Anatomical Characteristics of Duplicated Caudal Vena Cava in Cats—A Case Report
Abstract
:Simple Summary
Abstract
1. Introduction
| Species | Sex | Diagnostic Method | Number | Prevalence of dCVC Anomaly | Prevalence of Accompanied Pathologies | References |
|---|---|---|---|---|---|---|
| Domestic cat | Not specified | Necropsy | 301 | dCVC in 7%, of which 21% were accompanied with ureteric anomalies | Circumcaval/retrocaval ureter in 32%, of which 80% were affected on the right side | Bélanger et al., 2014 [31] |
| Domestic cat | 50.4% females and 49.6% males (90.4% castrated) | CT angiography, USG, and MRI | 272 | dCVC in 5.8% | Circumcaval/retrocaval ureter in 12.5%, of which 87.5% had dCVC | Pey et al., 2015 [23] |
| Domestic cat | Female | Necropsy | 1 | dCVC | Left retrocaval ureter | Casteleyn et al., 2015 [21] |
| Domestic cat | Female | Necropsy | 1 | dCVC | Circumcaval ureters and diaphragmatic hernia | Chisco et al., 2016 [25] |
| Domestic cat | Female | Necropsy | 1 | dCVC | None | Stocco et al., 2019 [28] |
| Domestic dog | Not specified | CT and USG | 7913 | Prevalence of dCVC was 2.08% on CT and 0.46% on USG; prevalence was significantly higher in small breeds than in large breeds | Extrahepatic portosystemic shunts | Bertolini et al., 2014 [4] |
| Domestic dog | Not specified | CT | 121 | CVC split in 14% (99% affected on the right side): partial duplication in 7% and complete duplication in 6% | Not specified | Ryu et al., 2019 [9] |
| Guinea pig | 50 males and 50 females | Embalming, observation of transverse sections | 100 | dCVC in 54% (30% in males and 24% in females) | None | Nakamura et al., 2019 [32] |
2. Materials and Methods
3. Results
4. Discussion
4.1. Pre-Hepatic Caudal Vena Cava in Domestic Mammals and Infrahepatic Human Inferior Vena Cava Development
4.2. Differences between the Duplicated Caudal Vena Cava and Inferior Vena Cava
4.3. Duplicated Caudal Vena Cava in Cats and Dogs
4.4. Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gil-Ortuño, C.; Sebastián-Marcos, P.; Sabater-Molina, M.; Nicolas-Rocamora, E.; Gimeno-Blanes, J.R.; Fernández Del Palacio, M.J. Genetics of feline hypertrophic cardiomyopathy. Clin. Genet. 2020, 98, 203–214. [Google Scholar] [CrossRef] [PubMed]
- DeHoff, M.E.; Clark, K.L.; Meganathan, K. Learning outcomes and student-perceived value of clay modeling and cat dissection in undergraduate human anatomy and physiology. Adv. Physiol. Educ. 2011, 35, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Cornillie, P.; Simoens, P. Prenatal development of the caudal vena cava in mammals: Review of the different theories with special reference to the dog. Anat. Histol. Embryol. 2005, 34, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Diana, A.; Cipone, M.; Drigo, M.; Caldin, M. Multidetector row computed tomography and ultrasound characteristics of caudal vena cava duplication in dogs. Vet. Radiol. Ultrasound. 2014, 55, 521–530. [Google Scholar] [CrossRef]
- Popesko, P. Anatomy of Domestic Animals; Príroda: Bratislava, Czechoslovakia, 1992; pp. 414, 449–452. (In Slovak) [Google Scholar]
- König, H.E.; Liebich, H.G. Part 2. In Veterinary Anatomy of Domestic Mammals; Hajko and Hajková: Bratislava, Slovakia, 2002; p. 192. (In Slovak) [Google Scholar]
- Reighard, J.; Jennings, H.S. Anatomy of the Cat/by Jacob Reighard and H.S. Jennings with One Hundred and Seventy-Three Original Figures Drawn by Louise Burridge Jennings; H. Holt and Company: New York, NY, USA, 1901; pp. 324–327. [Google Scholar]
- König, H.E.; Perez, W. Anatomie der Katze und ihr Verhalten aus der Sicht des Anatomen, eine Textsammlung; Cuvillier Verlag: Göttingen, Germany, 2022; pp. 149–150. [Google Scholar]
- Ryu, C.; Choi, S.; Choi, H.; Lee, Y.; Lee, K. CT variants of the caudal vena cava in 121 small breed dogs. Vet. Radiol. Ultrasound. 2019, 60, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Natsis, K.; Apostolidis, S.; Noussios, G.; Papathanasiou, E.; Kyriazidou, A.; Vyzas, V. Duplication of the inferior vena cava: Anatomy, embryology and classification proposal. Anat. Sci. Int. 2010, 85, 56–60. [Google Scholar] [CrossRef]
