Brown and Green Seaweed Antioxidant Properties and Effects on Blood Plasma Antioxidant Enzyme Activities, Hepatic Antioxidant Genes Expression, Blood Plasma Lipid Profile, and Meat Quality in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Experimental Design
2.2. Sample Collection
2.3. Antioxidant Property of Brown and Green Seaweed
2.3.1. Preparation of Seaweed Extract
2.3.2. Antioxidant Activity Assay
2.4. Plasma Antioxidant Enzymes Activities
2.4.1. Catalase Activity
2.4.2. Superoxide Dismutase Activity
2.4.3. Glutathione Peroxidase Activity
2.5. Hepatic Antioxidant Genes Expression
2.6. Plasma Lipid Profile
2.7. Proximate Analysis of Breast Meat
2.8. Meat Quality Determination
2.8.1. Meat pH
2.8.2. Meat Color
2.8.3. Water Holding Capacity of Meat
2.8.4. Meat Tenderness or Shear Force
2.9. Statistical Analysis
3. Results
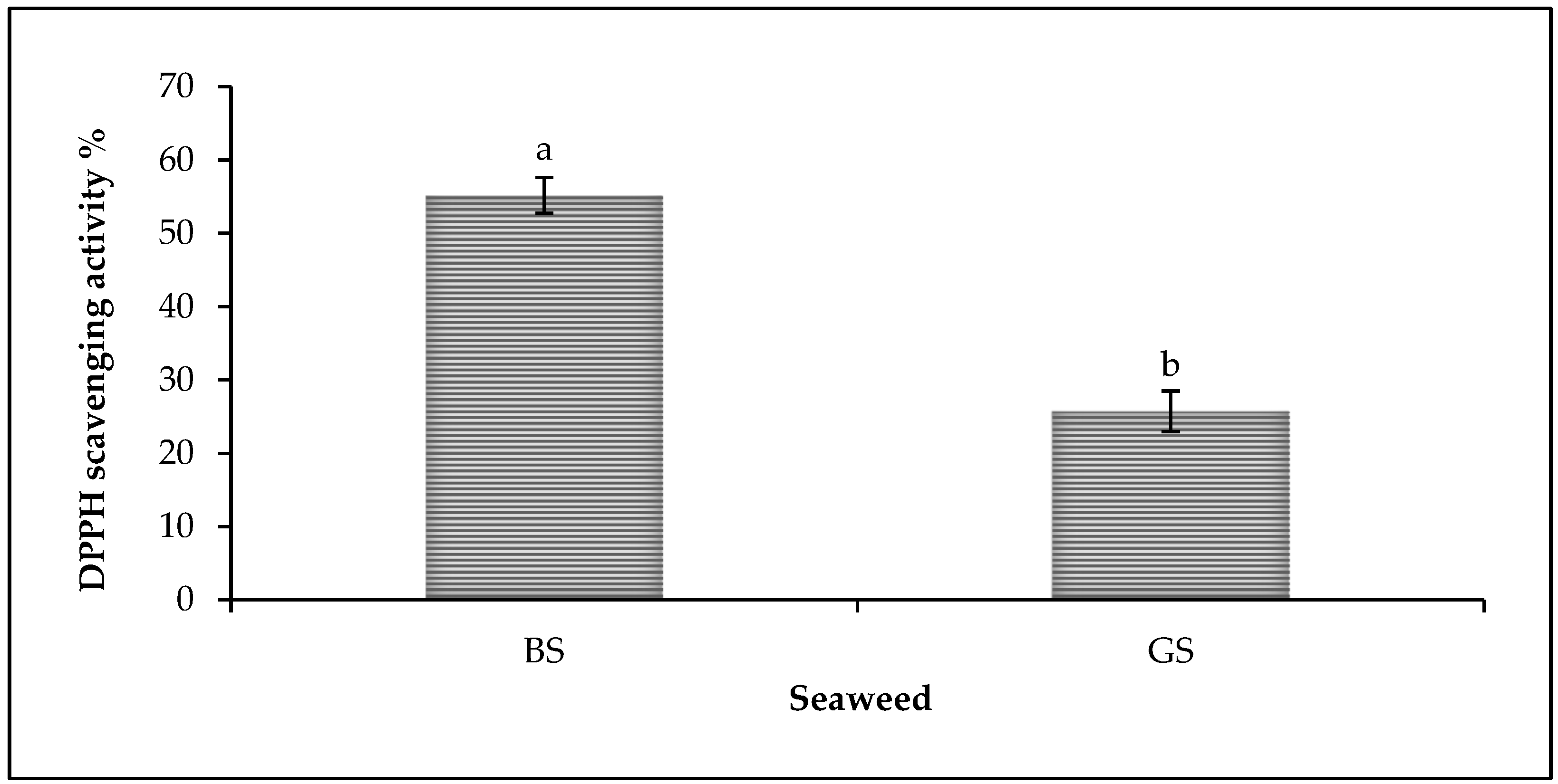
3.1. Antioxidant Property of Brown and Green Seaweed
3.2. Plasma Antioxidant Enzymes Activities
3.3. Hepatic Antioxidant Genes Expression
3.4. Plasma Lipid Profile
3.5. Proximate Analysis of Breast Meat
3.6. Meat Quality
4. Discussion
4.1. Antioxidant Properties of Brown and Green Seaweed
4.2. Plasma Antioxidant Enzymes Activities
4.3. Hepatic Antioxidant Genes Expression
4.4. Plasma Lipid Profile
4.5. Proximate Analysis of Breast Meat
4.6. Meat Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corino, C.; Modina, S.C.; Di Giancamillo, A.; Chiapparini, S.; Rossi, R. Seaweeds in pig nutrition. Animals 2019, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Kumar, M.; Mishra, G.; Sahoo, D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017, 226, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Waagbø, R.; Lock, E.J.; Bruckner, C.G. In-Depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- El-Deek, A.A.; Al-Harthi, M.A.; Abdalla, A.A.; Elbanoby, M.M. The use of brown algae meal in finisher broiler. Egyt. Poult. Sci. J. 2011, 31, 767–781. [Google Scholar]
- Azizi, M.N.; Loh, T.C.; Foo, H.L.; Akit, H.; Izuddin, W.I.; Shazali, N.; Teik Chung, E.L.; Samsudin, A.A. Chemical compositions of brown and green seaweed, and effects on nutrient digestibility in broiler chickens. Animals 2021, 11, 2147. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Gupta, D. Methods for Determination of Antioxidant Capacity: A Review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Abdulla, N.R.; Loh, T.C.; Akit, H.; Sazili, A.Q.; Foo, H.L.; Mohamad, R.; Abdul Rahim, R.; Ebrahimi, M.; Sabow, A.B. Fatty acid profile, cholesterol and oxidative status in broiler chicken breast muscle fed different dietary oil sources and calcium levels. S. Afr. J. Anim. Sci. 2015, 45, 153–163. [Google Scholar] [CrossRef]
- Rao, P.V.S.; Periyasamy, C.; Kumar, K.S.; Annabattula, S.R.; Anantharaman, P. Seaweeds: Distribution, Production and Uses. In Bioprospecting of Algae; Noor, M.M., Bhatnagar, S.K., Sinha, S.K., Eds.; Society for Plant Research India: Greater Noida, India, 2018; pp. 59–67. [Google Scholar]
- Hayes, M. Marine Bioactive Compounds: Sources, Characterrizaiton and Application; Library of Congress: Dublin, Ireland, 2012; ISBN 9788578110796.
