Evolution of Avian Eye Size Is Associated with Habitat Openness, Food Type and Brain Size
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
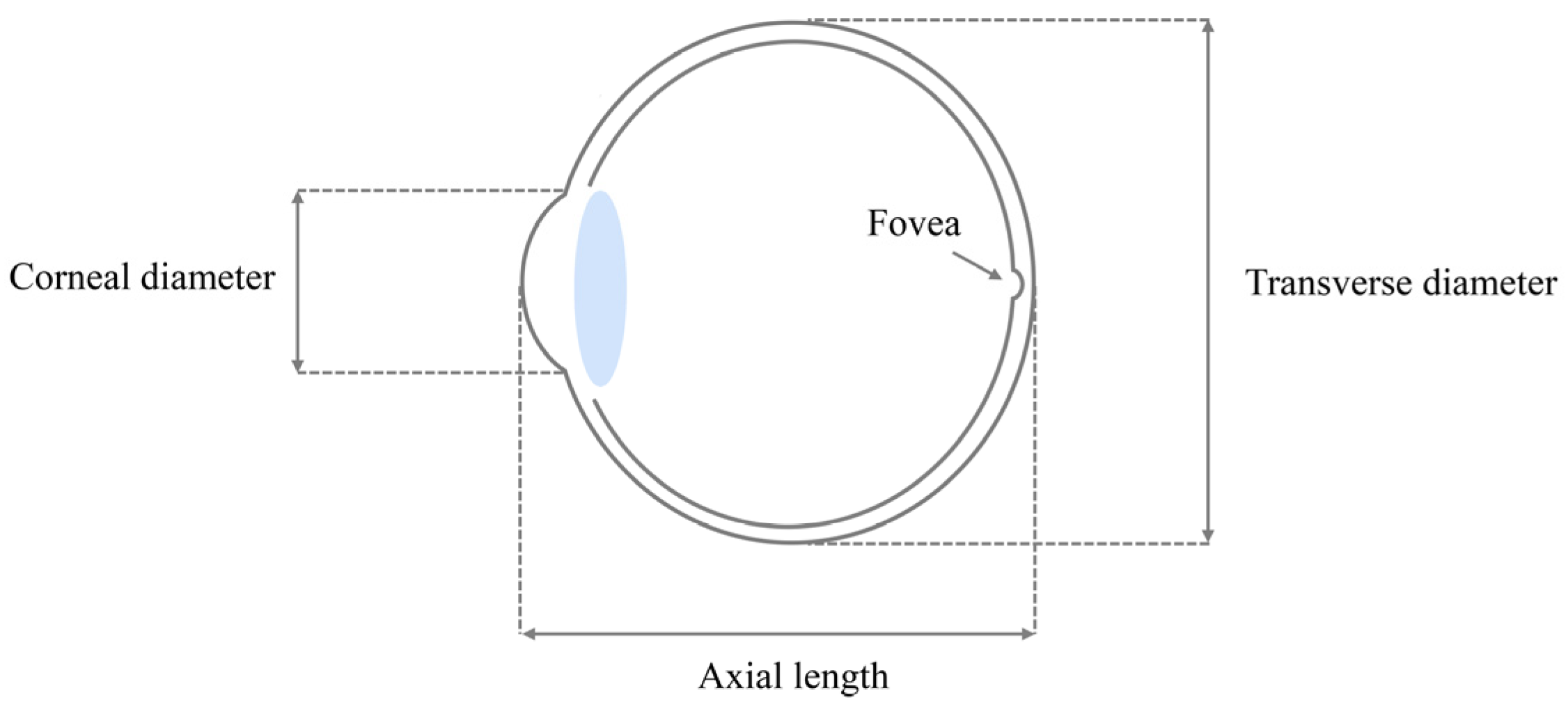
2.1. Data Collection
2.2. Phylogeny
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Land, M.F.; Nilsson, D.E. Animal Eyes; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Lythgoe, J.N. The Ecology of Vision; Clarendon Press: Oxford, UK, 1979. [Google Scholar]
- Ali, M.A.; Klyne, M.A. Vision in Vertebrates; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Kolb, H. Simple anatomy of the retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995; pp. 1–26. [Google Scholar]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Thomas, R.J.; Kelly, D.J.; Goodship, N.M. Eye design in birds and visual constraints on behavior. Ornitol. Neotrop. 2004, 15, 243–250. [Google Scholar]
- Thomas, K.N.; Gower, D.J.; Bell, R.C.; Fujita, M.K.; Schott, R.K.; Streicher, J.W. Eye size and investment in frogs and toads correlate with adult habitat, activity pattern and breeding ecology. Proc. R. Soc. B 2020, 287, 20201393. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.; Ruxton, G.D. Why did some ichthyosaurs have such large eyes? J. Exp. Biol. 2002, 205, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Cranbrook Institute of Science: Bloomfield Hills, MI, USA, 1942. [Google Scholar]
- Snyder, A.W.; Miller, W.H. Photoreceptor diameter and spacing for highest resolving power. J. Opt. Soc. Am. 1977, 67, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Rostgaard, J.; Qvortrup, K. A note about retinal structure and visual acuity. A light microscopic study of the cones in fovea centralis. Acta Opthalmol. Scand. 1999, 77, 45–49. [Google Scholar] [CrossRef]
- Martin, G.R. An owl’s eye: Schematic optics and visual performance in Strix aluco. J. Comp. Physiol. 1982, 145, 341–349. [Google Scholar] [CrossRef]
- Huang, C.H.; Zhong, M.J.; Liao, W.B.; Kotrschal, A. Investigating the role of body size, ecology, and behavior in anuran eye size evolution. Evol. Ecol. 2019, 33, 585–598. [Google Scholar] [CrossRef]
- Howland, H.C.; Merola, S.; Basarab, J.R. The allometry and scaling of the size of vertebrate eyes. Vis. Res. 2004, 44, 2043–2065. [Google Scholar] [CrossRef]
- Hall, M.I.; Ross, C.F. Eye shape and activity pattern in birds. J. Zool. 2007, 271, 437–444. [Google Scholar] [CrossRef]
- Hall, M.I.; Heesy, C.P. Eye size, flight speed and Leuckart’s Law in birds. J. Zool. 2011, 283, 291–297. [Google Scholar] [CrossRef]
- Clifton, I.T.; Chamberlain, J.D.; Gifford, M.E. Role of phenotypic plasticity in morphological differentiation between water snake populations. Integr. Zool. 2020, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, B.R.; Surkova, E.N.; Shenbrot, G.I.; Khokhlova, I.S. Latitudinal gradients in body size and sexual size dimorphism in fleas: Males drive Bergmann’s pattern. Integr. Zool. 2023, 18, 414–426. [Google Scholar] [CrossRef]
