A Simple Telemetry Sensor System for Monitoring Body Temperature in Rabbits—A Brief Report
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
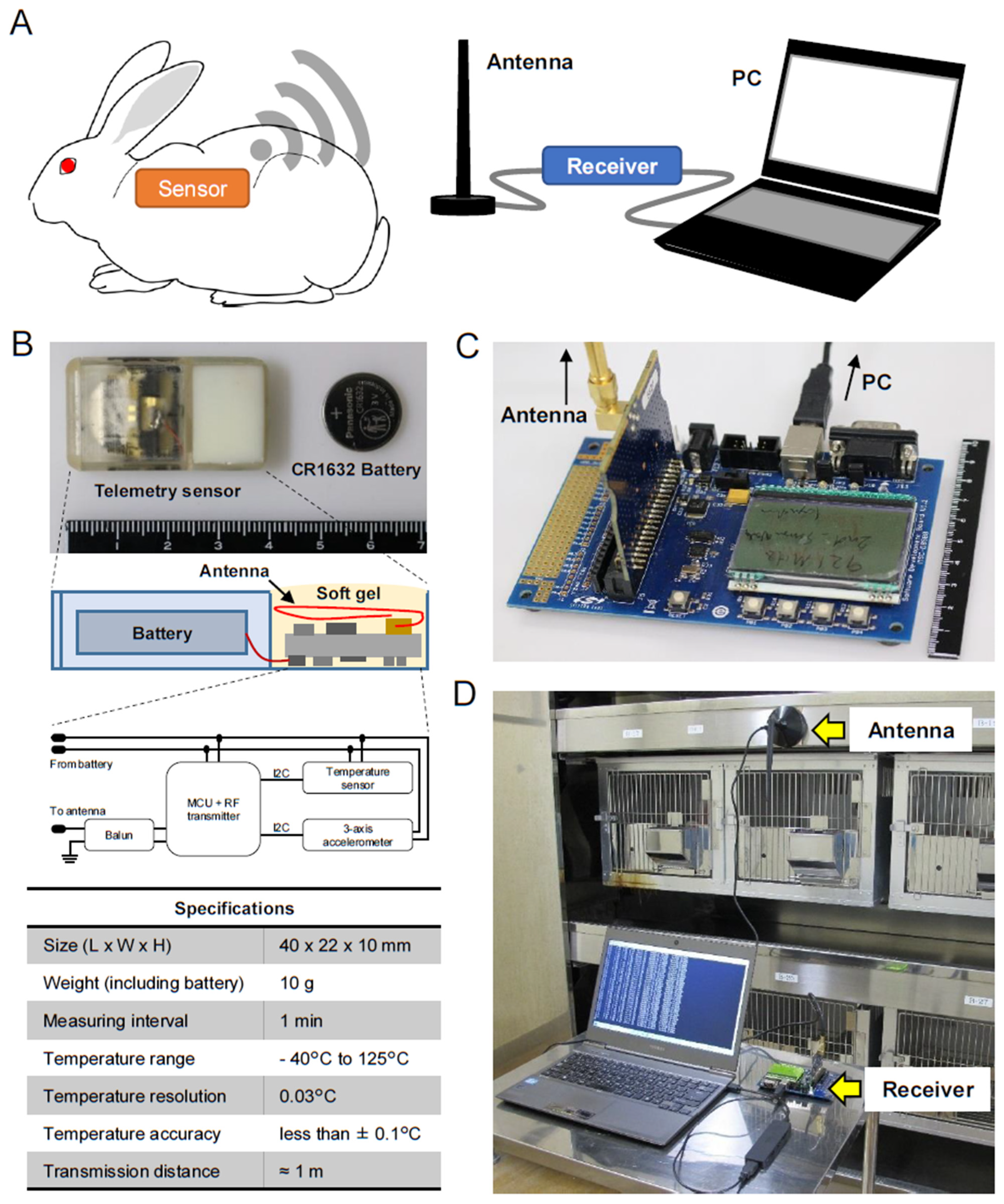
2.1. Telemetry Sensor System
2.2. Implantation of the Sensor
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kirschen, G.W.; Singer, D.D.; Thode, H.C., Jr.; Singer, A.J. Relationship between body temperature and heart rate in adults and children: A local and national study. Am. J. Emerg. Med. 2020, 38, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cao, J.; Niu, C.; Bao, M.; Xu, J.; Huo, D.; Liao, S.; Liu, W.; Speakman, J.R. Body temperature is a more important modulator of lifespan than metabolic rate in two small mammals. Nat. Metab. 2022, 4, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Riedel, N.; Grittner, U.; Endres, M.; Banneke, S.; Emmrich, J.V. Body temperature measurement in mice during acute illness: Implantable temperature transponder versus surface infrared thermometry. Sci. Rep. 2018, 8, 3526. [Google Scholar] [CrossRef]
- Hymczak, H.; Golab, A.; Mendrala, K.; Plicner, D.; Darocha, T.; Podsiadlo, P.; Hudziak, D.; Gocol, R.; Kosinski, S. Core Temperature Measurement-Principles of Correct Measurement, Problems, and Complications. Int. J. Environ. Res. Public Health 2021, 18, 10606. [Google Scholar] [CrossRef] [PubMed]
- Fulbrook, P. Core body temperature measurement: A comparison of axilla, tympanic membrane and pulmonary artery blood temperature. Intensive Crit. Care Nurs. 1997, 13, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Brunell, M.K. Comparison of noncontact infrared thermometry and 3 commercial subcutaneous temperature transponding microchips with rectal thermometry in rhesus macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 479–484. [Google Scholar]
- Wise, J. Rectal thermometer should be used for accurate temperature reading, analysis finds. BMJ 2015, 351, h6125. [Google Scholar] [CrossRef]
- Svantner, M.; Lang, V.; Skala, J.; Kohlschutter, T.; Honner, M.; Muzika, L.; Kosova, E. Statistical Study on Human Temperature Measurement by Infrared Thermography. Sensors 2022, 22, 8395. [Google Scholar] [CrossRef]
- Sarkar, S.; Misra, S. From Micro to Nano: The Evolution of Wireless Sensor-Based Health Care. IEEE Pulse 2016, 7, 21–25. [Google Scholar] [CrossRef]