- Qin, Y. Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Osborn, P., Ed.; Andre Gerhard Wolff: London, UK, 2018; ISBN 9780128133125. [Google Scholar]
- Shao, P.; Chen, M.; Pei, Y.; Sun, P. In intro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Int. J. Biol. Macromol. 2013, 59, 295–300. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Bergé, J.P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (eae) for the production of antiviral and antioxidant extracts from the green seaweed Ulva Armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Airanthi, M.K.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gumus, R.; Urcar Gelen, S.; Koseoglu, S.; Ozkanlar, S.; Ceylan, Z.G.; Imik, H. The Effects of fucoxanthin dietary inclusion on the growth performance, antioxidant metabolism and meat quality of broilers. Rev. Bras. Cienc. Avic. 2018, 20, 487–496. [Google Scholar] [CrossRef]
- Ji, X.; Rivers, L.; Zielinski, Z.; Xu, M.; MacDougall, E.; Stephen, J.; Zhang, S.; Wang, Y.; Chapman, R.G.; Keddy, P.; et al. Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities. Food Chem. 2012, 133, 1177–1187. [Google Scholar] [CrossRef]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Danladi, Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Tamrin, N.A.M.; Azizi, M.N. Effects of postbiotics and paraprobiotics as replacements for antibiotics on growth performance, carcass characteristics, small intestine histomorphology, immune status and hepatic growth gene expression in broiler chickens. Animals 2022, 12, 917. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2-δδct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of feeding different postbiotics produced by Lactobacillus Plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef]
- Delong, D.M.; Delong, E.R.; Wood, P.D.; Lippel, K.; Rifkind, B.M. A Comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol: The lipid research clinics prevalence study. J. Am. Med. Assoc. 1986, 256, 2372–2377. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Sazili, A.Q.; Parr, T.; Sensky, P.L.; Jones, S.W.; Bardsley, R.G.; Buttery, P.J. The relationship between slow and fast myosin heavy chain content, calpastatin and meat tenderness in different ovine skeletal muscles. Meat Sci. 2005, 69, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cavitt, L.C.; Youm, G.W.; Meullenet, J.F.; Owens, C.M.; Xiong, R. Prediction of poultry meat tenderness using razor blade shear, allo-kramer shear, and sarcomere length. J. Food Sci. 2004, 69, 11–15. [Google Scholar] [CrossRef]
- Al-Hmoud, H.A.; Ibrahim, N.E.; El-Hallous, E.I. Concentration and the other formulations effects on the drug release rate from a controlled-release matrix. Afr. J. Pharm. Pharmacol. 2014, 8, 364–371. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Bharathiaraja, S.; Mahendran, S. Extraction, partially purification and study on antioxidant property of fucoxanthin from Sargassum cinereum. J. Chem. Pharm. Res. 2016, 8, 610–616. [Google Scholar]
- Van Den Burg, S.; Stuiver, M.; Veenstra, F.; Bikker, P.; Contreras, A.L.; Palstra, A.; Broeze, J.; Jansen, H.; Jak, R.; Gerritsen, A.; et al. A Triple P Review of the Feasibility of Sustainable Offshore Seaweed Production in the North Sea; Wageningen: Wageningen, The Netherlands, 2013; ISBN 9789086156528. [Google Scholar]
