Effect of Neurosteroids on Basal and Stress-Induced Oxytocin Secretion in Luteal-Phase and Pregnant Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Brain Surgeries
2.3. Procedures and Design of Experiment 1: Sheep in the Luteal Phase
2.4. Procedures and Design of Experiment 2: Pregnant Sheep
2.5. Analysis of Gene Expression Levels: RNA Isolation, cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.6. Hormone Concentration Assay
2.7. Statistical Analyses
3. Results
3.1. Experiment 1: Sheep in the Luteal Phase
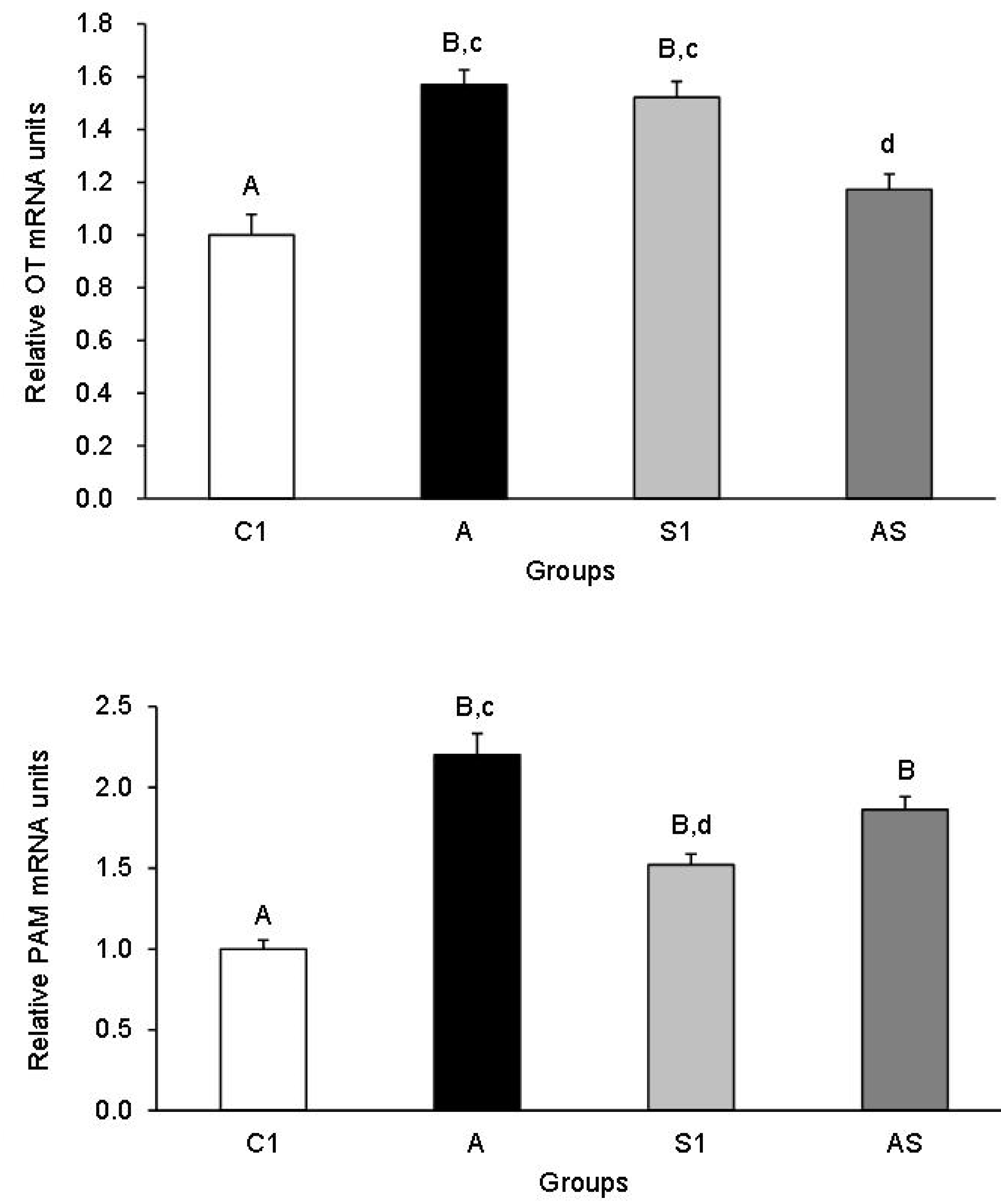
3.1.1. OT and PAM mRNA Expression in the Hypothalamic Nuclei
3.1.2. OT and PAM mRNA Expression in the PP
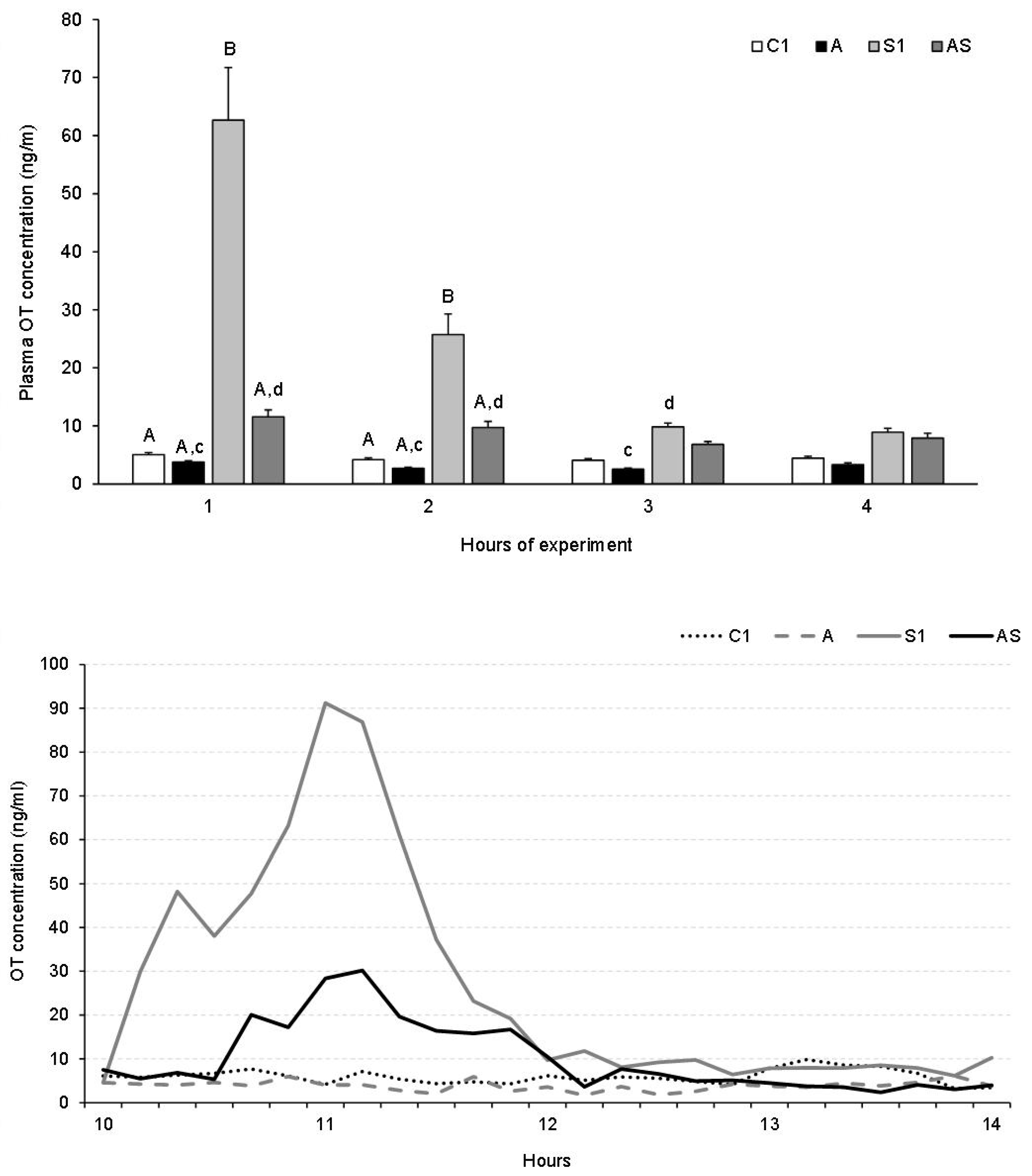
3.1.3. Plasma OT Concentration
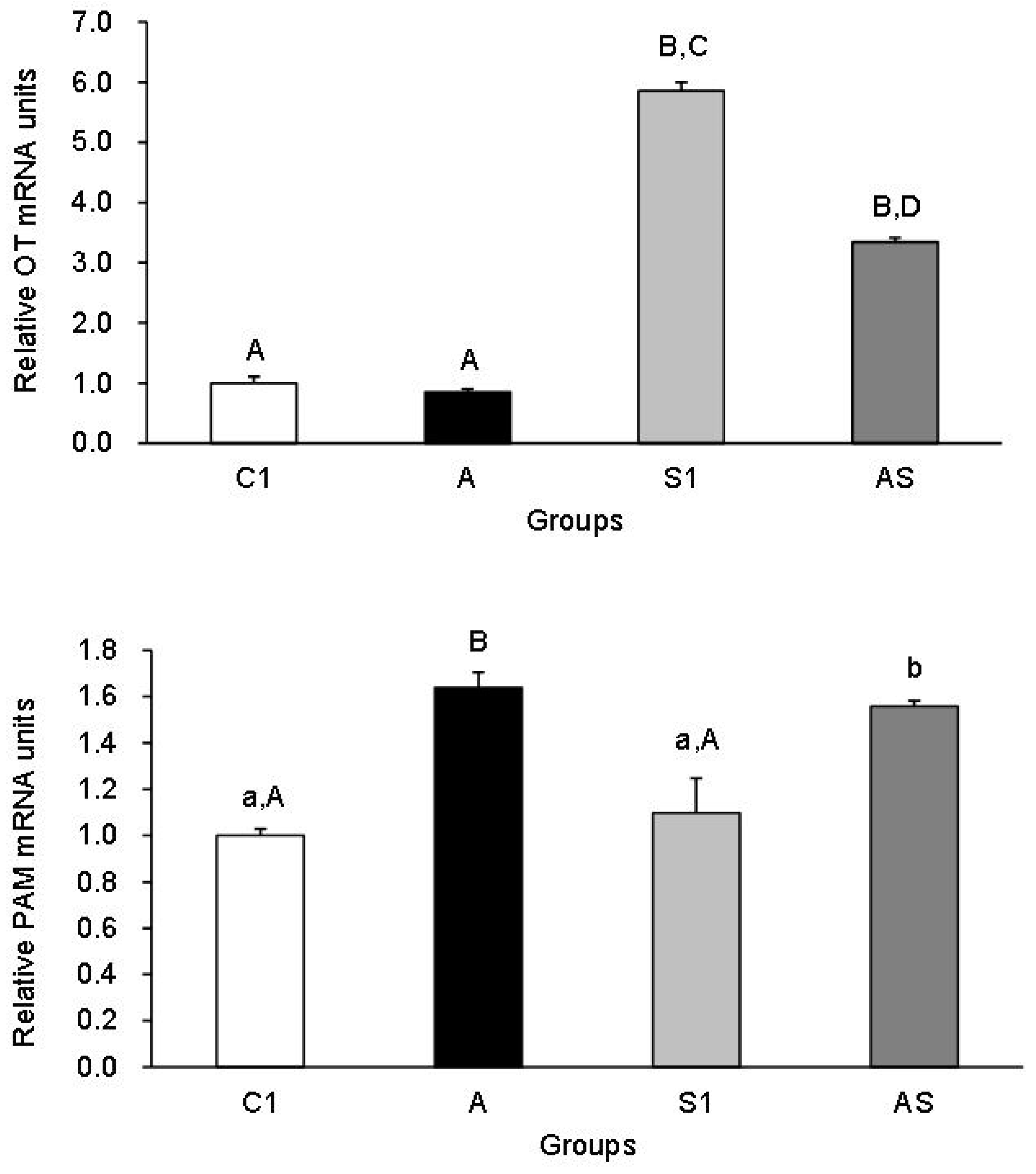
3.2. Experiment 2: Pregnant Sheep
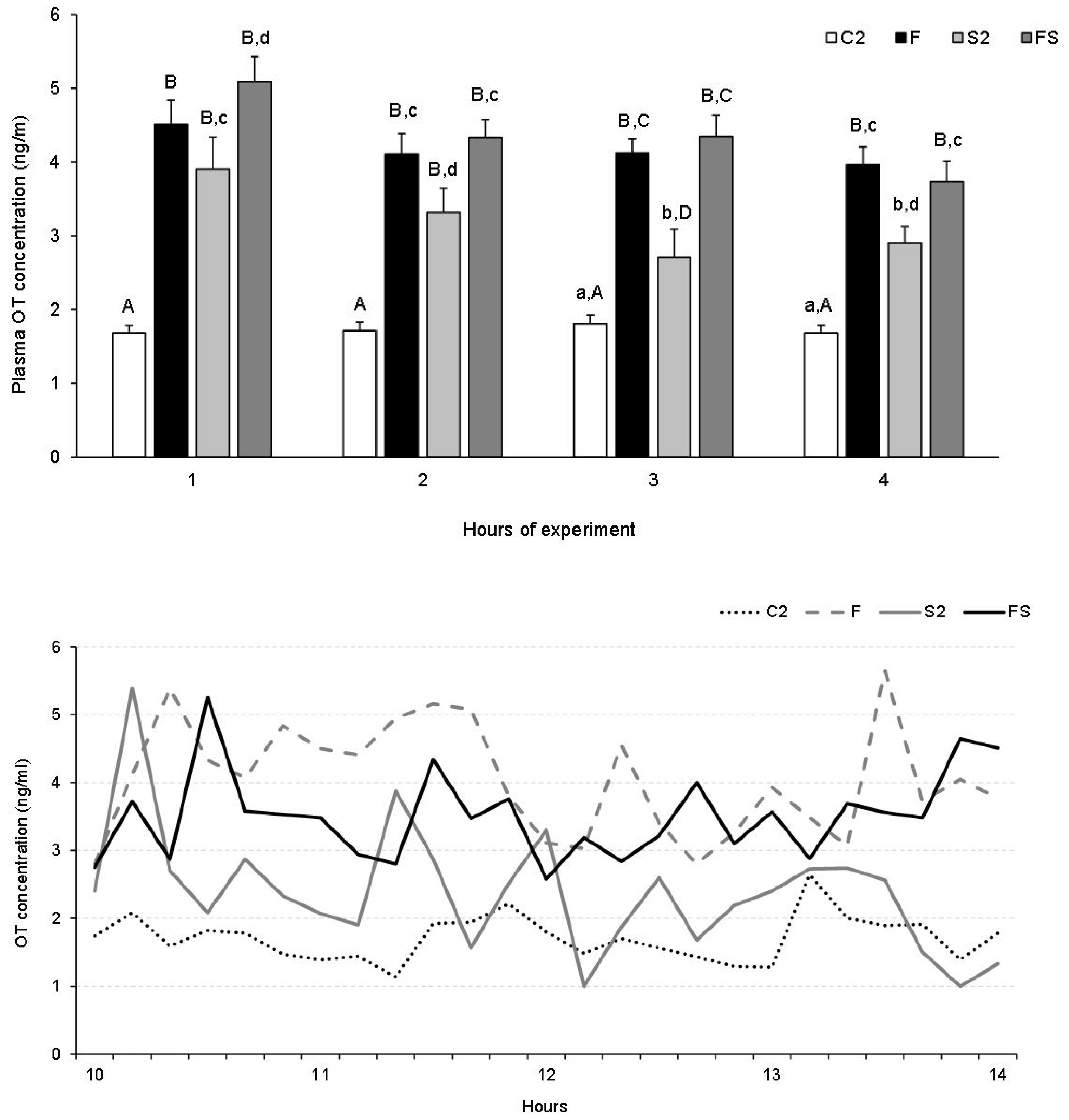
Plasma OT Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rault, J.L.; Carter, C.S.; Garner, J.P.; Marchant-Forde, J.N.; Richert, B.T.; Lay, D.C., Jr. Repeated intranasal OT administration in early life dysregulates the HPA axis and alters social behavior. Physiol. Behav. 2013, 112–113, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Svennersten-Sjaunja, K.; Olsson, K. Endocrinology of milk production. Domest. Anim. Endocrinol. 2005, 29, 241–258. [Google Scholar] [CrossRef]
- Ogi, A.; Mariti, C.; Pirrone, F.; Baragli, P.; Gazzano, A. The influence of OT on maternal care in lactating dogs. Animals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Onaka, T.; Takayanagi, Y. Role of OT in the control of stress and food intake. J. Neuroendocrinol. 2019, 3, e12700. [Google Scholar] [CrossRef] [PubMed]
- Sivukhina, E.V.; Jirikowski, G.F. Magnocellular hypothalamic system and its interaction with the hypothalamo-pituitary-adrenal axis. Steroids 2016, 111, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Czyzyk, T.A.; Ning, Y.; Hsu, M.-S.; Peng, B.; Mains, R.E.; Eipper, B.A.; Pintar, J.E. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev. Biol. 2005, 287, 301–313. [Google Scholar] [CrossRef]
