Correlation Analysis between Muskrat (Ondatra zibethicus) Musk and Traditional Musk
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling
2.3. Sample Preparation
2.4. GC–TOF–MS Analysis
2.5. Data Processing
3. Results
3.1. Identification and Quantification of Compounds by GC–TOF–MS
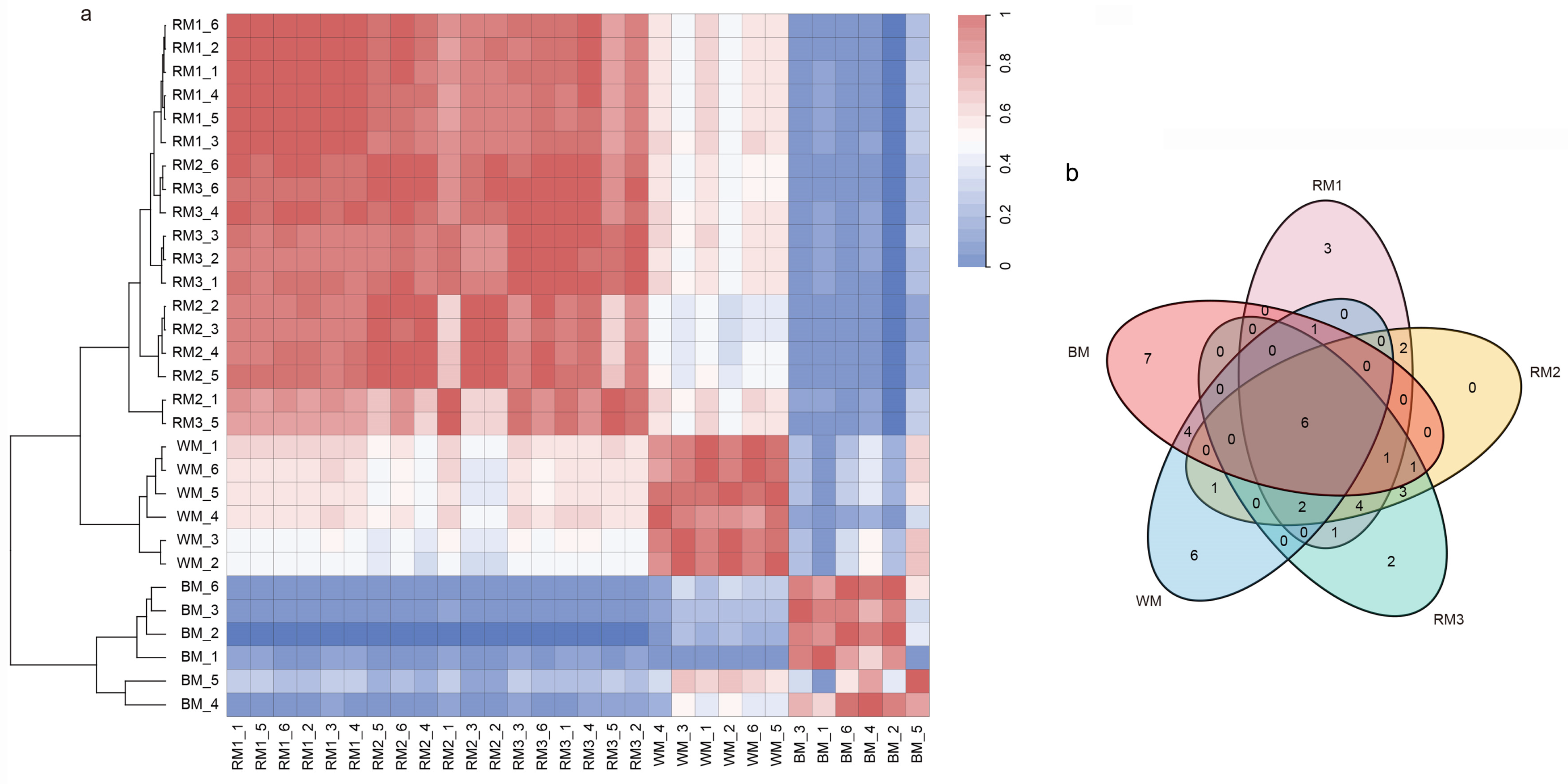
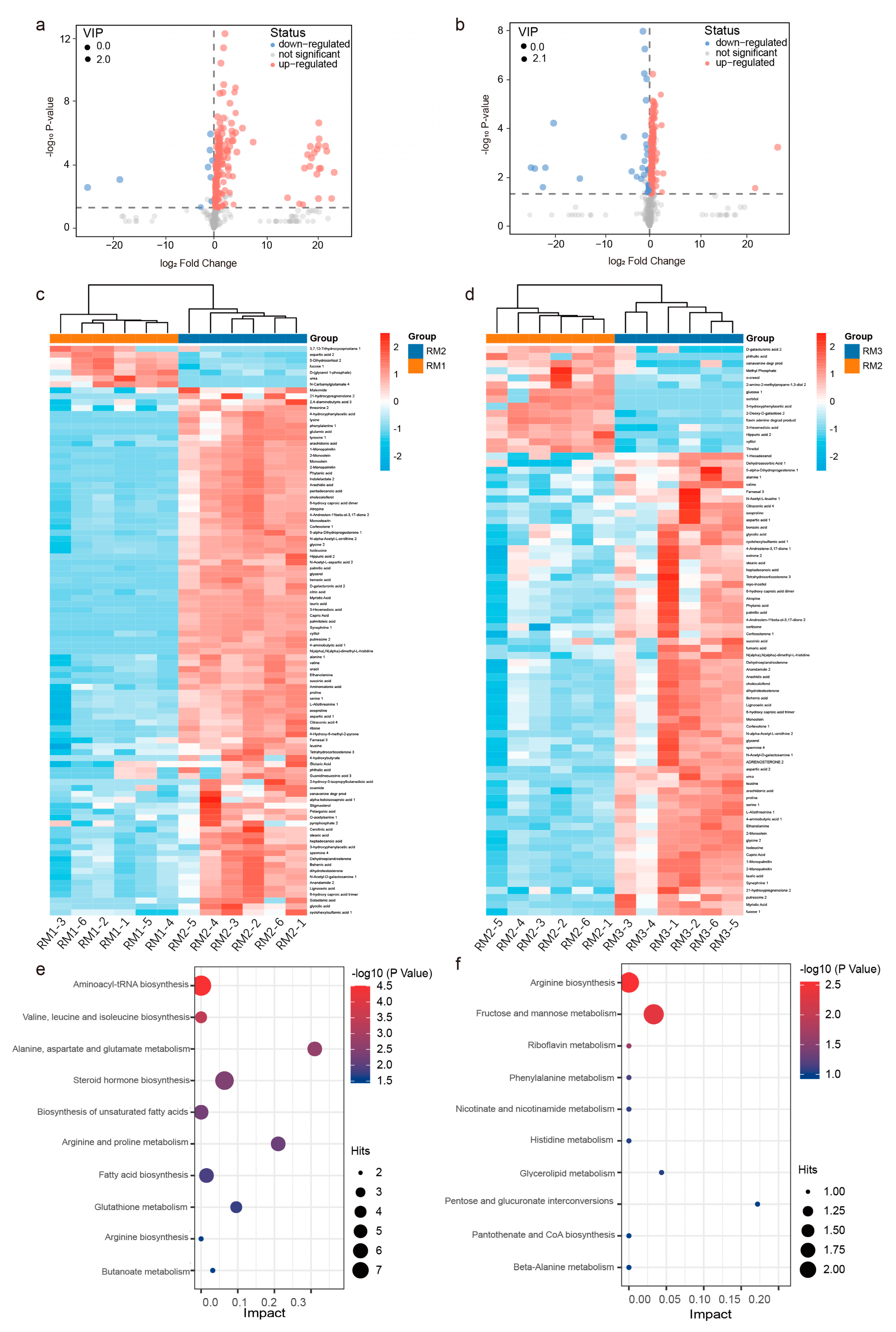
3.2. Multivariate Analysis of the Metabolomics Data
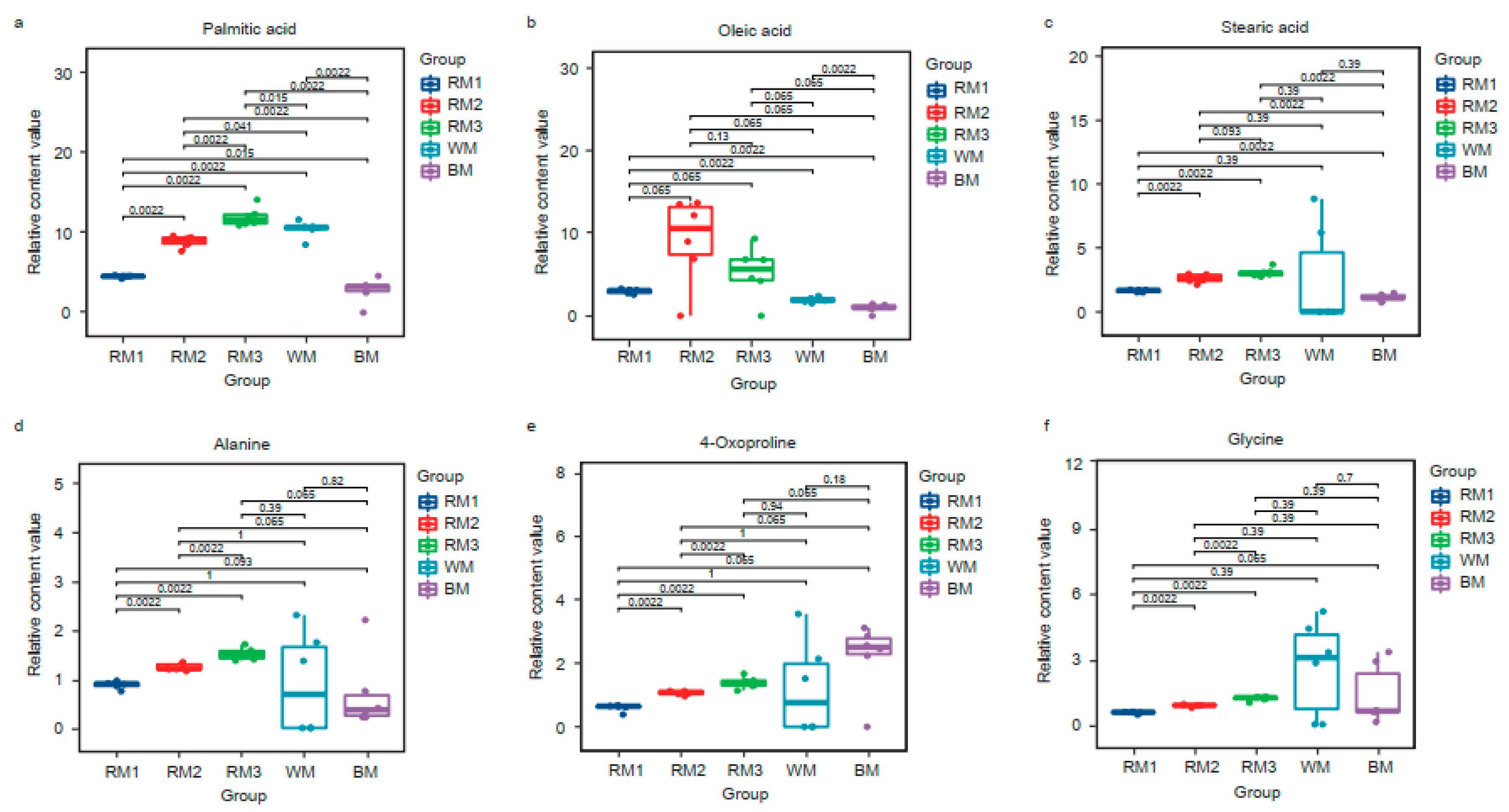
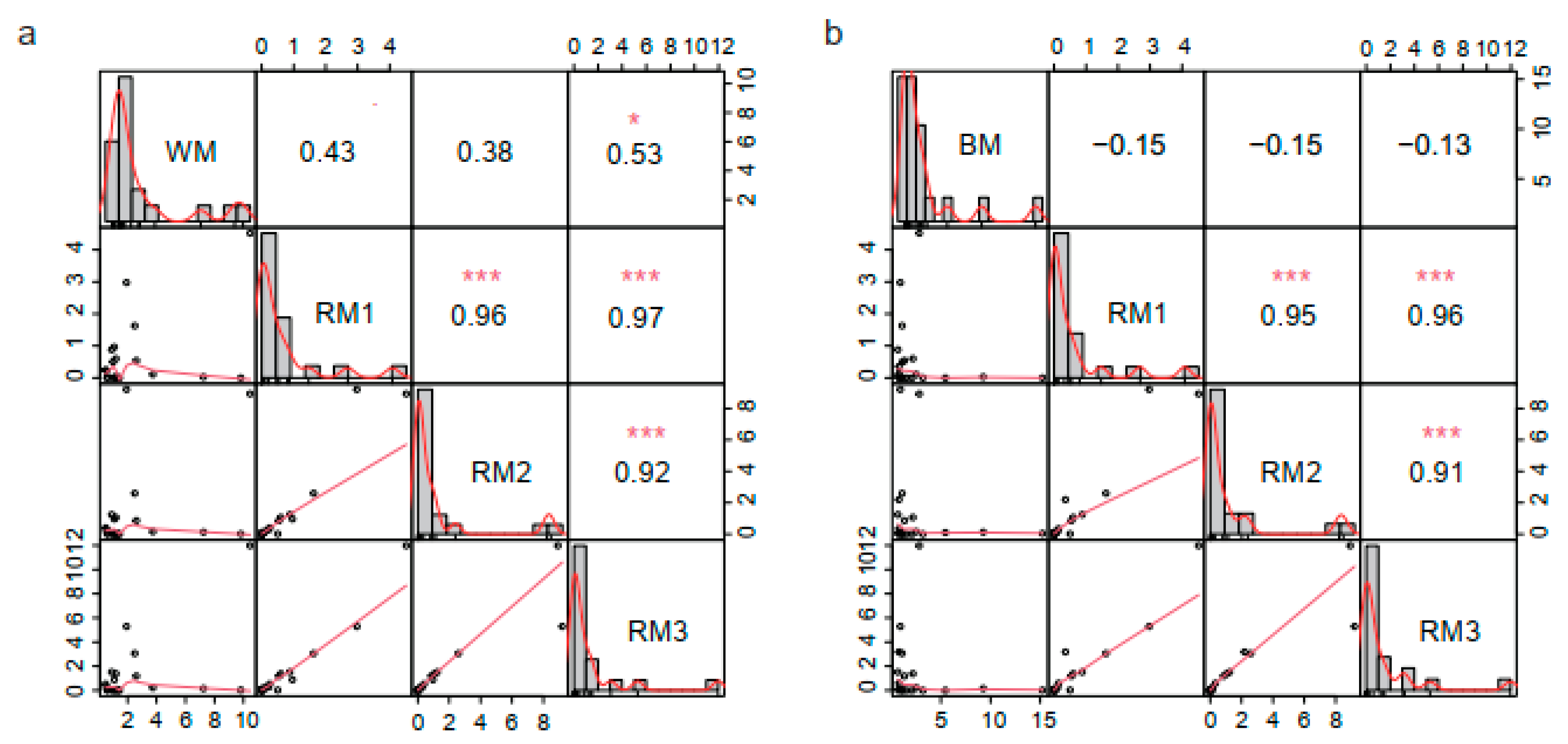
3.3. Correlation Analysis between Muskrat Musk and Musk
3.4. Metabolic Profiles of the Muskrats at Different Ages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Wang, N.; Zhang, Y.; Wang, Y.; Li, J.; Wu, Q.; Liu, Y. Neuroprotective effect of muscone on glutamate-induced apoptosis in PC12 cells via antioxidant and Ca2+ antagonism. Neurochem. Int. 2014, 70, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Chen, D.F.; Lai, X.P.; Liu, D.H.; Deng, R.D.; Zhou, J.H.; Zhang, S.X.; Li, Y.W.; Li, H.; Zhang, Q.D. Muscone exerts neuroprotection in an experimental model of stroke via inhibition of the fas pathway. Nat. Prod. Commun. 