- Kim, S.K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Wiley-Blackwall publishing: Oxford, UK, 2011; ISBN 9780470979181. [Google Scholar]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Bacou, E.; Walk, C.; Rider, S.; Litta, G.; Perez-Calvo, E. Dietary Oxidative Distress: A Review of nutritional challenges as models for poultry, swine and fish. Antioxidants 2021, 10, 525. [Google Scholar] [CrossRef]
- Latham, H. Temperature stress-induced bleaching of the coralline alga Corallina Officinalis: A role for the enzyme bromoperoxidase. Biosci. Horiz. 2008, 1, 104–113. [Google Scholar] [CrossRef]
- Ramirez-Higuera, A.; Quevedo-Corona, L.; Paniagua-Castro, N.; Chamorro-Ceballos, G.; Milliar-Garcia, A.; Jaramillo-Flores, M.E. Antioxidant enzymes gene expression and antihypertensive effects of seaweeds Ulva Linza and Lessonia Trabeculata in rats fed a high-fat and high-sucrose diet. J. Appl. Phycol. 2014, 26, 597–605. [Google Scholar] [CrossRef]
- Maheswari, M.; Das, A.; Datta, M.; Tyagi, A.K. Supplementation of tropical seaweed-based formulations improves antioxidant status, immunity and milk production in lactating Murrah buffaloes. J. Appl Phycol. 2021, 33, 2629–2643. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Mahrose, K. Seaweeds, intact and processed, as a valuable component of poultry feeds. J. Mar. Sci. Eng. 2020, 8, 620. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, S.R.; Oh, J.W. Effects of dietary fermented seaweed and seaweed fusiforme on growth performance, carcass parameters and immunoglobulin concentration in broiler chicks. Asian-Australas. J. Anim. Sci. 2014, 27, 862–870. [Google Scholar] [CrossRef]
- Kumar, A.K. Effect of Sargassum wightii on growth, carcass and serum qualities of broiler chickens. Open Access J. Vet. Sci. Res. 2018, 3, 000156. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J. Med. Food 2010, 13, 792–800. [Google Scholar] [CrossRef]
- Dousip, A.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H.; Lim, T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2014, 26, 999–1008. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Okab, A.B.; Aljumaah, R.S.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A. Nutritional Value of Green Seaweed (Ulva lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013, 12, 177–181. [Google Scholar] [CrossRef]
| Ingredients (%) | Dietary Treatments 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | |
| Corn | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 | 46.0 |
| Soybean meal | 40.0 | 40.0 | 39.8 | 39.5 | 39.3 | 39.0 | 38.8 | 39.8 | 39.5 | 39.3 | 39.0 | 38.8 |
| Wheat pollard | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Palm oil | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| L-Lysine 2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| DL-Methionine 3 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| DCP 4 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 | 2.60 |
| Calcium carbonate | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mineral mix 5 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin mix 6 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidants | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Toxin binder | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Seaweed | - | - | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 |