- Zedda, M.; Sathe, V.; Chakraborty, P.; Palombo, M.R.; Farina, V. A first comparison of bone histomorphometry in extant domestic horses (Equus caballus) and a Pleistocene Indian wild horse (Equus namadicus). Integr. Zool. 2020, 15, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Muñoz–Muñoz, F.; Pagès, N.; Durao, A.F.; England, M.; Werner, D.; Talavera, S. Narrow versus broad: Sexual dimorphism in the wing form of western European species of the subgenus Avaritia (Culicoides, Ceratopogonidae). Integr. Zool. 2021, 16, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Amshokova, A.; Balčiauskienė, L.; Benedek, A.M.; Cichocki, J.; Csanády, A.; De Mendonça, P.G.; Nistreanu, V. Geographical clines in the size of the herb field mouse (Apodemus uralensis). Integr. Zool. 2020, 15, 55–68. [Google Scholar] [CrossRef]
- Secondi, J.; Scriba, M.F.; Mondy, N.; Lengagne, T. Artificial light at night decreases the pupillary light response of dark-adapted toads to bright light. Integr. Zool. 2022. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Herzog, H.; Jiang, Y.G.; Zhao, Y.H.; Zhang, D.Y. Exquisite structure of the lateral line system in eyeless cavefish Sinocyclocheilus tianlinensis contrast to eyed Sinocyclocheilus macrophthalmus (Cypriniformes: Cyprinidae). Integr. Zool. 2020, 15, 314–328. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Liao, W.B. Anuran interorbital distance variation: The role of ecological and behavioral factors. Integr. Zool. 2022, 17, 777–786. [Google Scholar] [CrossRef]
- Ausprey, I.J. Adaptations to light contribute to the ecological niches and evolution of the terrestrial avifauna. Proc. R. Soc. B 2021, 288, 20210853. [Google Scholar] [CrossRef]
- Schmitz, L.; Wainwright, P.C. Nocturnality constrains morphological and functional diversity in the eyes of reef fishes. BMC Evol. Biol. 2011, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, C.C.; Lewis, R.J. Effects of habitat light intensity on mammalian eye shape. Anat. Rec. 2011, 294, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortega, C.; Santos, E.S.A.; Gil, D. Species-specific differences in relative eye size are related to patterns of edge avoidance in an Amazonian rainforest bird community. Ecol. Evol. 2014, 4, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Széskely, T.; Cuthill, I.C.; Harper, D.G.C.; Newson, S.E.; Frayling, T.D.; Wallis, P.D. Eye size in birds and the timing of song at dawn. Proc. R. Soc. Lond. B 2002, 269, 831–837. [Google Scholar] [CrossRef]
- Berg, K.S.; Brumfield, R.T.; Apanius, V. Phylogenetic and ecological determinants of the neotropical dawn chorus. Proc. R. Soc. B 2006, 273, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Ockendon, N.; Davis, S.E.; Toms, M.P.; Mukherjee, S. Eye size and the time of arrival of birds at garden feeding stations in winter. J. Ornithol. 2009, 150, 903–908. [Google Scholar] [CrossRef]
- Martin, G.R. Through birds’ eyes: Insights into avian sensory ecology. J. Ornithol. 2012, 153, 23–48. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Sensory basis of vigilance behavior in birds: Synthesis and future prospects. Behav. Process. 2012, 89, 143–152. [Google Scholar] [CrossRef]
- Martin, G.R. The subtlety of simple eyes: The tuning of visual fields to perceptual challenges in birds. Philos. Trans. R. Soc. B 2014, 369, 20130040. [Google Scholar] [CrossRef]
