Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Experimental Temperature Settings
2.2. Collection of Samples for Developmental Analysis and Multi-Method Analysis
2.3. DEGs Study
2.4. ATR-FTIR Study
2.5. CHCs Study
3. Results
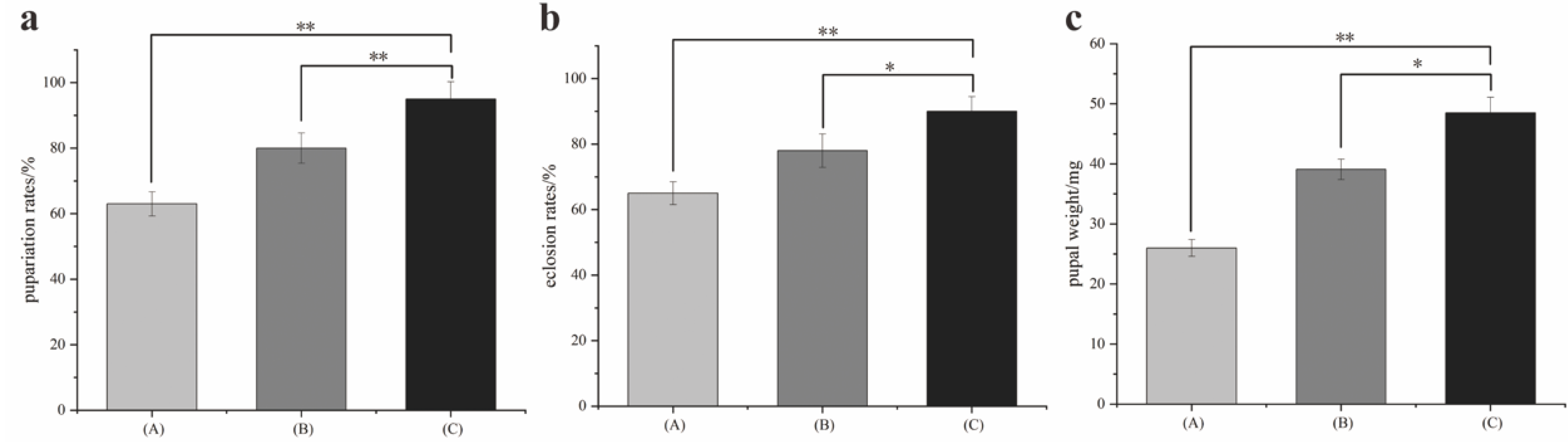
3.1. Development Analysis
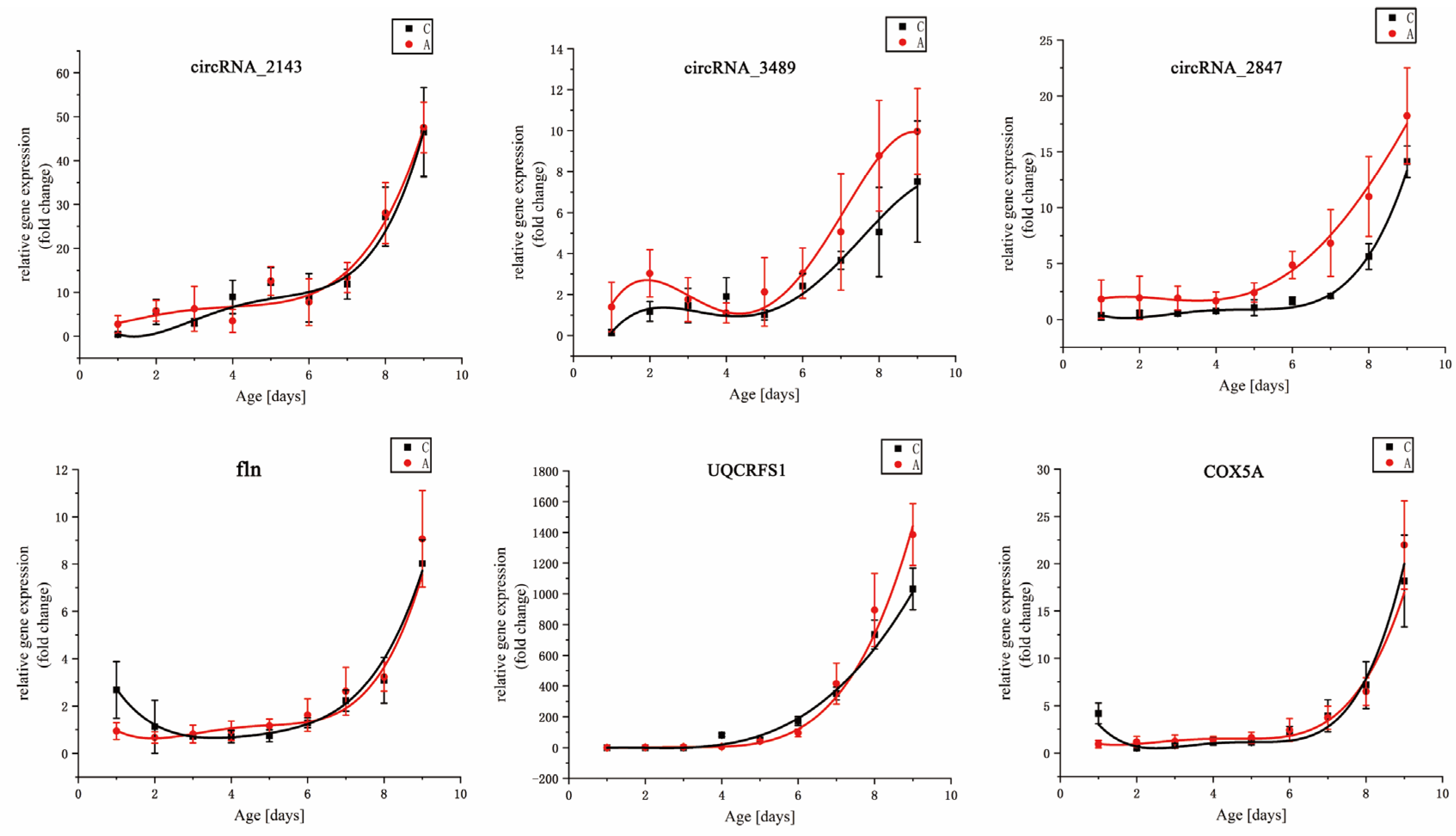
3.2. DEGs Analysis
3.3. ATR-FTIR Analysis
3.4. CHC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, K.; Herrmann, E.; Amendt, J.; Verhoff, M.A.; Zehner, R.E. Age-dependent gene expression of Calliphora vicina pupae (Diptera: Calliphoridae) at constant and fluctuating temperatures. Int. J. Leg. Med. 2021, 135, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Hall, M.J. Estimating the age of Calliphora vicina eggs (Diptera: Calliphoridae): Determination of embryonic morphological landmarks and preservation of egg samples. Int. J. Leg. Med. 2016, 130, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Wang, Y.; Gong, Q.; Liu, Z.-J.; Wang, M.; Xu, W.; Wang, Y.-H.; Wang, J.-F. Research Progress on Developmental Biology of Sarcosaprophagous Insect s. Fa Yi Xue Za Zhi 2021, 37, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Ma Yukun, H.C.; Min, J. Effects of temperature on the growth and development of four common necrophagous flies and their significance in forensic medicine. Chin. J. Forensic Med. 1998, 81–84, 81–84. [Google Scholar]
- Zhang, X.Y.; Li, Y.; Shang, Y.J.; Ren, L.P.; Chen, W.; Wang, S.W.; Guo, Y.D. Development of Sarcophaga dux (diptera: Sarcophagidae) at constant temperatures and differential gene expression for age estimation of the pupae. J. Therm. Biol. 2020, 93, 102735. [Google Scholar] [CrossRef] [PubMed]
- Ze-min, L. Pupal morphogenesis of Lucilia cuprina in different constant temperatures and its significance in Forensic Medicine. J. Forensic Med. 2008, 23, 324–325+326. [Google Scholar]
- Feng, D.-X.; Liu, G.-C. Pupal age estimation of forensically important Megaselia scalaris (Loe w) (Diptera: Phoridae). Forensic Sci. Int. 2014, 236, 133–137. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhang, Y.; Tao, L.; Wang, J. Development of Musca domestica at constant temperatures and the first case report of its application for estimating the minimum postmortem interval. Forensic Sci. Int. 2018, 285, 172–180. [Google Scholar] [CrossRef]
- Hu, G.; Wang, M.; Wang, Y.; Tang, H.; Chen, R.; Zhang, Y.; Zhao, Y.; Jin, J.; Wang, Y.; Wu, M.; et al. Development of Necrobia rufipes (De Geer, 1775) (Coleoptera: Cleridae) under constant temperatures and its implication in forensic entomology. Forensic Sci. Int. 2020, 311, 110275. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Liu, C.; Wang, J.; Hu, G.; Wang, M.; Yang, L.; Chu, J. Development of Nasonia vitripennis (Hymenoptera: Pteromalidae) at Constant Temperatures in China. J. Med. Entomol. 2019, 56, 368–377. [Google Scholar] [CrossRef]
- Chen, W.; Yang, L.; Ren, L.; Shang, Y.; Wang, S.; Guo, Y. Aldrichina grahami Impact of Constant Versus Fluctuating Temperatures on the Development and Life History Parameters of (Diptera: Calliphoridae). Insects 2019, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Niederegger, S.; Pastuschek, J.; Mall, G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci. Int. 2010, 199, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.-A.; Anderson, G.S. Effect of Fluctuating Temperatures on the Development of a Forensically Important Blow Fly, Protophormia terraenovae (Diptera: Calliphoridae). Environ. Entomol. 2013, 42, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Shiao, S.F.; Okuyama, T. Development of insects under fluctuating temperature: A review and case study. J. Appl. Entomol. 2015, 139, 592–599. [Google Scholar] [CrossRef]