| Vitamin E | - | 0.01 | - | - | - | - | - | - | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated analysis 7 | ||||||||||||
| ME (kcal/kg) 8 | 3040.2 | 3039.9 | 3041.0 | 3041.9 | 3042.7 | 3043.6 | 3044.5 | 3040.7 | 3041.3 | 3041.9 | 3042.5 | 3043.0 |
| Protein | 21.95 | 21.95 | 21.94 | 21.91 | 21.90 | 21.89 | 21.87 | 21.93 | 21.90 | 21.87 | 21.85 | 21.82 |
| Fat | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.98 | 5.97 | 5.97 |
| Fiber | 4.34 | 4.34 | 4.33 | 4.31 | 4.31 | 4.29 | 4.28 | 4.32 | 4.31 | 4.30 | 4.29 | 4.28 |
| Calcium | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 | 0.83 |
| Total phosphorous | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.00 | 1.00 | 1.01 | 1.01 | 1.00 | 1.00 | 1.00 |
| Available phosphorus | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Ingredients (%) | Dietary Treatments 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | |
| Corn | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 | 52.0 |
| Soybean meal | 32.0 | 32.0 | 31.8 | 31.5 | 31.3 | 31.0 | 30.8 | 31.8 | 31.5 | 31.3 | 31.0 | 30.8 |
| Wheat pollard | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Palm oil | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 | 5.10 |
| L-Lysine 2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| DL-Methionine 3 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| DCP 4 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 |
| Calcium carbonate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mineral mix 5 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Vitamin mix 6 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Antioxidants | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Toxin binder | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Seaweed | - | - | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 |
| Vitamin E | - | 0.01 | - | - | - | - | - | - | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Calculated analysis 7 | ||||||||||||
| ME (kcal/kg) 8 | 3149.8 | 3149.5 | 3150.7 | 3151.5 | 3152.4 | 3153.3 | 3154.1 | 3150.4 | 3150.9 | 3151.6 | 3152.1 | 3152.7 |
| Protein | 19.06 | 19.06 | 19.05 | 19.03 | 19.01 | 19.00 | 18.98 | 19.04 | 19.01 | 18.98 | 18.96 | 18.93 |
| Fat | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.19 | 7.18 | 7.18 | 7.18 |
| Fiber | 4.00 | 4.00 | 3.99 | 3.98 | 3.97 | 3.96 | 3.95 | 3.99 | 3.98 | 3.97 | 3.96 | 3.94 |
| Calcium | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Total phosphorous | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 |
| Available phosphorus | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 |
| Target Gene | Primer Sequence 5′-3′ | bp | Accession No. | |
|---|---|---|---|---|
| SOD1 | F-CACTGCATCATTGGCCGTACCA | R-GCTTGCACACGGAAGAGCAAGT | 224 | NM_205064.1 |
| GPX1 | F-GCTGTTCGCCTTCCTGAGAG | R-GTTCCAGGAGACGTCGTTGC | 118 | NM_001277853.2 |
| CAT | F-TGGCGGTAGGAGTCTGGTCT | R-GTCCCGTCCGTCAGCCATTT | 139 | NM_001031215.2 |
| GAPDH | F-CTGGCAAAGTCCAAGTGGTG | R-AGCACCACCCTTCAGATGAG | 275 | NM_204305.1 |