- Fite, K.V.; Rosenfield-Wessels, S. A comparative study of deep avian foveas. Brain Behav. Evol. 1975, 12, 97–115. [Google Scholar] [CrossRef]
- Inzunza, O.; Bravo, H.; Smith, R.L.; Angel, M. Topography and morphology of retinal ganglion cells in Falconiforms: A study on predatory and carrion-eating birds. Anat. Rec. 1991, 229, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.S.; Partridge, J.C.; Cuthill, I.C.; Bennett, A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: The blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A 2000, 186, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Székely, T.; Powell, R.F.; Cuthill, I.C. Eye size, foraging methods and the timing of foraging in shorebirds. Funct. Ecol. 2006, 20, 157–165. [Google Scholar] [CrossRef]
- Potier, S.; Mitkus, M.; Bonadonna, F.; Duriez, O.; Isard, P.F.; Dulaurent, T.; Mentek, M.; Kelber, A. Eye size, fovea, and foraging ecology in Accipitriform raptors. Brain Behav. Evol. 2017, 90, 232–242. [Google Scholar] [CrossRef]
- Garamszegi, L.Z.; Møller, A.P.; Erritzøe, J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. Lond. B 2002, 269, 961–967. [Google Scholar] [CrossRef]
- Laughlin, S.B.; De Ruyter van Steveninck, R.R.; Anderson, J.C. The metabolic cost of neural information. Nat. Neurosci. 1998, 1, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nijhout, H.F.; Emlen, D.J. Competition among body parts in the development and evolution of insect morphology. Proc. Natl. Acad. Sci. USA 1998, 95, 3685–3689. [Google Scholar] [CrossRef]
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804. [Google Scholar] [CrossRef]
- Moran, D.; Softley, R.; Warrant, E.J. The energetic cost of vision and the evolution of eyeless Mexican cavefish. Sci. Adv. 2015, 1, e1500363. [Google Scholar] [CrossRef]
- Martin, G.R.; Katzir, G. Sun shades and eye size in birds. Brain Behav. Evol. 2000, 56, 340–344. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Tran, E. Changes in vigilance and foraging behaviour with light intensity and their effects on food intake and predator detection in house finches. Anim. Behav. 2007, 74, 1381–1390. [Google Scholar] [CrossRef]
- Dowling, J.E. The Retina: An Approachable Part of the Brain; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—From eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Howell, K.J.; Beston, S.M.; Stearns, S.; Walsh, M.R. Coordinated evolution of brain size, structure, and eye size in Trinidadian killifish. Ecol. Evol. 2021, 11, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.F. The scaling of eye size in adult birds: Relationship to brain, head and body sizes. Vis. Res. 2008, 48, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Wylie, D.R.; Gutiérrez-Ibáñez, C.; Iwaniuk, A.N. Integrating brain, behavior, and phylogeny to understand the evolution of sensory systems in birds. Front. Neurosci. 2015, 9, 281. [Google Scholar] [CrossRef]
- Møller, A.P.; Erritzøe, J. Flight distance and eye size in birds. Ethology 2010, 116, 458–465. [Google Scholar] [CrossRef]