- Meyer, C.W.; Ootsuka, Y.; Romanovsky, A.A. Body Temperature Measurements for Metabolic Phenotyping in Mice. Front. Physiol. 2017, 8, 520. [Google Scholar] [CrossRef]
- Niemeyer, J.E. Telemetry for small animal physiology. Lab Anim. 2016, 45, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Ito, T.; Ohwada, K.; Mera, Y.; Matsushita, M.; Tomoike, H. Hereditary postprandial hypertriglyceridemic rabbit exhibits insulin resistance and central obesity—A novel model of metabolic syndrome. Arterioscl. Throm. Vas. 2006, 26, 2752–2757. [Google Scholar] [CrossRef] [PubMed]
- Waqar, A.B.; Koike, T.; Yu, Y.; Inoue, T.; Aoki, T.; Liu, E.; Fan, J. High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis 2010, 213, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chu, Y.; Zhang, C.; Lin, Y.; Xu, K.; Yang, P.; Fan, J.; Liu, E. Diet-induced central obesity and insulin resistance in rabbits. J. Anim. Physiol. Anim. Nutr. 2008, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, E.; Diaz-Villasenor, A.; Diaz, G.; Salazar, A.M.; Montufar-Chaveznava, R.; Ostrosky-Wegman, P.; Caldelas, I. Misadjustment of diurnal expression of core temperature and locomotor activity in lactating rabbits associated with maternal over-nutrition before and during pregnancy. PLoS ONE 2020, 15, e0232400. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Zhang, D.; Matsumoto, S.; Hiroshima, H.; Maeda, R.; Sato, M.; Toyoda, A.; Gotoh, T.; Ohkohchi, N. Development of Implantable Wireless Sensor Nodes for Animal Husbandry and MedTech Innovation. Sensors 2018, 18, 979. [Google Scholar] [CrossRef]
- Kong, Y.; Guan, M.; Zheng, S.; Jiang, P.; Wang, L.; Yao, X.; Lu, J.; Xie, C.; Wang, F. Locating Hazardous Chemical Leakage Source Based on Cooperative Moving and Fixing Sensors. Sensors 2019, 19, 1092. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Maeda, R. Real-time tracking of organ-shape and vessel-locations for surgical navigation using MEMS tri-axis magnetic sensors. Med. Eng. Phys. 2021, 93, 42–48. [Google Scholar] [CrossRef]
- Varosi, S.M.; Brigmon, R.L.; Besch, E.L. A simplified telemetry system for monitoring body temperature in small animals. Lab. Anim. Sci. 1990, 40, 299–302. [Google Scholar]
