Leishmania Animal Models Used in Drug Discovery: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Methodology
2.2. Study Eligibility
2.3. Data Extraction
2.4. Risk of Bias
2.5. Registration
3. Results
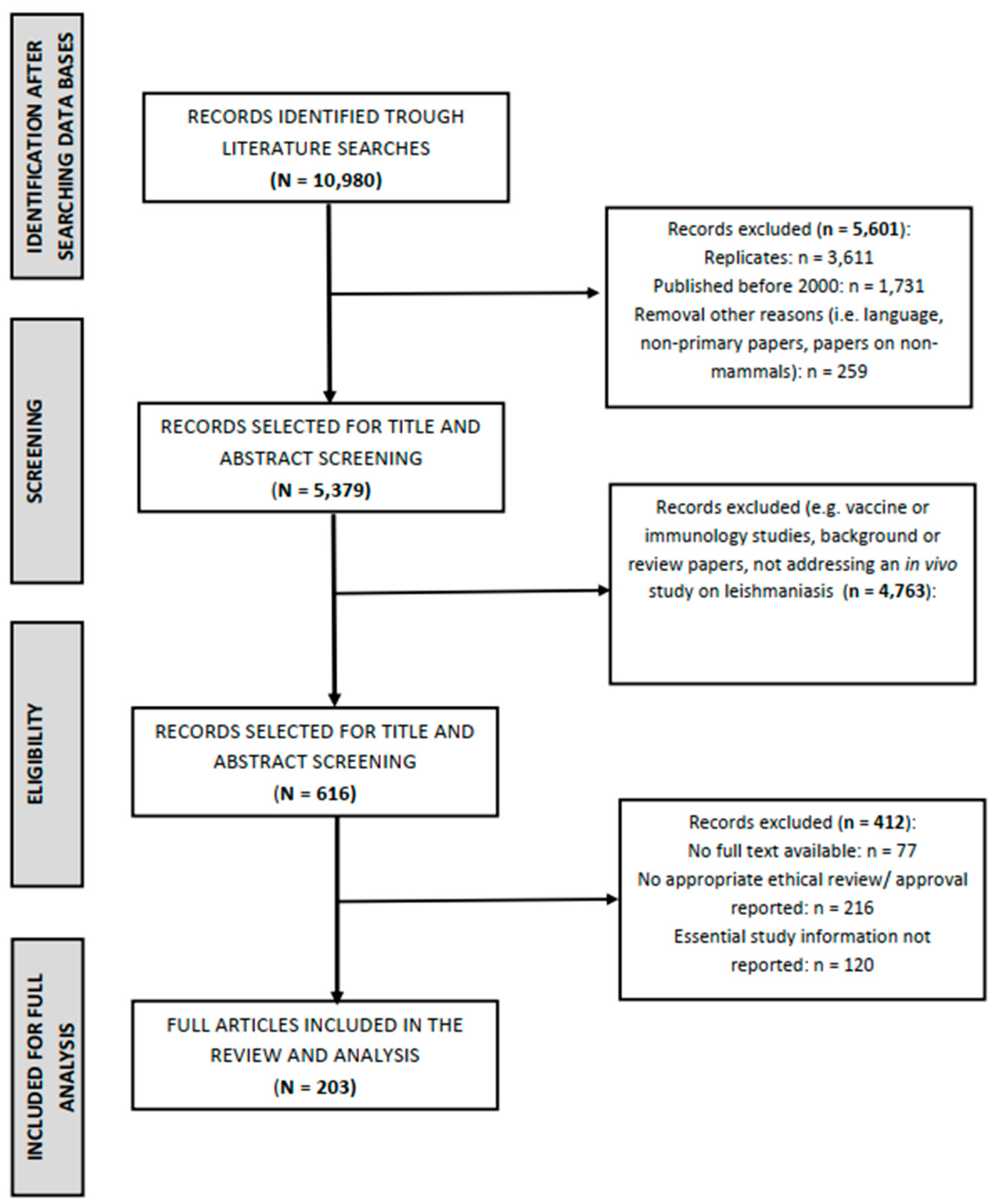
3.1. Search Results
3.2. Experimental Animals
3.3. Sample Size Calculations and Applied Statistics
3.4. Experimental Design
3.5. Animal Welfare Aspects
3.6. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef]
- Hu, R.V.P.F.; Ramdas, S.; Nieuwkerk, P.; Reis, R.; Lai AFat, R.F.M.; de Vries, H.J.C.; Schallig, H.D.F.H. Body location of “New World” cutaneous leishmaniasis lesions and its impact on the quality of life of patients in Suriname. PLoS Negl. Trop. Dis. 2020, 14, e0008759. [Google Scholar] [CrossRef]
- Ramdas, S.; van der Geest, S.; Schallig, H.D. Nuancing stigma through ethnography: The case of cutaneous leishmaniasis in Suriname. Soc. Sci. Med. 2016, 151, 139–146. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef]
- Gillespie, P.M.; Beaumier, C.M.; Strych, U.; Hayward, T.; Hotez, P.J.; Bottazzi, M.E. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 2016, 34, 2992–2995. [Google Scholar] [CrossRef]
- Akbari, M.; Oryan, A.; Hatam, G. Immunotherapy in treatment of leishmaniasis. Immunol. Lett. 2021, 233, 80–86. [Google Scholar] [CrossRef]
- Altamura, F.; Rajesh, R.; Catta-Preta, C.M.C.; Moretti, N.S.; Cestari, I. The current drug discovery landscape for trypanosomiasis and leishmaniasis: Challenges and strategies to identify drug targets. Drug Dev. Res. 2022, 83, 225–252. [Google Scholar] [CrossRef]
- Hendrickx, S.; Caljon, G.; Maes, L. Need for sustainable approaches in antileishmanial drug discovery. Parasitol. Res. 2019, 118, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Caridha, D.; Vesely, B.; van Bocxlaer, K.; Arana, B.; Mowbray, C.E.; Rafati, S.; Uliana, S.; Reguera, R.; Kreishman-Deitrick, M.; Sciotti, R.; et al. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug. Resist. 2019, 11, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Mears, E.R.; Modabber, F.; Don, R.; Johnson, G.E. A Review: The current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003889. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.B.; Hooijmans, C.R.; Tillema, A.; Leenaars, M.; Ritskes-Hoitinga, M. A search filter for increasing the retrieval of animal studies in Embase. Lab. Anim. 2011, 45, 268–270. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Tillema, A.; Leenaars, M.; Ritskes-Hoitinga, M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab. Anim. 2010, 44, 170–175. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Stokes, W.S. Humane endpoints for laboratory animals used in regulatory testing. ILAR J. 2002, 43 (Suppl. S1), S31–S38. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Leenaars, M.; Ritskes-Hoitinga, M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern. Lab. Anim. 2010, 38, 167–182. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Titus, R.G.; Marchand, M.; Boon, T.; Louis, J.A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985, 7, 545–555. [Google Scholar] [CrossRef]