- Potier, S.; Mitkus, M.; Kelber, A. Visual adaptations of diurnal and nocturnal raptors. Semin. Cell Dev. Biol. 2020, 106, 116–126. [Google Scholar] [CrossRef]
- Iwaniuk, A.N.; Nelson, J.E. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 2002, 80, 16–23. [Google Scholar] [CrossRef]
- Tobias, J.A.; Sheard, C.; Pigot, A.L.; Devenish, A.J.M.; Yang, J.; Sayol, F.; Neate-Clegg, M.H.C.; Alioravainen, N.; Weeks, T.L.; Barber, R.A.; et al. AVONET: Morphological, ecological and geographical data for all birds. Ecol. Lett. 2022, 25, 581–597. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; De Villemereuil, P. A general method for simultaneously accounting for phylogenetic and species sampling uncertainty via Rubin’s rules in comparative analysis. Syst. Biol. 2019, 68, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Nolazco, S.; Delhey, K.; Nakagawa, S.; Peters, A. Ornaments are equally informative in male and female birds. Nat. Commun. 2022, 13, 5917. [Google Scholar] [CrossRef]
- R Project for Statistical Computing. Available online: https://www.R--project.org/ (accessed on 1 December 2022).
- Brooke, M.D.L.; Hanley, S.; Laughlin, S.B. The scaling of eye size with body mass in birds. Proc. R. Soc. Lond. B 1999, 266, 405–412. [Google Scholar] [CrossRef]
- Freckleton, R.P.; Harvey, P.H.; Pagel, M. Phylogenetic analysis and comparative data: A test and review of evidence. Am. Nat. 2002, 160, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999, 48, 612–622. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Liao, W.B.; Jiang, Y.; Li, D.Y.; Jin, L.; Zhong, M.J.; Qi, Y.; Lüpold, S.; Kotrschal, A. Cognition contra camouflage: How the brain mediates predator–driven crypsis evolution. Sci. Adv. 2022, 8, eabq1878. [Google Scholar] [CrossRef]
- Chen, C.; Shao, W.J.; Zhu, X.; Yang, Y.J.; Jiang, Y.; Liao, W.B. Brain size predicts foraging and escaping abilities in the paddy frogs. Integr. Zool. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.R. R2s for correlated data: Phylogenetic models, LMMs, and GLMMs. Syst. Biol. 2019, 68, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.D. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Plummer, M.; Best, N.; Cowles, K.; Vines, K. CODA: Convergence diagnosis and output analysis for MCMC. R News 2006, 6, 7–11. [Google Scholar]
- Martin, P.R.; Bonier, F. Species interactions limit the occurrence of urban-adapted birds in cities. Proc. Natl. Acad. Sci. USA 2018, 115, E11495–E11504. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Huang, Z.S.; Huang, J.Q.; Zhang, C.G.; Meng, F.W. Phylogenetic analysis and expression differences of eye-related genes in cavefish genus Sinocyclocheilus. Integr. Zool. 2021, 16, 354–367. [Google Scholar] [CrossRef]
- Caves, E.M.; Sutton, T.T.; Johnsen, S. Visual acuity in ray-finned fishes correlates with eye size and habitat. J. Exp. Biol. 2017, 220, 1586–1596. [Google Scholar] [CrossRef]
- Borghi, C.E.; Giannoni, S.M.; Roig, V.G. Eye reduction in subterranean mammals and eye protective behavior in Ctenomys. Mastozool. Neotrop. 2002, 9, 123–134. [Google Scholar]
- Martin, G.R.; Wilson, K.J.; Wild, J.M.; Parsons, S.; Kubke, M.F.; Corfield, J. Kiwi forego vision in the guidance of their nocturnal activities. PLoS ONE 2007, 2, e198. [Google Scholar] [CrossRef]