- Kjærsgaard, A.; Pertoldi, C.; Loeschcke, V.; Blanckenhorn, W.U. The effect of fluctuating temperatures during development on fitness-r elated traits of Scatophaga stercoraria (Diptera: Scathophagidae). Environ. Entomol. 2013, 42, 1069–1078. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Ragland, G.J.; Diamond, S.E. Evolution in a constant environment: Thermal fluctuations and thermal sensitivity of laboratory and field populations of Manduca sexta. Evolution 2009, 63, 537–541. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.F.; Zhang, Y.N.; Tao, L.-Y.; Wang, M. Forensically Important Boettcherisca peregrina (Diptera: Sarcophagidae) in China: Development Pattern and Significance for Estimating Postmortem Interval. J. Med. Entomol. 2017, 54, 1491–1497. [Google Scholar] [CrossRef]
- Robertson, C.W. The metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J. Morphol. 1936, 59, 351–399. [Google Scholar] [CrossRef]
- Bainbridge, S.P.; Bownes, M. Staging the metamorphosis of Drosophila melanogaster. J. Embryol. Exp. Morphol. 1981, 66, 57–80. [Google Scholar] [CrossRef]
- Davies, K.; Harvey, M.L. Internal Morphological Analysis for Age Estimation of Blow Fly Pupae (Diptera: Calliphoridae) in Postmortem Interval Estimation. J. Forensic Sci. 2013, 58, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sato, C. Effects of x-irradiation on the development of the respiratory system and survival time in pupae of Sarcophaga peregrina. Tohoku J. Exp. Med. 1966, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nur Aliah, N.A.; Heo, C.C.; Noor Shafini, M.; Mohd Hafizi, M. Age estimation of forensically important blowfly, Chrysomya megacephala (Diptera: Calliphoridae) pupae using micro-computed tomography imaging. Trop. Biomed. 2019, 36, 640–653. [Google Scholar] [PubMed]
- Brown, K.; Harvey, M. Optical coherence tomography: Age estimation of Calliphora vicina pupae in vivo? Forensic Sci. Int. 2014, 242, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.J.; Ren, L.P.; Yang, L.; Wang, S.W.; Chen, W.; Dong, J.N.; Ma, H.M.; Qi, X.; Guo, Y.D. Differential Gene Expression for Age Estimation of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) Intrapuparial. J. Med. Entomol. 2020, 57, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.M.; Jennings, K.C.; Foran, D.R. Aging Blow Fly Eggs Using Gene Expression: A Feasibility Study. J. Forensic Sci. 2007, 52, 1350–1354. [Google Scholar] [CrossRef]
- de Lima, L.A.S.; Baia, T.C.; Gama, R.A.; da Silva Gasparotto, L.H.; Lima, K.M.G. Near Infrared Spectroscopy as an Emerging Tool for Forensic Entomotoxicology. NIR News 2014, 25, 5–7. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Q.; Liu, R.; Wei, X.; Li, Z.; Fan, S.; Wang, Z. Evaluating the effects of causes of death on postmortem interval estimation by ATR-FTIR spectroscopy. Int. J. Leg. Med. 2019, 134, 565–574. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Gajjar, K.; Theophilou, G.; Martin, F.L.; Martin-Hirsch, P.P.L. Vibrational spectroscopy of biofluids for disease screening or diagnosis: Translation from the laboratory to a clinical setting. J. Biophotonics 2014, 7, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Barauna, V.G.; Singh, M.N.; Barbosa, L.L.; Marcarini, W.D.; Vassallo, P.F.; Mill, J.G.; Ribeiro-Rodrigues, R.; Campos, L.C.G.; Warnke, P.H.-H.; Martin, F.L. Ultrarapid On-Site Detection of SARS-CoV-2 Infection Using Simple ATR-FTIR Spectroscopy and an Analysis Algorithm: High Sensitivity and Specificity. Anal. Chem. 2021, 93, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015, 37, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Botella-Cruz, M.; Velasco, J.; Millán, A.; Hetz, S.K.; Pallarés, S. Cuticle Hydrocarbons Show Plastic Variation under Desiccation in Saline Aquatic Beetles. Insects 2021, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-H.; Yè, G.; Hu, C.; Xu, X.H.; Li, K. Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: Potential for determining larval age. Med. Vet. Entomol. 2006, 20, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Drijfhout, F.P.; Tomberlin, J.K.; Bala, M. Cuticular hydrocarbons as a tool for determining the age of Chrysomya rufifacies (Diptera: Calliphoridae) larvae. J. Forensic Sci. 2020, 66, 236–244. [Google Scholar] [CrossRef]
- Alotaibi, F.; Alkuriji, M.A.; AlReshaidan, S.; Alajmi, R.; Metwally, D.M.; Almutairi, B.F.; Alorf, M.S.; Haddadi, R.; Ahmed, A. Body Size and Cuticular Hydrocarbons as Larval Age Indicators in the Forensic Blow Fly, Chrysomya albiceps (Diptera: Calliphoridae). J. Med. Entomol. 2020, 58, 1048–1055. [Google Scholar] [CrossRef]
- Zhu, G.-H.; Xu, X.H.; Yu, X.J.; Zhang, Y.T.; Wang, J.-f. Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci. Int. 2007, 169, 1–5. [Google Scholar] [CrossRef]
- Rodriguez, W.C.; Bass, W.M. Insect Activity and Its Relationship to Decay Rates of Human Cadavers in East Tennessee. J. Forensic Sci. 1983, 28, 423–432. [Google Scholar] [CrossRef]
- Byrd, J.; Butler, J.F. Effects of temperature on Sarcophaga haemorrhoidalis (Diptera: Sarcophagidae) development. J. Med. Entomol. 1998, 35, 694–698. [Google Scholar] [CrossRef]
- Szpila, K.; Mądra, A.; Jarmusz, M.; Matuszewski, S. Flesh flies (Diptera: Sarcophagidae) colonising large carcasses in Central Europe. Parasitol. Res. 2015, 114, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Frère, B.; Suchaud, F.; Bernier, G.; Cottin, F.; Vincent, B.; Dourel, L.; Lelong, A.L.M.; Arpino, P. GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal. Bioanal. Chem. 2013, 406, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Bunchu, N.; Chaiwong, T.; Moophayak, K.; Sukontason, K.L. Forensically important flesh fly species in Thailand: Morphology and developmental rate. Parasitol. Res. 2010, 106, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Samerjai, C.; Sukontason, K.L.; Sukontason, K.; Limsopatham, K.; Chareonviriyaphap, T.; Somboon, P.; Tomberlin, J.K.; Sanit, S. Ultrastructure of male terminalia of Boettcherisca peregrina and Boettcherisca nathani (Diptera: Sarcophagidae), flesh fly species of forensic importance. Acta Trop. 2021, 224, 106148. [Google Scholar] [CrossRef]
- Wells, J.D.; Stevens, J.R. Application of DNA-based methods in forensic entomology. Annu. Rev. Entomol. 2008, 53, 103–120. [Google Scholar] [CrossRef]