- Eipper, B.A.; Milgram, S.L.; Husten, E.J.; Yun, H.-Y.; Mains, R.E. Peptidylglycine α-amidating monooxygenase: A multifunctional protein with catalytic, processing and routing domains. Protein Sci. 1993, 2, 489–497. [Google Scholar] [CrossRef]
- Oyarce, A.M.; Eipper, B.A. Identification of subcellular compartments containing peptidylglycine α-amidating monooxygenase in rat anterior pituitary. J. Cell Sci. 1995, 108, 287–297. [Google Scholar] [CrossRef]
- Gaier, E.D.; Miller, M.B.; Ralle, M.; Aryal, D.; Wetsel, W.C.; Mains, R.E.; Eipper, B.A. Peptidylglycine alpha-amidating monooxygenase heterozygosity alters brain copper handling with region specificity. J. Neurochem. 2013, 127, 605–619. [Google Scholar] [CrossRef]
- Kumar, D.; Thomason, R.T.; Yankova, M.; Gitlin, J.D.; Mains, R.E.; Eipper, B.A.; King, S.M. Microvillar and ciliary defects in zebrafish lacking an actin-binding bioactive peptide amidating enzyme. Sci. Rep. 2018, 8, 4547–4561. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Yoshida, M.; Takashima, A.; Takanami, K.; Yoshida, S.; Nishimori, K.; Nishijima, I.; Sakamoto, H.; Yamagata, T.; Onaka, T. Activation of supraoptic OT neurons by secretin facilitates social recognition. Biol. Psychiatry 2017, 81, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Italiano, D.; Ferrara, D.; Mondello, S.; Conti-Nibali, V.; Salviera, C.; Bramanti, P. The hypothalamic-neurohypophyseal system: Current and future treatment of vasopressin and oxytocyn related disorders. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 235–250. [Google Scholar] [CrossRef]
- Bosch, O.J.; Kromer, S.A.; Brunton, P.J.; Neumann, I.D. Release of OT in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 2004, 124, 439–448. [Google Scholar] [CrossRef]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease: An update on current knowledge. Annu. Rev. Public Health 2013, 34, 337–354. [Google Scholar] [CrossRef]
- Duque-Wilckens, N.; Steinman, M.Q.; Busnelli, M.; Chini, B.; Yokoyama, S.; Pham, M.; Laredo, S.A.; Hao, R.; Perkeybile, A.M.; Minie, V.A.; et al. OT Receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female california mice. Biol. Psychiatry 2018, 83, 203–213. [Google Scholar] [CrossRef]