- Ito, T.; Ikeda, Y. A case of double inferior vena cava with renal, ovarian and iliac vein variation. Anat. Sci. Int. 2018, 93, 139–143. [Google Scholar] [CrossRef]
- Venieratos, D.; Panagouli, E.; Lolis, E. Variations of the iliac and pelvic venous systems with special attention to the drainage patterns of the ascending lumbar and iliolumbar veins. Ann. Anat. 2012, 194, 396–403. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 877–879. [Google Scholar]
- Shammas, N.W.; Rachwan, R.J.; Daher, G.; Bou Dargham, B. Double Inferior Vena Cava and its Implications during Endovascular and Surgical Interventions: A Word of Caution. J. Invasive Cardiol. 2017, 29, 51–53. [Google Scholar]
- Mayo, J.; Gray, R.; St Louis, E.; Grosman, H.; McLoughlin, M.; Wise, D. Anomalies of the inferior vena cava. Am. J. Roentgenol. 1983, 140, 339–345. [Google Scholar] [CrossRef]
- Gayer, G.; Luboshitz, J.; Hertz, M.; Zissin, R.; Thaler, M.; Lubetsky, A.; Bass, A.; Korat, A.; Apter, S. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. Am. J. Roentgenol. 2003, 180, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Gayer, G.; Zissin, R.; Strauss, S.; Hertz, M. IVC anomalies and right renal aplasia detected on CT: A possible link? Abdom. Imaging 2003, 28, 395–399. [Google Scholar] [CrossRef]
- Huntington, G.S.; McClure, C.F. The development of the veins in the domestic cat (Felis domestica) with especial reference, 1) to the share taken by the supracardinal veins in the development of the postcava and azygos veins and 2) to the interpretation of the variant conditions of the postcava and its tributaries, as found in the adult. Anat. Rec. 1920, 20, 1–30. [Google Scholar]
- Butler, E.G. The relative role played by the embryonic veins in the development of the mammalian vena cava posterior. Am. J. Anat. 1927, 39, 267–353. [Google Scholar] [CrossRef]
- Bass, J.E.; Redwine, M.D.; Kramer, L.A.; Huynh, P.T.; Harris, J.H., Jr. Spectrum of Congenital Anomalies of the Inferior Vena Cava: Cross-sectional Imaging Findings. Radiographics 2000, 20, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Casteleyn, C.; Cornillie, P.; Van Cruchten, S.; Van Ginneken, C. Left Retrocaval Ureter around the Ipsilateral Limb of a Double Caudal Vena Cava in a Cat. J. Comp. Pathol. 2015, 152, 313–316. [Google Scholar] [CrossRef]
- Hikspoors, J.P.; Soffers, J.H.; Mekonen, H.K.; Cornillie, P.; Köhler, S.E.; Lamers, W.H. Development of the human infrahepatic inferior caval and azygos venous systems. J. Anat. 2015, 226, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Pey, P.; Marcon, O.; Drigo, M.; Specchi, S.; Bertolini, G. Multidetector-row computed tomographic characteristics of presumed preureteral vena cava in cats. Vet. Radiol. Ultrasound. 2015, 56, 359–366. [Google Scholar] [CrossRef]
- Hikspoors, J.P.J.M.; Mekonen, H.K.; Mommen, G.M.C.; Cornillie, P.; Köhler, S.E.; Lamers, W.H. Infrahepatic inferior caval and azygos vein formation in mammals with different degrees of mesonephric development. J. Anat. 2016, 228, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Chisco, D.F.; Buriticá, E.F.; Ospina-Argüelles, D.A.; Echeverry Bonilla, D.F.; Vélez, J.F. Hernia diafragmática pleuroperitoneal congénita en un gato: Reporte de caso. Rev. Colomb. De Cienc. Anim. 2016, 9, 45–51. [Google Scholar]
- Abidu-Figueiredo, M.; Stocco, A.V.; Santos-Sousa, C.A.; Junior, P.S.; Pires, L.A.S.; Babinski, M.A. Right circumcaval ureter and double right renal vein in the Brazilian shorthair cat (Felis catus): Two case reports. Folia Morphol. 2019, 78, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G. Anomalies of the portal venous system in dogs and cats as seen on multidetector-row computed tomography: An overview and systematization proposal. Vet. Sci. 2019, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Stocco, A.V.; dos Santos-Sousa, C.A.; Souza-Junior, P.; Toledo, K.; Abidu-Figueiredo, M. Duplicity of caudal vena cava and renal veins in a Brazilian shorthaired cat-a case report. Vet. Arh. 2019, 89, 257–265. [Google Scholar] [CrossRef]
- International Federation of Associations of Anatomists. Available online: https://fipat.library.dal.ca/te2/ (accessed on 10 January 2023).