2012, 7, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; You, S.; Ding, X.; Luan, P.; Xu, J.; Cui, Q.; Wang, F.; Li, R.; Zhu, Y.; Zhang, J. Protective effect and underlying mechanism of muscone on acute cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 2023, 308, 116287. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhao, Y.; Yao, J.; Li, N.; Fang, T.; Wang, Y.; Meng, Z.; Liu, W. Proteomics on the role of muscone in the “consciousness-restoring resuscitation” effect of musk on ischemic stroke. J. Ethnopharmacol. 2022, 296, 115475. [Google Scholar] [CrossRef] [PubMed]
- Asada, R.; Kageyama, K.; Tanaka, H.; Saitoh, Y.; Miwa, N. Antitumor and anti-invasive effects of diverse musk-fragrant macrocyclic ketones and their enhancement by hyperthermia. Mol. Med. Rep. 2012, 5, 148–152. [Google Scholar] [PubMed]
- Li, Y.; Zhang, T.; Qi, L.; Yang, S.; Xu, S.; Cha, M.; Zhang, M.; Huang, Z.; Yu, J.; Hu, D.; et al. Microbiota Changes in the Musk Gland of Male Forest Musk Deer during Musk Maturation. Front. Microbiol. 2018, 9, 3048. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, W.; Yang, S.; Li, Y.; Zhang, M.; Shi, M.; Guo, X.; Li, D.; Zhang, B.; Liu, S.; et al. Study of compositions of musks in different types secreted by forest musk deer (Moschus berezovskii). PLoS ONE 2021, 16, e0245677. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Chang, F.; Wang, J.; Wang, Y.; Tang, J.; Zhang, K.; Gao, L.; Xue, X.; Wan, Y. The essential differences in microbial and chemical components of musk of different qualities secreted by captive male forest musk deer (Moschus berezovskii). Microb. Biotechnol. 2022, 15, 1783–1794. [Google Scholar] [CrossRef]
- Wang, Y.; Harris, R. Moschus berezovskii (errata version published in 2016). In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2015; p. e.T13894A103431781. [Google Scholar]
- Jiang, Z.; Jiang, J.; Wang, Y.; Zhang, E.; Zhang, Y.; Li, L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.; et al. Red List of China’s Vertebrates. Biodivers. Sci. 2016, 24, 500–551. [Google Scholar]
- Xie, W.; Tang, Z.; Xu, L.; Zhong, J.; Zhang, H.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal expressions of SF-1, StAR and P450scc in the scent glands of the muskrats (Ondatra zibethicus). J. Steroid Biochem. Mol. Biol. 2020, 204, 105766. [Google Scholar] [CrossRef]