| Enzymes 1 | Dietary Treatments 2 | SEM 3 | p-Values | Contrast p-Values 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| CAT (U/L) | 4.11 | 3.71 | 3.73 | 3.73 | 3.65 | 3.84 | 4.16 | 3.46 | 3.97 | 3.96 | 3.92 | 4.00 | 0.21 | 0.6222 | 0.3072 | 0.1506 |
| SOD (U/mL) | 3.10 | 3.11 | 3.14 | 3.08 | 3.11 | 3.09 | 3.15 | 3.04 | 3.10 | 3.12 | 3.14 | 3.11 | 0.04 | 0.9395 | 0.4899 | 0.8876 |
| GPx (U/L) | 0.48 | 0.48 | 0.51 | 0.46 | 0.49 | 0.47 | 0.43 | 0.51 | 0.44 | 0.46 | 0.46 | 0.47 | 0.03 | 0.6493 | 0.5120 | 0.6833 |
| Parameters 1 (mRNA Fold Change) | Dietary Treatments 2 | SEM 3 | p-Values | Contrast p-Values 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| SOD1 | 1 c | 1.053 c | 1.13 b,c | 1.517 a,b | 1.604 a | 0.798 c | 1.174 a,b,c | 0.935 c | 1.208 a,b,c | 1.176 a,b,c | 0.992 c | 1.211 a,b,c | 0.049 | 0.0193 | 0.5515 | 0.0597 |
| GPX1 | 1 | 1.038 | 1.096 | 0.763 | 1.020 | 0.979 | 0.972 | 0.834 | 0.643 | 1.013 | 0.779 | 0.912 | 0.046 | 0.4557 | 0.4080 | 0.3860 |
| CAT | 1 | 1.002 | 1.301 | 1.050 | 1.007 | 1.034 | 0.793 | 1.029 | 0.847 | 1.074 | 1.064 | 0.858 | 0.041 | 0.7925 | 0.2322 | 0.5524 |
| Parameters 1 (mmol/L) | Dietary Treatments 2 | SEM 3 | p-Values | Contrast p-Values 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| TC | 2.43 c | 2.76 b,c | 2.9 b,c | 2.95 b,c | 4.0 a | 3.5 a,b | 2.98 b,c | 3.05 b,c | 3.28 a,b,c | 3.18 a,b,c | 2.9 b,c | 2.6 b,c | 0.26 | 0.0287 | 0.0182 | 0.1473 |
| TG | 0.31 | 0.29 | 0.3 | 0.34 | 0.37 | 0.39 | 0.42 | 0.54 | 0.41 | 0.29 | 0.45 | 0.48 | 0.07 | 0.3979 | 0.0555 | 0.6822 |
| HDL | 1.85 c | 2.68 b,c | 2.35 b,c | 2.45 b,c | 3.68 a | 2.74 b | 2.44 b,c | 2.5 b,c | 2.64 b,c | 2.68 b,c | 2.42 b,c | 2.19 b,c | 0.22 | 0.0067 | 0.0110 | 0.0124 |
| LDL | 0.57 | 0.65 | 0.57 | 0.58 | 0.82 | 0.57 | 0.58 | 0.58 | 0.75 | 0.6 | 0.51 | 0.53 | 0.09 | 0.5385 | 0.1541 | 0.5914 |
| VLDL | 0.06 | 0.07 | 0.08 | 0.08 | 0.11 | 0.08 | 0.06 | 0.09 | 0.1 | 0.06 | 0.07 | 0.07 | 0.01 | 0.3979 | 0.0566 | 0.6360 |
| Nutrients 1 (%) | Dietary Treatments 2 | Contrast p-Values 4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | SEM 3 | p-Values | Line. | Quad. | |
| Moisture | 74.43 | 73.03 | 74.25 | 74.03 | 74.18 | 74.38 | 74.17 | 73.5 | 73.56 | 74.71 | 74.04 | 74.1 | 0.40 | 0.3168 | 0.0755 | 0.6850 |
| CP | 23.04 c | 23.85 b,c | 24.51 a,b | 24.69 a,b | 23.93 a,b,c | 23.79 b,c | 24.29 a,b,c | 24.33 a,b | 25.14 a | 24.04 a,b,c | 24.75 a,b | 23.71 b,c | 0.38 | 0.0404 | 0.0218 | 0.4461 |
| EE | 2.23 a | 2.19 a,b | 1.99 a,b,c | 1.88 b,c | 1.93 a,b,c | 1.92 a,b,c | 2.11 a,b | 2.05 a,b | 1.96 a,b,c | 1.70 c | 2.05 a,b | 1.87 b,c | 0.09 | 0.0258 | 0.7959 | 0.0619 |
| Ash | 1.79 | 1.89 | 1.68 | 1.66 | 1.75 | 1.67 | 1.76 | 1.78 | 1.74 | 1.67 | 1.7 | 1.64 | 0.07 | 0.4587 | 0.5104 | 0.2256 |
| Parameters | Dietary Treatments 1 | SEM 2 | p-Values | Contrast p-Values 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | BS 0.25 | BS 0.50 | BS 0.75 | BS 1 | BS 1.25 | GS 0.25 | GS 0.50 | GS 0.75 | GS 1 | GS 1.25 | Line. | Quad. | |||
| Day 1 | ||||||||||||||||
| pH | 6.02 | 5.90 | 5.78 | 5.87 | 6.03 | 5.87 | 6.05 | 5.94 | 5.79 | 5.97 | 5.83 | 5.83 | 0.07 | 0.2827 | 0.7491 | 0.9708 |
| Drip loss% | 1.80 | 1.55 | 1.72 | 1.70 | 1.44 | 1.72 | 1.38 | 1.46 | 1.39 | 1.07 | 1.23 | 1.56 | 0.23 | 0.6304 | 0.9737 | 0.9700 |
| Cooking loss% | 28.8 | 27.21 | 27.98 | 25.88 | 23.62 | 25.56 | 23.55 | 21.02 | 26.2 | 22.28 | 23.04 | 25.94 | 28.8 | 0.0939 | 0.2273 | 0.1439 |