- Bailey, H.; Bishop, W.J. Leishman-Donovan bodies and donovaniasis; Sir William Boog Leishman, 1865–1926; Charles Donovan, 1863–1951. Br. J. Vener. Dis. 1959, 35, 8–9. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.L.; Shaw, D.J.; Clutton, R.E. Animal welfare requirements in publishing guidelines. Lab. Anim. 2022, 56, 561–575. [Google Scholar] [CrossRef]
- Kiige, S.G.; Mutiso, J.M.; Laban, L.T.; Khayeka-Wandabwa, C.; Anjili, C.O.; Ingonga, J.; Gicheru, M.M. F₁ cross-breed between susceptible BALB/c and resistant Swiss mice infected with Leishmania major exhibit an intermediate phenotype for lesion sizes and type 1 cytokines but show low level of total IgG antibodies. Scand. J. Immunol. 2014, 79, 283–291. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-time PCR applications for diagnosis of leishmaniasis. Parasit. Vectors 2018, 11, 273. [Google Scholar] [CrossRef]
- Van der Meide, W.; Guerra, J.; Schoone, G.; Farenhorst, M.; Coelho, L.; Faber, W.; Peekel, I.; Schallig, H. Comparison between quantitative nucleic acid sequence-based amplification, real-time reverse transcriptase PCR, and real-time PCR for quantification of Leishmania parasites. J. Clin. Microbiol. 2008, 46, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Verrest, L.; Kip, A.E.; Musa, A.M.; Schoone, G.J.; Schallig, H.D.F.H.; Mbui, J.; Khalil, E.A.G.; Younis, B.M.; Olobo, J.; Were, L.; et al. Blood Parasite Load as an Early Marker to Predict Treatment Response in Visceral Leishmaniasis in Eastern Africa. Clin. Infect. Dis. 2021, 73, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Perez-Pertejo, Y.; Fresno, M.; Balana-Fouce, R.; Reguera, R.M. Infrared Fluorescent Imaging as a Potent Tool for In Vitro, Ex Vivo and In Vivo Models of Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Ferrua, B.; Lang, T.; Maddugoda, M.P.; Munro, P.; Pomares, C.; Lemichez, E.; Marty, P. Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, In Vivo, Ex Vivo and In Vitro. PLoS Negl. Trop. Dis. 2011, 5, e1323. [Google Scholar] [CrossRef] [PubMed]
| Mouse (Mus musculus) *; 179 Studies | Hamster (Mesocricetus auratus); 37 Studies | |
|---|---|---|
| Gender | 124 female; 22 male: 4 female and male mixed; 29 gender unreported. | 9 female; 19 male: 5 female and male mixed; 4 gender unreported. |
| Mean age (weeks) | 6.8 ± 0.2 [6.5–7.1] | 8.8 ± 1.0 [6.8–10.8] |
| Mean weight (grams) | 22.7 ± 0.7 [21.3–24.1] | 90.8 ± 7.1 [76.2–105.3] |
| Mean number in total per study | 35.9 ± 5.1 [25.5–46.4] | 36.4 ± 1.7 [32.9–39.8] |
| Mean number in total per study group | 6.5 ± 0.4 [5.6–7.4] | 7.4 ± 0.3 [6.9–7.9] |
| Mouse (Mus musculus) 1; 179 Studies | Hamster (Mesocricetus auratus); 37 Studies | |
|---|---|---|
| Treatment provided | ||
| Experimental treatment 2 | 179 (100%) | 37 (100%) |
| Competitor treatment 3 | 115 (64.2%) | 28 (75.7%) |
| Placebo treatment 4 | 133 (74.3) | 25 (67.6%) |