- Nasanbuyan, N.; Yoshida, M.; Takayanagi, Y.; Inutsuka, A.; Nishimori, K.; Yamanaka, A.; Onaka, T. OT–OT Receptor Systems Facilitate Social Defeat Posture in Male Mice. Endocrinology 2018, 159, 763–775. [Google Scholar] [CrossRef]
- Cavanaugh, J.; Carp, S.B.; Rock, C.M.; French, J.A. OT modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology 2016, 66, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Slattery, D.A. OT in general anxiety and social fear: A translational approach. Biol. Psychiatry 2016, 79, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.J.; Young, L.J. OT and social relationships: From attachment to bond disruption. Curr. Top. Behav. Neurosci. 2018, 35, 97–117. [Google Scholar]
- Papadimitriou, A.; Priftis, K.N. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [CrossRef]
- Billiards, S.S.; Walker, D.W.; Canny, B.J.; Hirst, J.J. Endotoxin increases sleep and brain allopregnanolone concentrations in newborn lambs. Pediatr. Res. 2002, 52, 892–899. [Google Scholar] [CrossRef]
- Sze, Y.; Gill, A.C.; Brunton, P.J. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J. Neuroendocrinol. 2018, 30, e12644. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.G.; Brown, A.R.; Lambert, J.J.; Belelli, D. Neurosteroids and GABA(A) receptor interactions: A focus on stress. Front. Neurosci. 2011, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, A.B.; Kits, K.S.; Baker, R.E.; Willems, W.P.; Leyting-Vermeulen, J.W.; Voorn, P.; Smit, A.B.; Bicknell, R.J.; Herbison, A.E. Plasticity in fast synaptic inhibition of adult OT neurons caused by switch in GABA(A) receptor subunit expression. Neuron 1997, 19, 1103–1114. [Google Scholar] [CrossRef]
- Misztal, T.; Młotkowska, P.; Marciniak, E.; Misztal, A. Allopregnanolone reduces neuroendocrine response to acute stressful stimuli in sheep. J. Endocrinol. 2020, 244, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Misztal, T.; Czauderna, M.R.; Młotkowska, P.; Misztal, A.; Marciniak, E. Temporal changes in the cerebrospinal fluid allopregnanolone concentration and hypothalamic-pituitary-adrenal axis activity in sheep during pregnancy and early lactation. Livest. Sci. 2020, 231, 103871. [Google Scholar] [CrossRef]