- Eagderi, S.; Adriaens, D. Cephalic morphology of Pythonichthys macrurus (Heterenchelyidae: Anguilliformes): Specializations for head-first burrowing. J. Morphol. 2010, 271, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Yovanovich, C.A.M.; Pierotti, M.E.R.; Rodrigues, M.T.; Grant, T. A dune with a view: The eyes of a neotropical fossorial lizard. Front. Zool. 2019, 16, 17. [Google Scholar] [CrossRef]
- Hughes, A. The topography of vision in mammals of contrasting lifestyles: Comparative optics and retinal organization. In Handbook of Sensory Physiology; Cresitelli, F., Ed.; Springer: New York, NY, USA, 1977; pp. 613–756. [Google Scholar]
- Boire, D.; Dufour, J.S.; Theoret, H.; Ptito, M. Quantitative analysis of the retinal ganglion cell layer in the ostrich, Struthio camelus. Brain Behav. Evol. 2001, 58, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Juricic, E.; Moore, B.A.; Doppler, M.; Freeman, J.; Blackwell, B.F.; Lima, S.L.; DeVault, T.L. Testing the terrain hypothesis: Canada geese see their world laterally and obliquely. Brain Behav. Evol. 2011, 77, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Lisney, T.J.; Iwaniuk, A.N.; Bandet, M.V.; Wylie, D.W. Eye shape and retinal topography in owls (Aves: Strigiformes). Brain Behav. Evol. 2012, 79, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Lisney, T.J.; Iwaniuk, A.N.; Kolominsky, J.; Bandet, M.V.; Corfield, J.R.; Wylie, D.R. Interspecifc variation in eye shape and retinal topography in seven species of galliform bird (Aves: Galliformes: Phasianidae). J. Comp. Physiol. A 2012, 198, 717–731. [Google Scholar] [CrossRef]
- Collin, S.P. A web-based archive for topographic maps of retinal cell distribution in vertebrates. Clin. Exp. Optom. 2008, 91, 85–95. [Google Scholar] [CrossRef]
- Land, M.F. Visual acuity in insects. Annu. Rev. Entomol. 1997, 42, 147–177. [Google Scholar] [CrossRef]
- Litherland, L.; Collin, S.P. Comparative visual function in elasmobranchs: Spatial arrangement and ecological correlates of photoreceptor and ganglion cell distributions. Vis. Neurosci. 2008, 25, 549–561. [Google Scholar] [CrossRef]
- Veilleux, C.C.; Kirk, E.C. Visual acuity in mammals: Effects of eye size and ecology. Brain Behav. Evol. 2014, 83, 43–53. [Google Scholar] [CrossRef]
- Hunt, S.P.; Webster, K.E. The projection of the retina upon the optic tectum of the pigeon. J. Comp. Neurol. 1975, 162, 433–445. [Google Scholar] [CrossRef]
- Marín, G.; Letelier, J.C.; Henny, P.; Sentis, E.; Farfán, G.; Fredes, F.; Pohl, N.; Karten, H.; Mpodozis, J. Spatial organization of the pigeon tectorotundal pathway: An interdigitating topographic arrangement. J. Comp. Neurol. 2003, 458, 361–380. [Google Scholar] [CrossRef]
- Hellmann, B.; Güntürkün, O.; Manns, M. Tectal mosaic: Organization of the descending tectal projections in comparison to the ascending tectofugal pathway in the pigeon. J. Comp. Neurol. 2004, 472, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Corral-López, A.; Garate-Olaizola, M.; Buechel, S.D.; Kolm, N.; Kotrschal, A. On the role of body size, brain size, and eye size in visual acuity. Behav. Ecol. Sociobiol. 2017, 71, 179. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.; Rattenborg, N.C.; Ruf, T.; McWilliams, S.R.; Cardinale, M.; Fusani, L. Sleeping unsafely tucked in to conserve energy in a nocturnal migratory songbird. Curr. Biol. 2019, 29, 2766–2772. [Google Scholar] [CrossRef] [PubMed]