- World Association of Veterinary Anatomists (WAVA). Available online: https://www.wava-amav.org/wava-documents.html (accessed on 10 January 2023).
- Bélanger, R.; Shmon, C.L.; Gilbert, P.J.; Linn, K.A. Prevalence of circumcaval ureters and double caudal vena cava in cats. Am. J. Vet. Res. 2014, 75, 91–95. [Google Scholar] [CrossRef]
- Nakamura, T.; Norimura, M.; Sumi, K.; Ichii, O.; Elewa, Y.H.A.; Kon, Y.; Tatsumi, O.; Hattori, H.; Yoshiyasu, T.; Nagasaki, K.I. Slc: Hartley guinea pigs frequently possess duplication of the caudal vena cava. Exp. Anim. 2019, 68, 465–470. [Google Scholar] [CrossRef]
- Korim, F.; Kuricová, M.; Lipták, T.; Vilhanová, Z.; Eberlová, L. Anomaly of the sixth cervical vertebra accompanied by atypical course of the vertebral artery in a dog. Vet. Rec. Case Rep. 2022, 10, e305. [Google Scholar] [CrossRef]
- Cornillie, P.; Van Den Broeck, W.; Simoens, P. Origin of the infrarenal part of the caudal vena cava in the pig. Anat. Histol. Embryol. 2008, 37, 387–393. [Google Scholar] [CrossRef]
- Kochmar, M.Y.; Hetsko, O.I.; Kochmar, O.M.; Holosh, Y.V. Development and formation of the topography of the inferior vena cava and pulmonary veins during the eighth month of prenatal human ontogenesis. Wiadomosci Lek. Wars. Pol. 2022, 75, 2491–2496. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 206–208. [Google Scholar]
- Sadler, T.W. Langmanova Lékařská Embryologie; Grada Publishing a.s.: Prague, Czech Republic, 2011; pp. 2016–2019. [Google Scholar]