- Guo, Y.D.; Cai, J.F.; Li, X.; Xiong, F.; Su, R.-n.; Chen, F.L.; Liu, Q.L.; Wang, X.H.; Chang, Y.F.; Zhong, M.; et al. Identification of the forensically important sarcophagid flies Boerttcherisca peregrina, Parasarcophaga albiceps and Parasarcophaga dux (Diptera: Sarcophagidae) based on COII gene in China. Trop. Biomed. 2010, 27, 451–460. [Google Scholar]
- Yè, G.; Li, K.; Zhu, J.; Zhu, G.; Hu, C. Cuticular Hydrocarbon Composition in Pupal Exuviae for Taxonomic Differentiation of Six Necrophagous Flies. J. Med. Entomol. 2007, 44, 450–456. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, Y.; Ren, L.; Qu, H.; Zhu, G.; Guo, Y. A Study of Cuticular Hydrocarbons of All Life Stages in Sarcophaga peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 2021, 59, 108–119. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, Y.; Ngando, F.J.; Qu, H.; Shang, Y.; Ren, L.; Guo, Y. Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models. Insects 2022, 13, 808. [Google Scholar] [CrossRef]
- Ren, L.P.; Shang, Y.J.; Yang, L.; Wang, S.W.; Wang, X.; Chen, S.; Bao, Z.G.; An, D.; Meng, F.M.; Cai, J.F.; et al. Chromosome-level de novo genome assembly of Sarcophaga peregrina provides insights into the evolutionary adaptation of flesh flies. Mol. Ecol. Resour. 2021, 21, 251–262. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, H.Y.; Shin, S.E.; Cha, H.K.; Seo, J.-H.; Kim, S.-K.; Park, S.H.; Son, G.H. Comprehensive transcriptome analysis of Sarcophaga peregrina, a forensically important fly species. Sci. Data 2018, 5, 180220. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L.; Brown, W.A.; Hewadikaram, K.A.; Omori, A. Effect of heroin in decomposing tissues on the development rate of Boettcherisca peregrina (Diptera, Sarcophagidae) and implications of this effect on estimation of postmortem intervals using arthropod development patterns. J. Forensic Sci. 1991, 36, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L.; Omori, A.; Goodbrod, J.R. Effect of cocaine in tissues on the development rate of Boettcherisca peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 1989, 26, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Guoxing, W.; Jiaying, Z.; Kai, L.; Xi, G.; Cui, H.; Jiaan, C.; Gong-yin, Y. A proteomic analysis of larval midguts of Boettcherisca peregrina in response to cadmium exposure. Bull. Insectology 2013, 66, 225–229. [Google Scholar]
- Chen, L.S. Necrophagous Flies in China; Guizhou Science and Technology Press: Guiyang, China, 2013. [Google Scholar]
- Ren, L.; Shang, Y.; Zhang, X.; Chen, S.; Zheng, Y.; Zou, Y.; Qu, Y.; Cai, J.; Zhang, C.; Guo, Y. Temporal Expression Profiles Reveal Potential Targets during Postembryonic Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae). Insects 2022, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Andersen, C.M.; Bro, R. Variable selection in regression—A tutorial. J. Chemom. 2010, 24, 728–737. [Google Scholar] [CrossRef]
- Ren, L.P.; Zhang, X.Y.; Li, Y.; Shang, Y.J.; Chen, S.; Wang, S.W.; Qu, Y.H.; Cai, J.F.; Guo, Y.D. Comparative analysis of mitochondrial genomes among the subfamily Sarcophaginae (Diptera: Sarcophagidae) and phylogenetic implications. Int. J. Biol. Macromol. 2020, 161, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kováts, E.; Weisz, P. Über den Retentionsindex und seine Verwendung zur Aufstellung einer Polaritätsskala für Lösungsmittel. Ber. Bunsenges. Phys. Chem. 1965, 69, 812–820. [Google Scholar] [CrossRef]