- Lank, D.B. Why fly by night? Inferences from tidally-induced migratory departures of sandpipers. J. Field Ornithol. 1989, 60, 154–161. [Google Scholar]
- Fusani, L.; Bertolucci, C.; Frigato, E.; Foà, A. Cryptochrome expression in the eye of migratory birds depends on their migratory status. J. Exp. Biol. 2014, 217, 918–923. [Google Scholar] [CrossRef]
- Kiltie, R.A. Scaling of visual acuity with body size in mammals and birds. Funct. Ecol. 2000, 14, 226–234. [Google Scholar] [CrossRef]
- Olsson, P.; Lind, O.; Mitkus, M.; Delhey, K.; Kelber, A. Lens and cornea limit UV vision of birds—A phylogenetic perspective. J. Exp. Biol. 2021, 224, jeb243129. [Google Scholar] [CrossRef]
- Fristoe, T.S.; Iwaniuk, A.N.; Botero, C.A. Big brains stabilize populations and facilitate colonization of variable habitats in birds. Nat. Ecol. Evol. 2017, 1, 1706–1715. [Google Scholar] [CrossRef]
- Garnett, S.T.; Duursma, D.E.; Ehmke, G.; Guay, P.; Stewart, A.; Szabo, J.K.; Weston, M.A.; Bennett, S.; Crowley, G.M.; Drynan, D.; et al. Biological, ecological, conservation and legal information for all species and subspecies of Australian bird. Sci. Data 2015, 2, 150061. [Google Scholar] [CrossRef]
- Ksepka, D.T.; Balanoff, A.M.; Smith, N.A.; Bever, G.S.; Bhullar, B.A.S.; Bourdon, E.; Braun, E.L.; Burleigh, J.G.; Clarke, J.A.; Colbert, M.W.; et al. Tempo and pattern of avian brain size evolution. Curr. Biol. 2020, 30, 2026–2036.e3. [Google Scholar] [CrossRef]
- Lindsay, W.R.; Houck, J.T.; Giuliano, C.E.; Day, L.B. Acrobatic courtship display coevolves with brain size in manakins (Pipridae). Brain Behav. Evol. 2015, 85, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Minias, P.; Podlaszczuk, P. Longevity is associated with relative brain size in birds. Ecol. Evol. 2017, 7, 3558–3566. [Google Scholar] [CrossRef]
- Sayol, F.; Downing, P.A.; Iwaniuk, A.N.; Maspons, J.; Sol, D. Predictable evolution towards larger brains in birds colonizing oceanic islands. Nat. Commun. 2018, 9, 2820. [Google Scholar] [CrossRef] [PubMed]
- Sayol, F.; Sol, D.; Pigot, A.L. Brain size and life history interact to predict urban tolerance in birds. Front. Ecol. Evol. 2020, 8, 58. [Google Scholar] [CrossRef]
- Sol, D.; Garcia, N.; Iwaniuk, A.; Davis, K.; Meade, A.; Boyle, W.A.; Székely, T. Evolutionary divergence in brain size between migratory and resident birds. PLoS ONE 2010, 5, e9617. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G. Flocking in birds is associated with diet, foraging substrate, timing of activity, and life history. Behav. Ecol. Sociobiol. 2022, 76, 74. [Google Scholar] [CrossRef]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. Birds of the World. Ithaca, NY: Cornell Laboratory of Ornithology. Available online: https://birdsoftheworld.org/bow/ (accessed on 29 September 2022).