| Leishmania species | ||
| L. amazonensis | 67 | 4 |
| L. major | 48 | 1 |
| L. donovani | 29 | 12 |
| L. infantum | 24 | 6 |
| L. chagasi 5 | 1 | 3 |
| L. brazaliensis | 6 | 8 |
| L. mexicana | 2 | - |
| L. tropica | 2 | 1 |
| L. panamensis | - | 2 |
| Stage of Leishmania used for inoculation | ||
| Amastigote | 21 | 13 |
| Promastigote | 158 | 24 |
| Mean number (log) of parasites per inoculum to establish experimental infection | 1.25 × 107 ± 2.31 × 107 | 2.36 × 107 ± 3.26 × 107 |
| Route of inoculation | ||
| Intravenous | 30 | - |
| Intracardial | 7 | 14 |
| Intraperitoneal | 15 | 7 |
| Subcutaneous | 125 | 16 |
| Other | 2 | - |
| Mean duration of inoculation (in days) ± standard and 95% CI | 22.9 ± 1.6 [26.8–33.0] | 31.4 ± 3.3 [24.7–38.1] |
| Experimental technique used as read-out to assess the effect of treatment 6 | ||
| Lesion size or increased thickness of the foot pad | 109 | 15 |
| Microscopy (Leishman–Donovan units) | 28 | 6 |
| Microscopy (Histopathology) | 20 | 12 |
| Limiting dilution assay | 80 | 12 |
| (q)PCR | 31 | 2 |
| Other methods | 18 | 2 |
| Primary organ targeted for read-out 7 | ||
| Skin (foot pad) | 57 | 13 |
| Skin (base of tail) | 36 | 0 |
| Skin (other location) | 7 | 3 |
| Spleen and/or liver | 77 | 21 |
| Bone marrow or other lymphatic tissue | 36 | 9 |
| Other organs | 21 | 3 |
| Other physiological parameters reported | ||
| Weight (gain or loss) | 30 | 5 |
| Blood parameters (inc. cytokines, antibodies, inflammatory markers) | 67 | 6 |
| Reported status of the experimental animals at the end of the experiment | ||
| Death | 162 | 32 |
| Alive | 3 | 1 |
| Not reported/unclear | 19 | 4 |
| Mouse (Mus musculus) *; 179 Studies | Hamster (Mesocricetus auratus); 37 Studies | |
|---|---|---|
| Sourcing | ||
| Commercial breeder | 108 (60.3%) | 22 (59.5%) |
| In-house, non-commercial breeder | 17 (9.5%) | 3 (8.1%) |
| Not reported | 54 (30.2%) | 12 (32.4%) |
| Housing | ||
| Individual | 21 (11.7%) | 4 (10.8%) |
| In groups | 6 (3.4%) | 4 (10.8%) |
| Not reported | 152 (84.9%) | 29 (78.4%) |
| Definition of human endpoint reported | 0 (0.0%) | 0 (0.0%) |
| 3Rs considered | 10 (5.6%) | 2 (5.4%) |
| Other welfare issues reported | 77 (43.0%) | 14 (37.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Ende, J.; Schallig, H.D.F.H. Leishmania Animal Models Used in Drug Discovery: A Systematic Review. Animals 2023, 13, 1650. https://doi.org/10.3390/ani13101650
van der Ende J, Schallig HDFH. Leishmania Animal Models Used in Drug Discovery: A Systematic Review. Animals. 2023; 13(10):1650. https://doi.org/10.3390/ani13101650
Chicago/Turabian Stylevan der Ende, Jacob, and Henk D. F. H. Schallig. 2023. "Leishmania Animal Models Used in Drug Discovery: A Systematic Review" Animals 13, no. 10: 1650. https://doi.org/10.3390/ani13101650
APA Stylevan der Ende, J., & Schallig, H. D. F. H. (2023). Leishmania Animal Models Used in Drug Discovery: A Systematic Review. Animals, 13(10), 1650. https://doi.org/10.3390/ani13101650