- Shin, D.S.; Sandstrom, C.K.; Ingraham, C.R.; Monroe, E.J.; Johnson, G.E. The inferior vena cava: A pictorial review of embryology, anatomy, pathology, and interventions. Abdom. Radiol. 2019, 44, 2511–2527. [Google Scholar] [CrossRef]
- Malaki, M.; Willis, A.P.; Jones, R.G. Congenital anomalies of the inferior vena cava. Clin. Radiol. 2012, 67, 165–171. [Google Scholar] [CrossRef]
- Tobias, K.M.; Rohrbach, B.W. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2400 cases (1980–2002). J. Am. Vet. Med. Assoc. 2003, 223, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.L.; Culligan, D.J.; Watson, H.G. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br. J. Haematol. 2001, 114, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.M. Current perspectives on the optimal age to spay/castrate dogs and cats. Vet. Med. 2015, 6, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pastore, G.E.; Lamb, C.R.; Lipscomb, V. Comparison of the Results of Abdominal Ultrasonography and Exploratory Laparotomy in the Dog and Cat. J. Am. Anim. Hosp. Assoc. 2007, 43, 264–269. [Google Scholar] [CrossRef]
- Vignesh, S.; Bhat, T.A. Unique Medley of Cardinal Veins: Duplicated Superior and Inferior Venae Cavae with Left Renal Agenesis and Hemiazygos Continuation of Left Inferior Vena Cava with Drainage into Left Atrium. Vasc. Endovasc. Surg. 2022, 56, 330–334. [Google Scholar] [CrossRef]
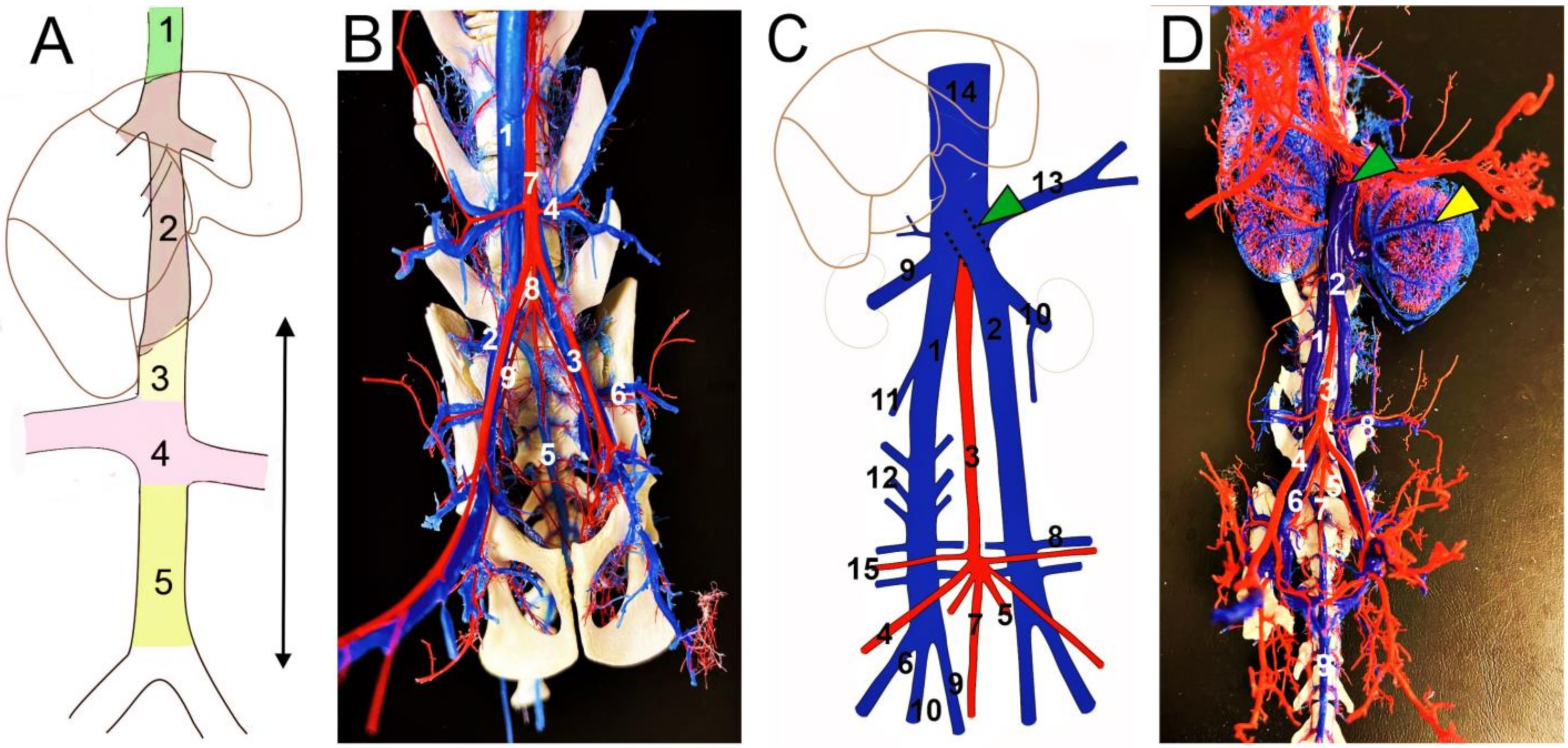
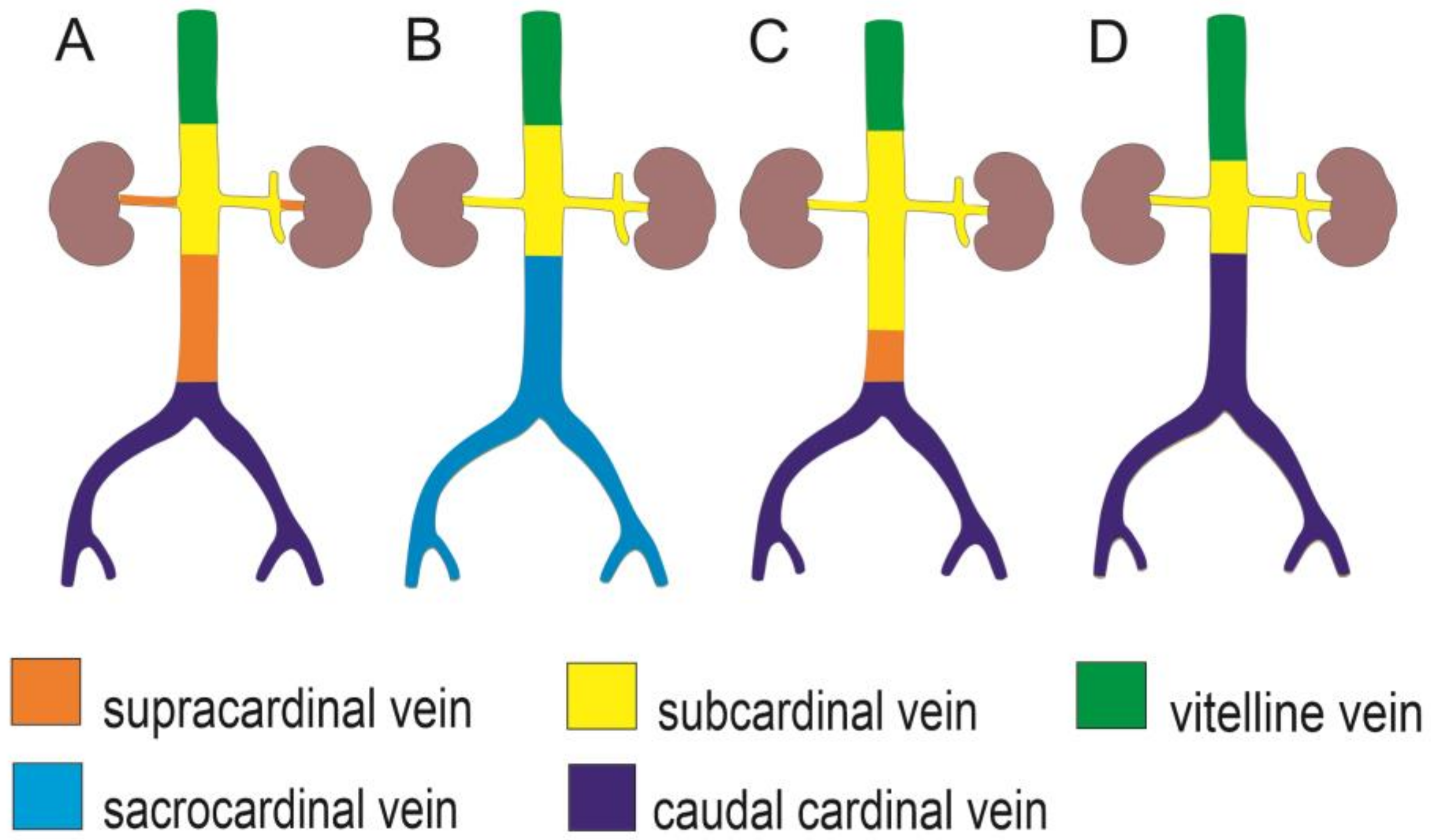