- Hall, M.I.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C. Optic foramen morphology and activity pattern in birds. Anat. Rec. 2009, 292, 1827–1845. [Google Scholar] [CrossRef]
- Healy, K.; Guillerme, T.; Finlay, S.; Kane, A.; Kelly, S.B.A.; McClean, D.; Kelly, D.J.; Donohue, I.; Jackson, A.L.; Cooper, N. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B 2014, 281, 20140298. [Google Scholar] [CrossRef]
- Iwaniuk, A.N.; Keirnan, A.R.; Janetzki, H.; Mardon, K.; Murphy, S.; Leseberg, N.P.; Weisbecker, V. The endocast of the Night Parrot (Pezoporus occidentalis) reveals insights into its sensory ecology and the evolution of nocturnality in birds. Sci. Rep. 2020, 10, 9258. [Google Scholar] [CrossRef]
- La, V.T. Diurnal and nocturnal birds vocalize at night: A review. Condor 2012, 114, 245–257. [Google Scholar]
- Mikula, P.; Morelli, F.; Lučan, R.K.; Jones, D.N.; Tryjanowski, P. Predation of bats by diurnal birds. Mamm. Rev. 2016, 46, 160–174. [Google Scholar] [CrossRef]
- Zheng, K.D.; Liang, D.; Wang, X.W.; Han, Y.Q.; Griesser, M.; Liu, Y.; Fan, P.F. Contrasting coloured ventral wings are a visual collision avoidance signal in birds. Proc. R. Soc. B 2022, 289, 20220678. [Google Scholar] [CrossRef] [PubMed]


| Variables | Posterior Mean | Lower 95% CI | Upper 95% CI | Pmcmc |
|---|---|---|---|---|
| (Intercept) | −1.347 | −1.510 | −1.182 | <0.001 |
| Relative brain size | 0.342 | 0.227 | 0.455 | <0.001 |
| Habitat openness (dense habitat vs. open habitat) | −0.057 | −0.083 | −0.031 | <0.001 |
| Habitat openness (dense habitat vs. semi-open habitat) | −0.025 | −0.043 | −0.007 | 0.007 |
| Food type (animals vs. plants) | −0.076 | −0.108 | −0.045 | <0.001 |
| Food type (animals vs. omnivorous) | −0.037 | −0.064 | −0.010 | 0.009 |
| Body mass | 0.725 | 0.700 | 0.750 | <0.001 |
| Variables | Posterior Mean | Lower 95% CI | Upper 95% CI | Pmcmc |
|---|---|---|---|---|
| (Intercept) | 0.496 | 0.433 | 0.559 | <0.001 |
| Relative brain size | 0.155 | 0.114 | 0.195 | <0.001 |
| Habitat openness (dense habitat vs. open habitat) | −0.019 | −0.028 | −0.010 | <0.001 |
| Habitat openness (dense habitat vs. semiopen habitat) | −0.008 | −0.014 | −0.001 | 0.024 |
| Food type (animals vs. plants) | −0.028 | −0.039 | −0.016 | <0.001 |
| Food type (animals vs. omnivorous) | −0.014 | −0.024 | −0.003 | 0.009 |
| Food type (animals vs. carrion or refuse) | −0.064 | −0.120 | −0.008 | 0.031 |
| Activity pattern (diurnal vs. nocturnal) | 0.020 | −0.010 | 0.049 | 0.206 |
| Body mass | 0.236 | 0.227 | 0.245 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, Y.; Xu, J.; Liao, W. Evolution of Avian Eye Size Is Associated with Habitat Openness, Food Type and Brain Size. Animals 2023, 13, 1675. https://doi.org/10.3390/ani13101675
Liu Y, Jiang Y, Xu J, Liao W. Evolution of Avian Eye Size Is Associated with Habitat Openness, Food Type and Brain Size. Animals. 2023; 13(10):1675. https://doi.org/10.3390/ani13101675
Chicago/Turabian StyleLiu, Yating, Ying Jiang, Jiliang Xu, and Wenbo Liao. 2023. "Evolution of Avian Eye Size Is Associated with Habitat Openness, Food Type and Brain Size" Animals 13, no. 10: 1675. https://doi.org/10.3390/ani13101675
APA StyleLiu, Y., Jiang, Y., Xu, J., & Liao, W. (2023). Evolution of Avian Eye Size Is Associated with Habitat Openness, Food Type and Brain Size. Animals, 13(10), 1675. https://doi.org/10.3390/ani13101675