- Di Girolamo, N.; Toth, G.; Selleri, P. Prognostic value of rectal temperature at hospital admission in client-owned rabbits. J. Am. Vet. Med. Assoc. 2016, 248, 288–297. [Google Scholar] [CrossRef]
- Ferrian, S.; Blas, E.; Larsen, T.; Sanchez, J.P.; Friggens, N.C.; Corpa, J.M.; Baselga, M.; Pascual, J.J. Comparison of immune response to lipopolysaccharide of rabbit does selected for litter size at weaning or founded for reproductive longevity. Res. Vet. Sci. 2013, 94, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.C.; Jan, B.; Stephane, B.; Antoine, G.; Nicolas, B. Performances assessment of Anipill® device prototype designed for continuous temperature monitoring. Biomed. Phys. Eng. Express 2018, 4, 055020. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Ruskoski, N.; van Saun, M.; Ying, G.S.; Oh, J.; Fraser, N.W. Weight loss and reduced body temperature determine humane endpoints in a mouse model of ocular herpesvirus infection. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 277–285. [Google Scholar]
- Guo, Y.; Wang, Q.J.; Zhang, K.H.; Yao, C.Y.; Huang, J.; Li, Q.; Liu, Z.Y.; Zhang, Y.; Shan, C.H.; Liu, P.; et al. Night-restricted feeding improves locomotor activity rhythm and modulates nutrient utilization to accelerate growth in rabbits. FASEB J. 2021, 35, e21166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Guo, Y.; Zhang, K.H.; Zhang, L.; Geng, S.X.; Shan, C.H.; Liu, P.; Zhu, M.Q.; Jin, Q.Y.; Liu, Z.Y.; et al. Night-Restricted Feeding Improves Gut Health by Synchronizing Microbe-Driven Serotonin Rhythm and Eating Activity-Driven Body Temperature Oscillations in Growing Rabbits. Front. Cell. Infect. Microbiol. 2021, 11, 771088. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Niimi, M.; Zhang, L.; Tang, X.; Lu, J.; Fan, J. A Simple Telemetry Sensor System for Monitoring Body Temperature in Rabbits—A Brief Report. Animals 2023, 13, 1677. https://doi.org/10.3390/ani13101677
Chen Y, Niimi M, Zhang L, Tang X, Lu J, Fan J. A Simple Telemetry Sensor System for Monitoring Body Temperature in Rabbits—A Brief Report. Animals. 2023; 13(10):1677. https://doi.org/10.3390/ani13101677
Chicago/Turabian StyleChen, Yajie, Manabu Niimi, Lan Zhang, Xiangming Tang, Jian Lu, and Jianglin Fan. 2023. "A Simple Telemetry Sensor System for Monitoring Body Temperature in Rabbits—A Brief Report" Animals 13, no. 10: 1677. https://doi.org/10.3390/ani13101677