| IVC/CVC Segment | Supracardinal Model (Huntington and McClure, 1920) [18] | Caudal Cardinal Model (Butler, 1927; Hikspoors et al., 2015) [19,22] | Sacrocardinal Model (Sadler, 2011) [37] |
|---|---|---|---|
| Renal | Right supracardinal vein, supracardinal-subcardinal anastomosis | Subcardinal veins | Subcardinal veins |
| Infrarenal/Pre-renal | Right supracardinal vein | Right caudal cardinal vein | Right sacrocardinal vein |
| Confluence of the common iliac veins | Posterior cardinal veins | ||
| Duplicated CVC precursor | Persistent left supracardinal vein (Bertolini et al., 2014) [4] | Persistent intersubcardinal anastomosis (Sadler, 2011) [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korim, F.; Kuricová, M.; Eberlová, L. Anatomical Characteristics of Duplicated Caudal Vena Cava in Cats—A Case Report. Animals 2023, 13, 1585. https://doi.org/10.3390/ani13101585
Korim F, Kuricová M, Eberlová L. Anatomical Characteristics of Duplicated Caudal Vena Cava in Cats—A Case Report. Animals. 2023; 13(10):1585. https://doi.org/10.3390/ani13101585
Chicago/Turabian StyleKorim, Filip, Mária Kuricová, and Lada Eberlová. 2023. "Anatomical Characteristics of Duplicated Caudal Vena Cava in Cats—A Case Report" Animals 13, no. 10: 1585. https://doi.org/10.3390/ani13101585
APA StyleKorim, F., Kuricová, M., & Eberlová, L. (2023). Anatomical Characteristics of Duplicated Caudal Vena Cava in Cats—A Case Report. Animals, 13(10), 1585. https://doi.org/10.3390/ani13101585