- Strzetelski, J.A.; Brzóska, F.; Kowalski, Z.M.; Osięgłowski, S. Zalecenia Żywieniowe dla Przeżuwaczy i Tabele Wartości Pokarmowej Pasz; Instytut Zootechniki PIB: Kraków, Poland, 2014. (In Polish) [Google Scholar]
- Welento, J.; Szteyn, S.; Milart, Z. Observations on the stereotaxic configuration of the hypothalamus nuclei in the sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar]
- Traczyk, W.; Przekop, F. Methods of investigation of the function of the hypothalamus and hypophysis in chronic experiments in sheep. Acta Physiol. Pol. 1963, 14, 227–236. (In Polish) [Google Scholar]
- Misztal, T.; Młotkowska, P.; Marciniak, E.; Roszkowicz-Ostrowska, K.; Misztal, A. Involvement of neurosteroids in the control of hypothalamic-pituitary-adrenal axis activity in pregnant sheep under basal and stressful conditions. Theriogenology 2021, 174, 114–120. [Google Scholar] [CrossRef]
- Hasiec, M.; Tomaszewska-Zaremba, D.; Misztal, T. Suckling and salsolinol attenuate responsiveness of the hypothalamic-pituitary-adrenal axis to stress: Focus on catecholamines, corticotrophin-releasing hormone, adrenocorticotrophic hormone, cortisol and prolactin secretion in lactating sheep. J. Neuroendocrinol. 2014, 26, 844–852. [Google Scholar] [CrossRef]
- Handa, R.J.; Kudwa, A.E.; Donner, N.C.; McGivern, R.F.; Brown, R. Central 5-alpha reduction of testosterone is required for testosterone’s inhibition of the hypothalamo-pituitary-adrenal axis response to restraint stress in adult male rats. Brain Res. 2013, 1529, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempflfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Górski, K.; Marciniak, E.; Zielińska-Górska, M.; Misztal, T. Salsolinol Up-Regulates OT Expression and Release During Lactation in Sheep. J. Neuroendocrinol. 2016, 28, 12362. [Google Scholar] [CrossRef] [PubMed]
- Mohr, E.; Schmitz, E. Functional characterization of estrogen and glucocorticoid responsive elements in the rat OT gene. Brain Res. Mol. Brain Res. 1991, 9, 293–298. [Google Scholar] [CrossRef]