- Espelie, K.E.; Bernays, E.A. Diet-related differences in the cuticular lipids of Manduca sexta larvae. J. Chem. Ecol. 1989, 15, 2003–2017. [Google Scholar] [CrossRef]
- Bernier, U.R.; Carlson, D.A.; Geden, C.J. Gas chromatography/mass spectrometry analysis of the cuticular hydrocarbons from parasitic wasps of the genus Muscidifurax. J. Am. Soc. Mass. Spectrom. 1998, 9, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Bernier, U.R.; Sutton, B.D. Elution Patterns from Capillary GC for Methyl-Branched Alkanes. J. Chem. Ecol. 2004, 24, 1845–1865. [Google Scholar] [CrossRef]
- Pang, T.; Zhu, S.; Lu, X.; Xu, G. Identification of unknown compounds on the basis of retention index data in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2007, 30, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Krkosová, Z.; Kubinec, R.; Soják, L.; Amann, A. Temperature-programmed gas chromatography linear retention indices of all C4-C30 monomethylalkanes on methylsilicone OV-1 stationary phase. Contribution towards a better understanding of volatile organic compounds in exhaled breath. J. Chromatogr. A 2008, 1179, 59–68. [Google Scholar] [CrossRef]
- Park, S.J.; Pandey, G.; Castro-Vargas, C.; Oakeshott, J.G.; Taylor, P.W.; Mendez, V. Cuticular Chemistry of the Queensland Fruit Fly Bactrocera tryoni (Froggatt). Molecules 2020, 25, 4185. [Google Scholar] [CrossRef]
- de Paula, M.C.; Michelutti, K.B.; Eulalio, A.D.M.d.M.; Mendonça, A.; Cardoso, C.A.L.; Andrade, L.H.C.; Lima, S.M.; Antonialli-Júnior, W.F. New approach to application of mid-infrared photoacoustic spectroscopy in forensic analysis: Study with the necrophagous blow fly Chrysomya megacephala (Diptera: Calliphoridae). J. Photochem. Photobiol. B Biol. 2020, 209, 111934. [Google Scholar] [CrossRef]
- Yu, K.; Wang, G.; Cai, W.; Wu, D.; Wei, X.; Zhang, K.; Liu, R.; Sun, Q.; Wang, Z. Identification of antemortem, perimortem and postmortem fractures by FTIR spectroscopy based on a rabbit tibial fracture model. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 239, 118535. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Romero-González, M.E.; Banwart, S.A. Analysis of bacteria on steel surfaces using reflectance micro-Fourier transform infrared spectroscopy. Anal. Chem. 2009, 81, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, S. The effect of temperature change on the development rate of two insects. Insect Knowl. 1994, 31, 237–240. [Google Scholar]
- Shi, P.; Ge, F. A comparison of different thermal performance functions describing temperature-dependent development rates. J. Therm. Biol. 2010, 35, 225–231. [Google Scholar] [CrossRef]
- Boehme, P.; Spahn, P.N.; Amendt, J.; Zehner, R.E. The analysis of temporal gene expression to estimate the age of forensically important blow fly pupae: Results from three blind studies. Int. J. Leg. Med. 2013, 128, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Han, H.; Chen, W.; Wang, S.W.; Meng, F.M.; Cai, J.F.; Guo, Y.D. Evaluation of Reference Genes and Age Estimation of Forensically Useful Aldrichina grahami (Diptera: Calliphoridae) During Intrapuparial Period. J. Med. Entomol. 2021, 58, 47–55. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.-Y.; Xia, S.-X.; Wang, J.-G.; Zhang, Y.; Tao, L.-Y. Estimating the age of Lucilia illustris during the intrapuparial period using two approaches: Morphological changes and differential gene expression. Forensic Sci. Int. 2018, 287, 1–11. [Google Scholar] [CrossRef]
