSM 29784 and Enzymes, Alone or in Combination, as Alternatives for Antibiotics to Improve Growth Performance, Digestive Enzyme Activity, Anti-Oxidative Status, Immune Response and the Intestinal Barrier of Broiler Chickens. Br. J. Nutr. 2021, 125, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.H.; Lee, S.J.; Gadde, U.D.; Oh, S.T.; Han, H.; Lillehoj, H.S. Effects of dietary Allium Hookeri Root on Growth Performance and Antioxidant Activity in Young Broiler Chickens. Res. Vet. Sci. 2018, 118, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.S.; Deng, W.; Liu, H.W. Effects of Chlorogenic Acid-Enriched Extract from Eucommia Ulmoides Leaf on Performance, Meat Quality, Oxidative Stability, and Fatty Acid Profile of Meat in Heat-Stressed Broilers. Poult. Sci. 2019, 98, 3040–3049. [Google Scholar] [CrossRef]
- Kawalilak, L.T.; Ulmer Franco, A.M.; Fasenko, G.M. Impaired intestinal villi growth in broiler chicks with unhealed navels. Poult. Sci. 2010, 89, 82–87. [Google Scholar] [CrossRef]
- Youssef, I.M.I.; Männer, K.; Zentek, J. Effect of Essential Oils or Saponins Alone or in Combination on Productive Performance, Intestinal Morphology and Digestive Enzymes’ Activity of Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2021, 105, 99–107. [Google Scholar] [CrossRef]
- Paul, S.S.; Vantharam Venkata, H.G.R.; Raju, M.V.; Rama Rao, S.V.; Nori, S.S.; Suryanarayan, S.; Kumar, V.; Perveen, Z.; Prasad, C.S. Dietary Supplementation of Extracts of Red Sea Weed (Kappaphycus alvarezii) Improves Growth, Intestinal Morphology, Expression of Intestinal Genes and Immune Responses in Broiler Chickens. J. Sci. Food Agric. 2021, 101, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.; Ibrahim, D.; Kishawy, A.T.Y.; Abdallah, H.M.; Hernandez-Santana, A.; Attia, G. Effect of Cereal Type and Plant Extract Addition on the Growth Performance, Intestinal Morphology, Caecal Microflora, and Gut Barriers Gene Expression of Broiler Chickens. Anim. Int. J. Anim. Biosci. 2021, 15, 100056. [Google Scholar] [CrossRef]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.D.; Delzenne, N.M.; Muccioli, G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1849998. [Google Scholar] [CrossRef]
- Lin, H.; Guo, Q.; Ran, Y.; Lin, L.; Chen, P.; He, J.; Chen, Y.; Wen, J. Multiomics Study Reveals Enterococcus and Subdoligranulum Are Beneficial to Necrotizing Enterocolitis. Front. Microbiol. 2021, 12, 752102. [Google Scholar] [CrossRef]
- Bunesova, V.; Lacroix, C.; Schwab, C. Mucin Cross-Feeding of Infant Bifidobacteria and Eubacterium hallii. Microb. Ecol. 2018, 75, 228–238. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Wang, Y.; Li, J.; Bai, G.; Liu, L.; Zhong, R.; Ma, T.; Pan, H.; Zhang, H. Supplementation of Multi-Enzymes Alone or Combined with Inactivated Lactobacillus Benefits Growth Performance and Gut Microbiota in Broilers Fed Wheat Diets. Front. Microbiol. 2022, 13, 927932. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wu, C.; Yu, J.; Luo, J.; Peng, X. Angelica Sinensis Polysaccharide Improves Rheumatoid Arthritis by Modifying the Expression of Intestinal Cldn5, Slit3 and Rgs18 through Gut Microbiota. Int. J. Biol. Macromol. 2022, 209, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Bai, P.; Yang, B.; Feng, X.; Qiu, F. Asiatic Acid Alleviates Metabolism Disorders in ob/ob Mice: Mechanistic Insights. Food Funct. 2022, 13, 6934–6946. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Zheng, K.; Ma, Y.J.; He, R.X.; Arowolo, M.A.; Zhou, Y.J.; Xiao, D.F.; He, J.H. Effects of Fenugreek Seed Extracts on Growth Performance and Intestinal Health of Broilers. Poult. Sci. 2022, 101, 101939. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, P.; Geng, Q.; Fan, H.; Gong, Y.; Hu, Y.; Shan, L.; Sun, Y.; Shen, W.; Zhou, Y. Disrupted Spermatogenesis in a Metabolic Syndrome Model: The Role of Vitamin A Metabolism in the Gut-Testis Axis. Gut 2022, 71, 78–87. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; Severs, E.L.; Hendrickson, J.D.; Mehta, G.; Whitney, A.K.; Ir, D.; Ohri, N.; Robertson, C.E.; et al. Bacteroidales Recruit IL-6-Producing Intraepithelial Lymphocytes in the Colon to Promote Barrier Integrity. Mucosal Immunol. 2018, 11, 357–368. [Google Scholar] [CrossRef]