- Crowley, R.S.; Insel, T.R.; O’Keefe, J.A.; Kim, N.B.; Amico, J.A. Increased accumulation of OT messenger ribonucleic acid in the hypothalamus of the female rat: Induction by long-term estradiol and progesterone administration and subsequent progesterone withdrawal. Endocrinology 1995, 136, 224–231. [Google Scholar] [CrossRef]
- Jirikowski, G.F.; Ochs, S.D.; Caldwell, J.D. OT and steroid actions. Curr. Top. Behav. Neurosci. 2018, 35, 77–95. [Google Scholar]
- Młotkowska, P.; Marciniak, E.; Roszkowicz-Ostrowska, K.; Misztal, T. Effects of allopregnanolone on central reproductive functions in sheep under natural and stressful conditions. Theriogenology 2020, 158, 138–147. [Google Scholar] [CrossRef]
- Webb, R.; Mitchell, M.D.; Falconer, J.; Robinson, J.S. Temporal relationships between peripheral plasma concentrations of OT, progesterone and 13,14-dihydro-15-keto prostaglandin F2si during the estrous cycle and early pregnancy in the ewe. Prostaglandins 1981, 22, 443–453. [Google Scholar] [CrossRef]
- Schams, D.; Lahlou-Kassi, A.; Glatzel, P. OT concentrations in peripheral blood during the oestrous cycle and after ovariectomy in two breeds of sheep with low and high fecundity. J. Endocrinol. 1982, 92, 9–13. [Google Scholar] [CrossRef]
- Olsen, R.W. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Widmer, H.; Ludwig, M.; Bancel, F.; Leng, G.; Dayanithi, G. Neurosteroid regulation of OT and vasopressin release from the rat supraoptic nucleus. J. Physiol. 2003, 548, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Trembleau, A.; Morales, M.; Bloom, F.E. Aggregation of vasopressin mRNA in a subset of axonal swellings of the median eminence and posterior pituitary: Light and electron microscopic evidence. J. Neurosci. 1994, 14, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Misztal, T.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central injection of exogenous IL-1β in the control activities of hypothalamic–pituitary–gonadal axis in anestrous ewes. Reprod. Domest. Anim. 2012, 47, 44–52. [Google Scholar] [CrossRef]
- Kaplan, B.B.; Lavina, Z.S.; Gioio, A.E. Subcellular compartmentation of neuronal protein synthesis: New insights into the biology of the neuron. Ann. N. Y. Acad. Sci. 2004, 1018, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Jezova, D.; Skultetyova, I.; Tokarev, D.I.; Bakos, P.; Vigas, M. Vasopressin and OT in stress. Ann. N. Y. Acad. Sci. 1995, 771, 192–203. [Google Scholar] [CrossRef]