- Lucas, E.R.; Darby, A.C.; Torr, S.J.; Donnelly, M.J. A gene expression panel for estimating age in males and females of the sleeping sickness vector Glossina morsitans. PLoS Negl. Trop. Dis. 2021, 15, e0009797. [Google Scholar] [CrossRef]
- Baqué, M.; Amendt, J.; Verhoff, M.A.; Zehner, R.E. Descriptive analyses of differentially expressed genes during larval development of Calliphora vicina (Diptera: Calliphoridae). Int. J. Leg. Med. 2015, 129, 891–902. [Google Scholar] [CrossRef]
- Boehme, P.; Spahn, P.N.; Amendt, J.; Zehner, R.E. Differential gene expression during metamorphosis: A promising approach for age estimation of forensically important Calliphora vicina pupae (Diptera: Calliphoridae). Int. J. Leg. Med. 2012, 127, 243–249. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; Isabelle, M.; Bazant-Hegemark, F.; Hutchings, J.; Orr, L.; Babrah, J.; Baker, R.; Stone, N. Vibrational spectroscopy: A clinical tool for cancer diagnostics. Analyst 2009, 134, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Jales, J.T.; Barbosa, T.M.; Medeiros, J.R.; Lima, L.A.S.; Lima, K.M.G.; Gama, R.A. Infrared spectroscopy and forensic entomology: Can this union work? A literature review. J. Forensic Sci. 2021, 66, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.G.; Trevisan, J.; Scott, A.D.; Carmichael, P.L.; Pollock, H.M.; Martin-Hirsch, P.L.; Martin, F.L. Biospectroscopy to metabolically profile biomolecular structure: A mul tistage approach linking computational analysis with biomarkers. J. Proteome Res. 2011, 10, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, L.A.; Yee, H.T.; Diem, M. Infrared Spectroscopy of Human Cells and Tissue. Part VI: A Comparative Study of Histopathology and Infrared Microspectroscopy of Normal, Cirrhotic, and Cancerous Liver Tissue. Appl. Spectrosc. 2000, 54, 1–8. [Google Scholar] [CrossRef]
- Obinaju, B.E.; Martin, F.L. Novel biospectroscopy sensor technologies towards environmental health monitoring in urban environments. Environ. Pollut. 2013, 183, 46–53. [Google Scholar] [CrossRef]
- Perez-Mendoza, J.; Dowell, F.E.; Broce, A.B.; Throne, J.E.; Wirtz, R.A.; Xie, F.; Fabrick, J.A.; Baker, J.E. Chronological age-grading of house flies by using near-infrared spectr oscopy. J. Med. Entomol. 2002, 39, 499–508. [Google Scholar] [CrossRef]
- Pickering, C.L.; Hands, J.R.; Fullwood, L.M.; Smith, J.A.; Baker, M.J. Rapid discrimination of maggots utilising ATR-FTIR spectroscopy. Forensic Sci. Int. 2015, 249, 189–196. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Blomquist, G.J. Insect Hydrocarbons: Biochemistry and Chemical Ecology. In Extracellular Composite Matrices in Arthropods; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Stepnowski, P. Cuticular lipids of insects as potential biofungicides: Methods of lip id composition analysis. Anal. Bioanal. Chem. 2011, 399, 3177–3191. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical Ecology, Biochemistry, and Molecular Biology of Insect Hydroc arbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Armold, M.T.; Regnier, F.E. A developmental study of the cuticular hydrocarbons of Sarcophaga bullata. J. Insect Physiol. 1975, 21, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.E.; Pechal, J.L.; Benbow, M.E.; Drijfhout, F.P. The potential use of cuticular hydrocarbons and multivariate analysis to age empty puparial cases of Calliphora vicina and Lucilia sericata. Sci. Rep. 2017, 7, 1933. [Google Scholar] [CrossRef] [PubMed]