| Ingredients (%) | 1~21 d (Early Growth Stage) | 22~42 d (Late Growth Stage) |
|---|---|---|
| Corn | 55.21 | 62.21 |
| Soybean meal | 36.40 | 29.20 |
| soybean oil | 4.70 | 4.90 |
| Limestone | 1.52 | 1.60 |
| CaHPO4 | 1.00 | 1.00 |
| L-Lysine | 0.35 | 0.30 |
| Methionine | 0.16 | 0.13 |
| L-Threonine | 0.06 | 0.06 |
| Salt | 0.30 | 0.30 |
| Premix 1 | 0.30 | 0.30 |
| Nutrient levels 2 | ||
| Metabolic energy, MJ/kg | 12.72 | 13.02 |
| Curde protein, % | 20.65 | 18.28 |
| Lysine, % | 1.27 | 1.09 |
| Methionine, % | 0.47 | 0.41 |
| Calcium, % | 0.90 | 0.91 |
| Total phosphorus, % | 0.54 | 0.52 |
| Indicators | Standard Curve | R2 | Contents (%) |
|---|---|---|---|
| general flavones | y = 0.8566x + 0.0497 | 0.9963 | 9.77 ± 0.12 |
| triterpene | y = 2.2275x + 0.0082 | 0.9918 | 5.20 ± 0.09 |
| lignans | y = 0.7335x + 0.0055 | 0.9910 | 28.07 ± 1.54 |
| polysaccharide | y = 3.8662x + 0.0234 | 0.9974 | 6.70 ± 0.23 |
| Items | CK | PD | LD | MD | HD |
|---|---|---|---|---|---|
| 1~21 d | |||||
| Initial weight (g) | 46 | 46 | 46 | 46 | 46 |
| Final weight (g) | 708.57 ± 34.83 a | 620.56 ± 98.10 b | 603.7 ± 95.80 b | 680.65 ± 22.28 ab | 653.89 ± 66.34 ab |
| ADG (g) | 31.81 ± 1.85 a | 25.40 ± 5.26 b | 25.40 ± 5.71 b | 30.06 ± 1.14 a | 28.64 ± 3.08 ab |
| ADFI (g) | 42.59 ± 2.57 ab | 40.00 ± 8.13 b | 44.10 ± 5.46 ab | 46.31 ± 1.53 a | 43.10 ± 2.81 ab |
| F/G | 1.34 ± 0.05 b | 1.53 ± 0.19 b | 1.82 ± 0.49 a | 1.54 ± 0.36 ab | 1.51 ± 0.13 b |
| 22~42 d | |||||
| Final weight (g) | 2476.67 ± 70.90 b | 2607.69 ± 384.75 ab | 2510.91 ± 217.27 ab | 2739.20 ± 105.95 a | 2664.35 ± 81.33 ab |
| ADG (g) | 83.70 ± 3.21 b | 93.97 ± 14.32 a | 87.64 ± 5.76 ab | 97.10 ± 6.28 a | 91.87 ± 5.74 ab |
| ADFI (g) | 134.03 ± 11.67 ab | 140.92 ± 18.39 ab | 125.81 ± 11.53 b | 142.03 ± 14.81 ab | 149.16 ± 11.62 a |
| F/G | 1.60 ± 0.16 ab | 1.51 ± 0.13 ab | 1.44 ± 0.19 b | 1.47 ± 0.15 ab | 1.62 ± 0.10 a |
| 1~42 d | |||||
| ADG (g) | 57.87 ± 1.69 b | 60.99 ± 9.16 ab | 58.69 ± 5.17 ab | 64.12 ± 2.52 a | 62.34 ± 1.94 ab |
| ADFI (g) | 88.31 ± 6.86 ab | 90.49 ± 11.14 ab | 84.95 ± 6.11 b | 94.17 ± 7.23 a | 96.13 ± 5.01 a |
| F/G | 1.53 ± 0.10 | 1.49 ± 0.07 | 1.46 ± 0.22 | 1.46 ± 0.09 | 1.54 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhou, D.; Wang, X.; Xiao, T.; Wu, L.; Tang, Q.; Lu, Y. Effects of Kadsura coccinea L. Fruit Extract on Growth Performance, Meat Quality, Immunity, Antioxidant, Intestinal Morphology and Flora of White-Feathered Broilers. Animals 2023, 13, 93. https://doi.org/10.3390/ani13010093
Zhang T, Zhou D, Wang X, Xiao T, Wu L, Tang Q, Lu Y. Effects of Kadsura coccinea L. Fruit Extract on Growth Performance, Meat Quality, Immunity, Antioxidant, Intestinal Morphology and Flora of White-Feathered Broilers. Animals. 2023; 13(1):93. https://doi.org/10.3390/ani13010093
Chicago/Turabian StyleZhang, Tianlu, Dong Zhou, Xin Wang, Tian Xiao, Lingxi Wu, Qi Tang, and Ying Lu. 2023. "Effects of Kadsura coccinea L. Fruit Extract on Growth Performance, Meat Quality, Immunity, Antioxidant, Intestinal Morphology and Flora of White-Feathered Broilers" Animals 13, no. 1: 93. https://doi.org/10.3390/ani13010093