- Douglas, A.J.; Johnston, H.; Brunton, P.; Russell, J.A. Sex-steroid induction of endogenous opioid inhibition on OT secretory responses to stress. J. Neuroendocrinol. 2000, 12, 343–350. [Google Scholar] [CrossRef]
- Laguna-Abreu, M.T.; Koenigkam-Santos, M.; Colleta, A.M.; Elias, P.C.; Moreira, A.C.; Antunes-Rodrigues, J.; Elias, L.L.; Castro, M. Time course of vasopressin and OT secretion after stress in adrenalectomized rats. Horm. Metabol. Res. 2005, 37, 84–88. [Google Scholar] [CrossRef]
- Yoshida, M.; Takayanagi, Y.; Onaka, T. The medial amygdala-medullary PrRP synthesizing neuron pathway mediates neuroendocrine responses to contextual conditioned fear in male rodents. Endocrinology 2014, 155, 2996–3004. [Google Scholar] [CrossRef]
- Onaka, Y.; Shintani, N.; Nakazawa, T.; Haba, R.; Ago, Y.; Wang, H.; Kanoh, T.; Hayata-Takano, A.; Hirai, H.; Nagata, K.Y.; et al. CRTH2, a prostaglandin D2 receptor, mediates depression-related behavior in mice. Behav. Brain Res. 2015, 284, 131–137. [Google Scholar] [CrossRef]
- Vacher, C.M.; Frétier, P.; Créminon, C.; Calas, A.; Hardin-Pouzet, H. Activation by serotonin and noradrenaline of vasopressin and OT expression in the mouse paraventricular and supraoptic nuclei. J. Neurosci. 2002, 22, 1513–1522. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Interactions between dopamine and OT in the control of sexual behavior. Prog. Brain Res. 2008, 170, 277–290. [Google Scholar]
- Baskerville, T.A.; Allard, J.; Wayman, C.; Douglas, A.J. Dopamine-OT interactions in penile erection. Eur. J. Neurosci. 2009, 3, 2151–2564. [Google Scholar] [CrossRef]
- Pow, D.V.; Morris, J.F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 1989, 32, 435–439. [Google Scholar] [CrossRef]
- Love, T.M. The impact of OT on stress: The role of sex. Curr. Opin. Behav. Sci. 2018, 23, 136–142. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The OT Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Peters, S.T.; Bowen, M.T.; Bohrer, K.; McGregor, I.S.; Neumann, I.D. OT inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict. Biol. 2017, 22, 702–711. [Google Scholar] [CrossRef]