- Roux, O.; Gers, C.; Legal, L. Ontogenetic study of three Calliphoridae of forensic importance through cuticular hydrocarbon analysis. Med. Vet. Entomol. 2008, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Toolson, E.C.; Kuper-Simbrón, R. Laboratory Evolution of Epicuticular Hydrocarbon Composition and Cutic Ular Permeability in Drosophila Pseudoobscura: Effects on Sexual Dimor Phism and Thermal-Acclimation Ability. Evolution 1989, 43, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ferveur, J.-F. Cuticular hydrocarbons: Their evolution and roles in Drosophila pherom onal communication. Behav. Genet. 2005, 35, 279–295. [Google Scholar] [CrossRef]
- Tregenza, T.; Buckley, S.H.; Pritchard, V.L.; Butlin, R.K. Inter- and Intrapopulation Effects of Sex and Age on Epicuticular Composition of Meadow Grasshopper, Chorthippus Parallelus. J. Chem. Ecol. 2000, 26, 257–278. [Google Scholar] [CrossRef]
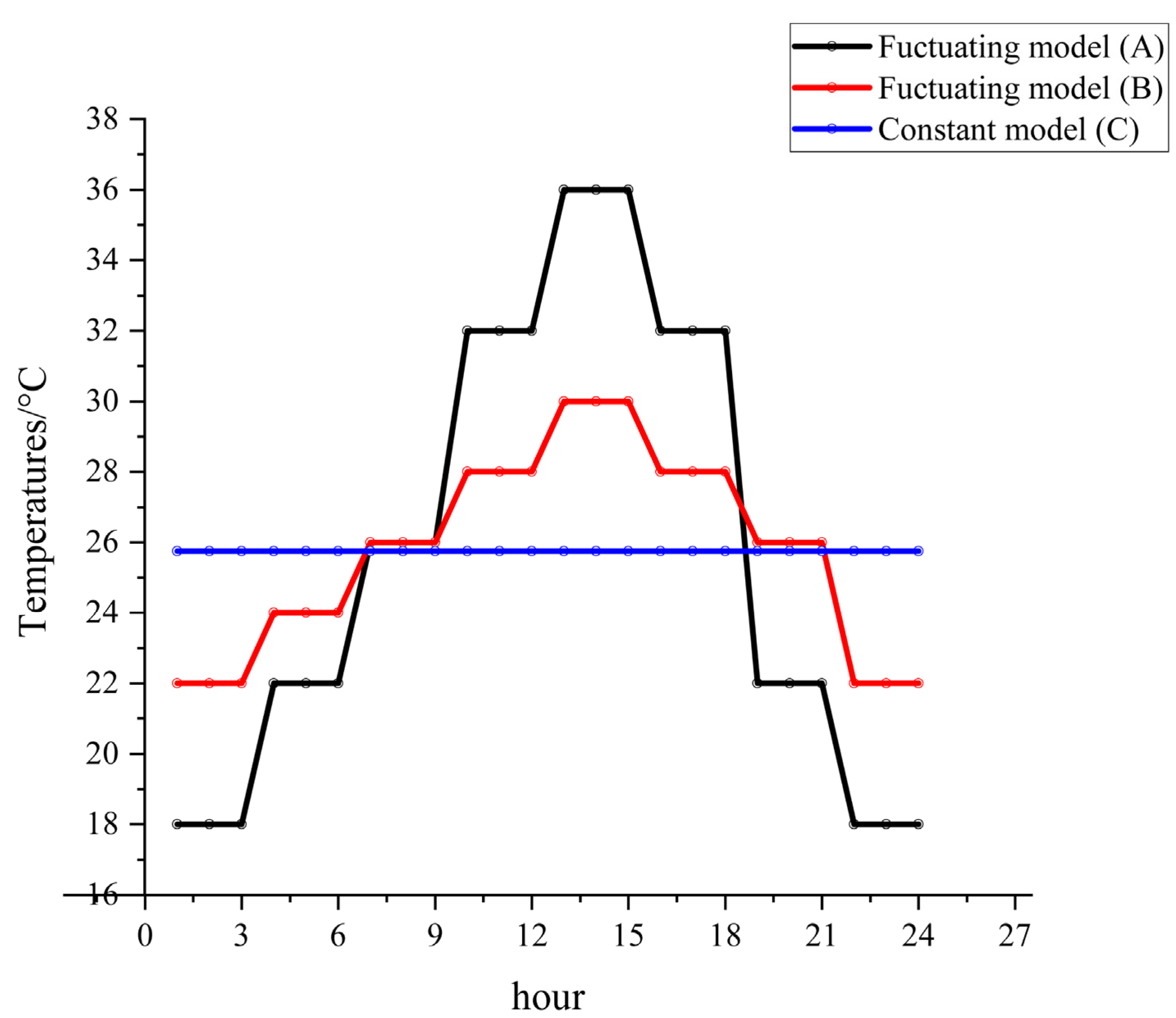
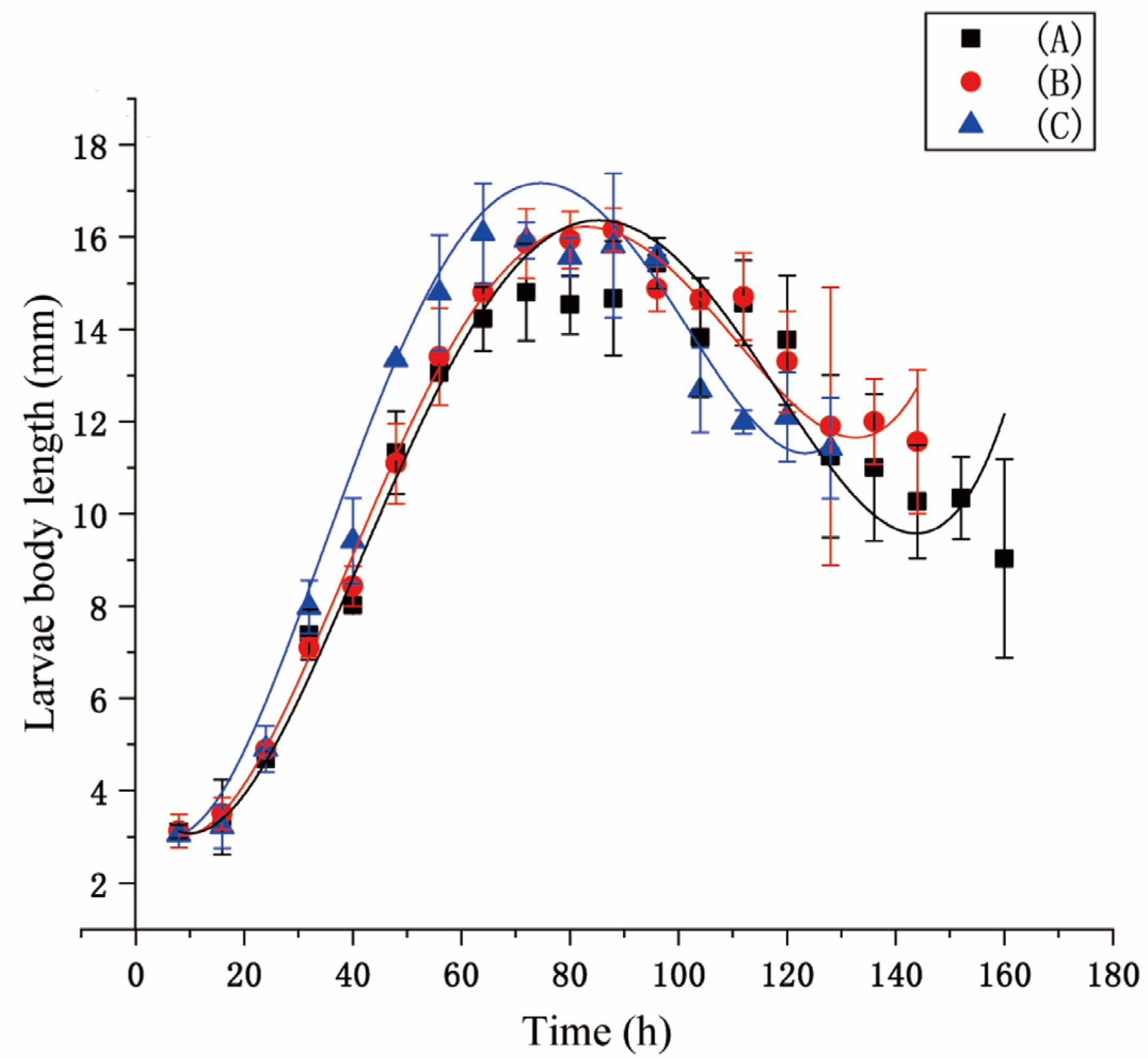

| Temperature (°C) | Equation | df | p | R2 |
|---|---|---|---|---|
| Fluctuating (A) | y = 4.21804 − 0.24142 × x+ 0.01422 × x2 − 1.55605 × 10-4 × x3 + 4.88045 × 10-7 × x4 | 15 | <0.001 | 0.99118 |
| Fluctuating (B) | y = 3.92763 − 0.21602 × x + 0.01444 × x2 − 1.67742 × 10-4 × x3 + 5.61143 × 10-7 × x4 | 13 | <0.001 | 0.99808 |
| Constant(C) | y = 3.70744 − 0.20709 × x + 0.0174 × x2 − 2.25212 × 10-4 × x3 + 8.25105 × 10-7 × x4 | 11 | <0.001 | 0.99991 |
| Developmental Stages | FirstInstar | SecondInstar | ThirdInstar | Wandering | Pupariation | Total Duration |
|---|---|---|---|---|---|---|
| Fluctuating (A) | 23.7 ± 2.51 | 27.7 ± 3.51 | 70.6 ± 5.1 | 37.6 ± 5.85 | 223 ± 11.35 | 382.6 ± 9.07 |
| Fluctuating (B) | 22.6 ± 2.57 | 25.6 ± 2.08 | 67.6 ± 7.2 | 35.6 ± 4.5 | 218.6 ± 5.5 | 370.3 ± 10.7 |
| Constant (C) | 22.3 ± 2.08 | 23.3 ± 2.08 | 65.3 ± 4.1 | 30.3 ± 2.08 | 217.3 ± 6.1 | 358.6 ± 15.04 |
| Gene | Temperature (°C) | Simulation Equation | F | p | R2 |
|---|---|---|---|---|---|
| circRNA_2143 | A | y = 1.3774 + 1.04846 × x+ 0.81578 × x2 − 0.30251 × x3 + 0.02911 × x4 | 4.52766 | <0.001 | 0.93603 |
| C | y = 7.63272 − 12.85128 × x + 6.8054 × x2 − 1.21003 × x3 + 0.07401 × x4 | 5.33739 | <0.001 | 0.92318 | |
| circRNA_3489 | A | y = 0.46214 + 2.31708 × x − 1.10734 × x2 + 0.17956 × x3 − 0.00686 × x4 | 0.37142 | <0.001 | 0.98587 |
| C | y = 2.22275 − 3.19588 × x + 1.62495 × x2 − 0.31026 × x3 + 0.02049 × x4 | 4.67951 | <0.001 | 0.9675 | |
| −circRNA_2847 | A | y = − 4.19518 + 8.98938 × x − 3.86827 × x2 + 0.60917 × x3 − 0.03011 × x4 | 0.41914 | <0.001 | 0.98452 |
| C | y = − 3.59782 + 5.57439 × x − 2.12695 × x2 + 0.31238 × x3 − 0.01444 × x4 | 1.89332 | <0.001 | 0.97677 | |
| fln | A | y = 12.56685 − 29.36167 × x + 23.64982 × x2 − 7.276 × x3 + 0.77481 × x4 | 3.52198 | <0.001 | 0.96925 |
| C | y = − 20.85524 + 38.63413 × x − 21.49614 × x2 + 3.80815 × x3 − 0.05313 × x4 | 8.86323 | <0.001 | 0.91811 | |
| UQCRFS1 | A | y = 2.57914 − 2.57384 × x + 1.15168 × x2 − 0.2 × x3 + 0.01229 × x4 | 1.78359 | <0.001 | 0.94842 |
| C | y = 6.03845 − 4.55755 × x + 1.42931 × x2 − 0.20105 × x3 + 0.0112 × x4 | 1.34105 | <0.001 | 0.98228 | |
| COX5A | A | y = 2.39587 − 2.59247 × x + 1.44986 × x2 − 0.29885 × x3 + 0.02108 × x4 | 2.5781 | <0.001 | 0.93142 |