| Shear force (g) | 978.3 | 811.5 | 1112.9 | 832.9 | 913.2 | 971.2 | 788.7 | 869.3 | 1058.6 | 1016.6 | 869.9 | 978.8 | 88.8 | 0.1726 | 0.2048 | 0.1535 |
| Colour 4 | ||||||||||||||||
| L* | 57.47 a | 50.93 b,c | 47.15 c,d | 44.06 d | 50.65 b,c | 50 b,c | 50.25 b,c | 48.59 b,c,d | 50.48 b,c | 50.63 b,c | 49.49 b,c,d | 53.43 a,b | 1.73 | 0.0018 | 0.8265 | 0.0002 |
| a* | 4.75 | 5.73 | 6.54 | 6.72 | 5.76 | 6.74 | 5.60 | 5.80 | 6.08 | 6.72 | 7.80 | 6.77 | 0.94 | 0.7647 | 0.0745 | 0.5778 |
| b* | 17.43 b,c | 20.45 a,b | 18.74 a,b,c | 20.35 a,b | 15.98 c | 19.48 a,b | 20.46 a,b | 19.57 a,b | 20.99 a,b | 20.12 a,b | 21.66 a | 20.73 a,b | 1.07 | 0.0390 | 0.0227 | 0.2827 |
| Day 7 | ||||||||||||||||
| pH | 6.07 | 5.97 | 6.04 | 6.13 | 6.18 | 6.09 | 5.89 | 5.84 | 5.95 | 5.87 | 5.96 | 5.95 | 0.07 | 0.0968 | 0.5520 | 0.4604 |
| Drip loss% | 3.53 a | 3.27 | 3.33 a,b | 3.05 a,b | 2.18 b | 3.25 a,b | 2.65 a,b | 3.16 b | 2.38 a,b | 3.31 a,b | 3.38 ab | 3.5 a | 0.39 | 0.3103 | 0.0128 | 0.9328 |
| Cooking loss% | 25.45 a | 19.47 | 22.22 a,b | 23.81 a | 22.13 a,b | 23.43 a | 18.17 b | 18.83 b | 20.59 a,b | 24.04 a,b | 19.83 a,b | 22.63 a,b | 1.91 | 0.4316 | 0.0206 | 0.7182 |
| Shear force (g) | 1148.9 | 1152.1 | 1101.6 | 1137.3 | 1226.8 | 1008.2 | 945.2 | 1133.2 | 995.2 | 820.1 | 898.2 | 1067.9 | 105.3 | 0.3495 | 0.5628 | 0.8429 |
| Color | ||||||||||||||||
| L* | 55.01 | 47.79 | 51.22 | 52.82 | 52.95 | 51.52 | 50.25 | 50.24 | 50.76 | 51.43 | 50.33 | 50.89 | 1.51 | 0.2781 | 0.1628 | 0.4581 |
| a* | 6.28 | 6.73 | 7.19 | 6.20 | 7.15 | 7.09 | 7.00 | 7.58 | 7.58 | 6.57 | 7.14 | 6.57 | 0.69 | 0.9400 | 0.1152 | 0.8437 |
| b* | 20.61 | 18.11 | 20.79 | 20.83 | 20.29 | 21.67 | 22.21 | 21 | 21.25 | 20.18 | 19.92 | 19.43 | 0.81 | 0.1433 | 0.0571 | 0.7256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, M.N.; Loh, T.C.; Foo, H.L.; Akit, H.; Izuddin, W.I.; Yohanna, D. Brown and Green Seaweed Antioxidant Properties and Effects on Blood Plasma Antioxidant Enzyme Activities, Hepatic Antioxidant Genes Expression, Blood Plasma Lipid Profile, and Meat Quality in Broiler Chickens. Animals 2023, 13, 1582. https://doi.org/10.3390/ani13101582
Azizi MN, Loh TC, Foo HL, Akit H, Izuddin WI, Yohanna D. Brown and Green Seaweed Antioxidant Properties and Effects on Blood Plasma Antioxidant Enzyme Activities, Hepatic Antioxidant Genes Expression, Blood Plasma Lipid Profile, and Meat Quality in Broiler Chickens. Animals. 2023; 13(10):1582. https://doi.org/10.3390/ani13101582
Chicago/Turabian StyleAzizi, Mohammad Naeem, Teck Chwen Loh, Hooi Ling Foo, Henny Akit, Wan Ibrahim Izuddin, and Danladi Yohanna. 2023. "Brown and Green Seaweed Antioxidant Properties and Effects on Blood Plasma Antioxidant Enzyme Activities, Hepatic Antioxidant Genes Expression, Blood Plasma Lipid Profile, and Meat Quality in Broiler Chickens" Animals 13, no. 10: 1582. https://doi.org/10.3390/ani13101582
APA StyleAzizi, M. N., Loh, T. C., Foo, H. L., Akit, H., Izuddin, W. I., & Yohanna, D. (2023). Brown and Green Seaweed Antioxidant Properties and Effects on Blood Plasma Antioxidant Enzyme Activities, Hepatic Antioxidant Genes Expression, Blood Plasma Lipid Profile, and Meat Quality in Broiler Chickens. Animals, 13(10), 1582. https://doi.org/10.3390/ani13101582