- Torner, L.; Plotsky, P.M.; Neumann, I.D.; de Jong, T.R. Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology 2017, 77, 165–174. [Google Scholar] [CrossRef]
- Herman, J.P.; Nawreen, N.; Smail, M.A.; Cotella, E.M. Brain mechanisms of HPA axis regulation: Neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress 2020, 23, 617–632. [Google Scholar] [CrossRef]
- Ditzen, B.; Schaer, M.; Gabriel, B.; Bodenmann, G.; Ehlert, U.; Heinrichs, M. Intranasal OT increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatr. 2009, 65, 728–731. [Google Scholar] [CrossRef]
- Windle, R.J.; Kershaw, Y.M.; Shanks, N.; Wood, S.A.; Lightman, S.L.; Ingram, C.D. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 2004, 24, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jurek, B. The interplay between OT and the CRF system: Regulation of the stress response. Cell Tissue Res. 2019, 375, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R. Progesterone in the brain: Hormone, neurosteroid and neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef]
- Douglas, A.J.; Neumann, I.; Meeren, H.K.M.; Leng, G.; Johnstone, L.E.; Munro, G.; Russell, J.A. Central endogenous opioid inhibition of supraoptic OT neurons in pregnant rats. J. Neurosci. 1995, 15, 5049–5057. [Google Scholar] [CrossRef]
- Russell, J.A.; Brunton, P.J. Neuroactive steroids attenuate OT stress responses in late pregnancy. Neuroscience 2006, 138, 879–889. [Google Scholar] [CrossRef]
- Tilbrook, A.J.; Clarke, I.J. Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamo-pituitary adrenal axis to stress. Front. Neuroendocrinol. 2006, 27, 285–307. [Google Scholar] [CrossRef]
- Schams, D.; Lahlou-Kassi, A. Circulating concentrations of OT during pregnancy in ewes. Acta Endocrinol. 1984, 106, 277–281. [Google Scholar]
- Rhodes, L.; Nathanielsz, P.W. Myometrial activity and plasma progesterone and OT concentrations in cycling and early-pregnant ewes. Biol. Reprod. 1990, 42, 834–841. [Google Scholar] [CrossRef]