| C | y = 10.4182 − 11.11295 × x + 4.40356 × x2 − 0.72446 × x3 + 0.04283 × x4 | 4.32924 | <0.001 | 0.89024 |
| Baseline Points (cm−1) | Wavenumber (cm−1) | Infrared Band |
|---|---|---|
| 1760~1730 | 1740 | Lipid (C = O stretching vibration) |
| 1680~1610 | 1640 | Amide I (C = O stretching) |
| 1580~1510 | 1544 | Amide II (N-H bending coupled to C-N stretching) |
| 1480~1420 | 1458 | C–H bending from CH2 and CH3 |
| 1420~1350 | 1405 | C=O vibrations of COO− from free fatty acids, free amino acids and polypeptides |
| 1330–1277 | 1309 | Amide III |
| 1245–1230 | 1241 | CH3–CO Symmetric stretching |
| 1161–1095 | 1121 | C–O, C–OH and P–O vibration |
| 1083–1078 | 1080 | PO2-symmetric stretching |
| 1100~1000 | 1041 | C–O(H) stretching vibration |
| 945~906 | 927 | C–O or C–OH vibrations from carbohydrates |
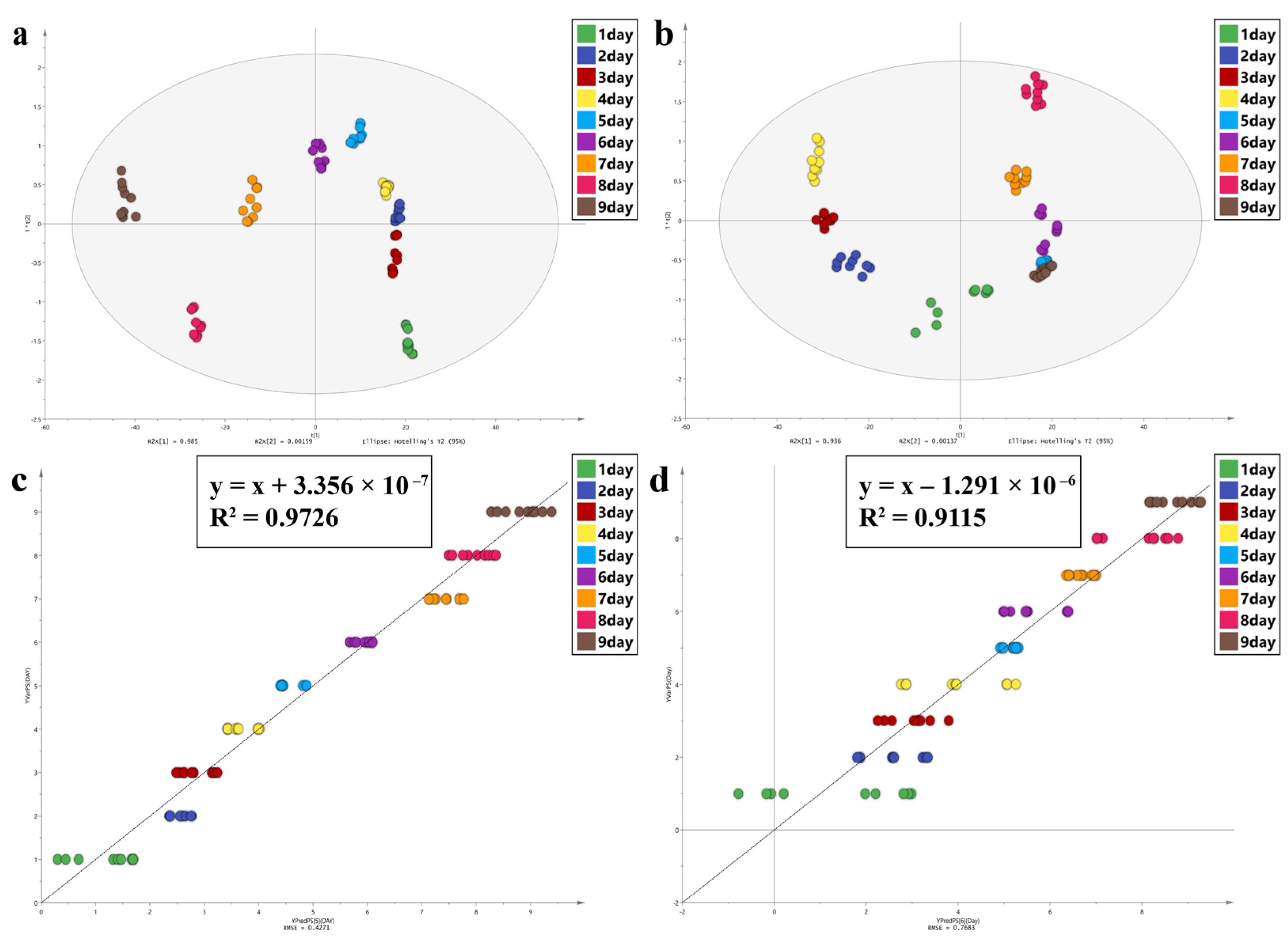
| Model Evaluation Parameters and Regression Equations of ATR-FTIR Study | ||||||
| PLS | OPLS-DA | |||||
| Equation | R2 | RMSE(DAY) | R2X (cum) | R2Y (cum) | Q2 (cum) | |
| A | y = x + 3.356 × 10−7 | 0.9726 | 0.4271 | 1 | 0.801 | 0.793 |
| C | y = 1x − 1.2916 × 10−6 | 0.9115 | 0.7683 | 1 | 0.744 | 0.707 |
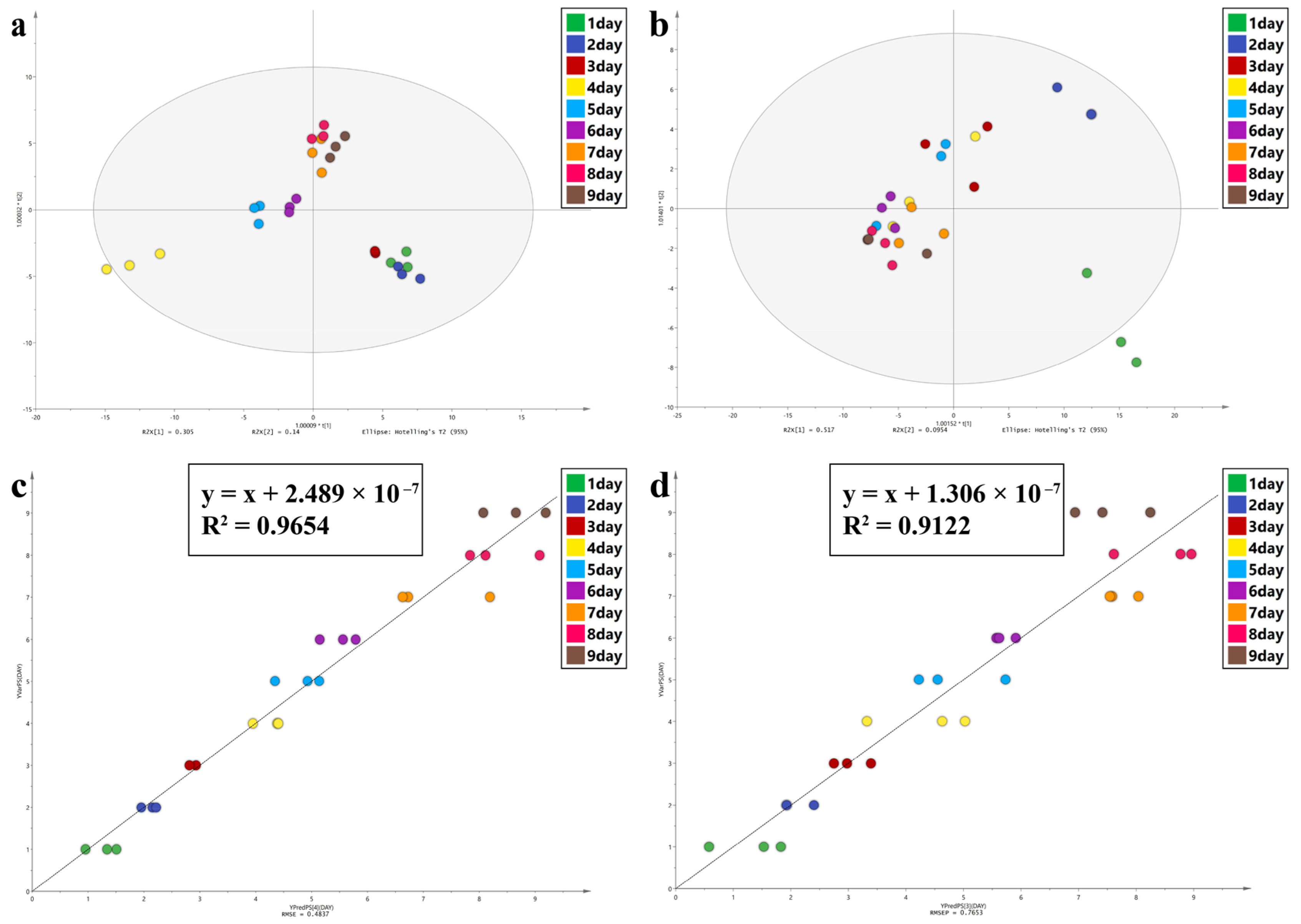
| Model evaluation parameters and regression equations of CHCs study | ||||||
| PLS | OPLS-DA | |||||
| Equation | R2 | RMSE(DAY) | R2X (cum) | R2Y (cum) | Q2 (cum) | |
| A | y = x + 2.489e × 10−7 | 0.9654 | 0.4837 | 0.973 | 0.927 | 0.551 |
| C | y = x + 1.306 × 10−7 | 0.9122 | 0.7653 | 0.949 | 0.824 | 0.44 |
| Fluctuating Temperature (Group A) | |||||||||
| Compounds | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | 6 Day | 7 Day | 8 Day | 9 Day |
| n-alkanes(21) * | 40.92% | 28.11% | 31.42% | 39.46% | 39.66% | 41.51% | 43.87% | 45.69% | 51.19% |
| branched alkanes(17) * | 55.61% | 67.85% | 63.46% | 55.47% | 56.88% | 54.83% | 50.41% | 46.16% | 39.54% |
| alkenes(3) * | 3.47% | 4.04% | 5.12% | 5.07% | 3.46% | 3.66% | 5.72% | 8.14% | 9.27% |
| Constant temperature (group C) | |||||||||
| Compounds | 1 day | 2 day | 3 day | 4 day | 5 day | 6 day | 7 day | 8 day | 9 day |
| n-alkanes(21) * | 41.54% | 31.99% | 31.05% | 38.17% | 27.79% | 37.42% | 38.40% | 39.28% | 47.20% |
| branched alkanes(17) * | 52.93% | 63.94% | 64.87% | 58.40% | 68.29% | 58.51% | 53.48% | 49.31% | 40.68% |
| alkenes(3) * | 5.52% | 4.07% | 4.08% | 3.43% | 3.92% | 4.07% | 8.12% | 11.41% | 12.11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.; Yang, F.; Ngando, F.J.; Zhang, X.; Feng, Y.; Ren, L.; Guo, Y. Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures. Animals 2023, 13, 1607. https://doi.org/10.3390/ani13101607
Shang Y, Yang F, Ngando FJ, Zhang X, Feng Y, Ren L, Guo Y. Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures. Animals. 2023; 13(10):1607. https://doi.org/10.3390/ani13101607
Chicago/Turabian StyleShang, Yanjie, Fengqin Yang, Fernand Jocelin Ngando, Xiangyan Zhang, Yakai Feng, Lipin Ren, and Yadong Guo. 2023. "Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures" Animals 13, no. 10: 1607. https://doi.org/10.3390/ani13101607
APA StyleShang, Y., Yang, F., Ngando, F. J., Zhang, X., Feng, Y., Ren, L., & Guo, Y. (2023). Development of Forensically Important Sarcophaga peregrina (Diptera: Sarcophagidae) and Intra-Puparial Age Estimation Utilizing Multiple Methods at Constant and Fluctuating Temperatures. Animals, 13(10), 1607. https://doi.org/10.3390/ani13101607