- An, S.M.; Kim, M.J.; Jeong, J.S.; Kim, S.Y.; Kim, D.S.; An, B.S.; Kim, S.C. OT modulates steroidogenesis-associated genes and estradiol levels in the placenta. Syst. Biol. Reprod. Med. 2023, 14, 1–11. [Google Scholar]
- Russell, J.A.; Leng, G.; Douglas, A.J. The magnocellular OT system, the fount of maternity: Adaptations in pregnancy. Front. Neuroendocrinol. 2003, 24, 27–61. [Google Scholar] [CrossRef]
- Jain, V.; McDonald, S.D.; Mundle, W.R.; Farine, D. Guideline no. 398: Progesterone for prevention of spontaneous preterm birth. J. Obs. Gynaecol. Can. 2020, 42, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.J.; Bales, J.; Russell, J.A. Allopregnanolone and induction of endogenous opioid inhibition of OT responses to immune stress in pregnant rats. J. Neuroendocrinol. 2012, 24, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Mostallino, M.C.; Porcu, P.; Follesa, P.; Barbaccia, M.L.; Trabucchi, M.; Purdy, R.H.; Grisenti, P.; Biggio, G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. USA 1998, 95, 13284–13289. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence (5′-3′) | GeneBank Access No. |
| OT | F | GCCTTCTCCCAGCACTGA | X16052 |
| R | CCTGGGGATGATCAGAGG | ||
| PAM | F | CCCAAAGGTGTTGGATTCAG | XM_004009099 |
| R | CACCAGAGCAGTCCTTGTGA | ||
| GAPDH | F | GGGTCATCATCTCTGCACCT | NM_001190390.1 |
| R | GGTCATAAGTCCCTCCACGA | ||
| PPIC | F | TGGAAAAGTCGTGCCCAAGA | XM_004008676.1 |
| R | TGCTTATACCACCAGTGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młotkowska, P.; Marciniak, E.; Misztal, A.; Misztal, T. Effect of Neurosteroids on Basal and Stress-Induced Oxytocin Secretion in Luteal-Phase and Pregnant Sheep. Animals 2023, 13, 1658. https://doi.org/10.3390/ani13101658
Młotkowska P, Marciniak E, Misztal A, Misztal T. Effect of Neurosteroids on Basal and Stress-Induced Oxytocin Secretion in Luteal-Phase and Pregnant Sheep. Animals. 2023; 13(10):1658. https://doi.org/10.3390/ani13101658
Chicago/Turabian StyleMłotkowska, Patrycja, Elżbieta Marciniak, Anna Misztal, and Tomasz Misztal. 2023. "Effect of Neurosteroids on Basal and Stress-Induced Oxytocin Secretion in Luteal-Phase and Pregnant Sheep" Animals 13, no. 10: 1658. https://doi.org/10.3390/ani13101658