- Li, H.; Shi, M.; Wang, Q.; Xia, T.; Sahu, S.K.; Zhang, Y.; Wang, J.; Li, T.; Ma, Y.; Liu, T.; et al. Chromosome-level Genome of the Muskrat (Ondatra zibethicus). Genome Biol. Evol. 2022, 14, evac138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Qiu, S.; Yu, H.; Tu, H.; Wen, Q.; George James, J.; Meng, Y.; Wu, Y.; Yang, N.; et al. Genomic evidence sheds light on the genetic mechanisms of musk secretion in muskrats. Int. J. Biol. Macromol. 2020, 145, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Fan, M.; Zhou, J.; Yang, S.; Zhang, M.; Qi, L.; Lin, S.; Hu, D.; Liu, S. Comparison of amino acid profiles and metabolic gene expression in muskrat scented glands in secretion and non-secretion season. Sci. Rep. 2017, 7, 41158. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, S.; Shi, M.; Zhang, S.; Zhang, T.; Li, Y.; Xu, S.; Cha, M.; Meng, Y.; Lin, S.; et al. Regulatory Roles of Peroxisomal Metabolic Pathways Involved in Musk Secretion in Muskrats. J. Membr. Biol. 2019, 252, 61–75. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.S.; Song, J.; Kim, H. Neuroprotective Effects of Musk of Muskrat on Transient Focal Cerebral Ischemia in Rats. Evid.-Based Complement. Altern. Med. eCAM 2019, 2019, 9817949. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, C.; Sun, F.; Zhang, L.; Song, F. Determination of chemical composition of muskrat musk. Chin. Pharm. J. 1994, 29, 396–397. [Google Scholar]
- Zhao, W.G.; Chen, Y.S.; Zhao, M.; Li, X.W.; Liu, D.Y. Study on the Comparison for the Muskrat Perfume Secretion of Fragrant Gland Cell Cultured In Vitro and that in Nature. Spec. Wild Econ. Anim. Plant Res. 2009, 4, 50–53. [Google Scholar]
- Li, D.; Chen, B.; Zhang, L.; Gaur, U.; Ma, T.; Jie, H.; Zhao, G.; Wu, N.; Xu, Z.; Xu, H.; et al. The musk chemical composition and microbiota of Chinese forest musk deer males. Sci. Rep. 2016, 6, 18975. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Wang, J.; Xing, H.; Qin, X.; Ren, Q.; Yang, J.; Li, L. Pharmacological effects and mechanisms of muscone. J. Ethnopharmacol. 2020, 262, 113120. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.L.; Chen, J.; Chen, Y.W.; Chai, Y.; Liu, Q.S.; Cheng, Y. Muscone with Attenuation of Neuroinflammation and Oxidative Stress Exerts Antidepressant-Like Effect in Mouse Model of Chronic Restraint Stress. Oxid. Med. Cell. Longev. 2022, 2022, 3322535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Yao, M.; Tian, Z.R.; Liu, S.F.; Song, Y.J.; Ye, J.; Li, G.; Sun, Y.L.; Cui, X.J.; Wang, Y.J. Muscone suppresses inflammatory responses and neuronal damage in a rat model of cervical spondylotic myelopathy by regulating Drp1-dependent mitochondrial fission. J. Neurochem. 2020, 155, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, Z.; Li, Z.; Li, J.; Li, Y. Muscone reduced the hypnotic and analgesic effect of ketamine in mice. J. Chin. Med. Assoc. JCMA 2020, 83, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Z.; Liang, D.; Yu, H.; Zheng, X.; Huang, Y. GC-MS was used to analyze the chemical composition differences between muskrat musk and musk. Res. Dev. Nat. Prod. 2021, 33, 1643–1648+1712. [Google Scholar]
- Van Dorp, D.A.; Klok, R.; Nugteren, D.H. New macrocyclic compounds from the secretions of the civetCat and the muskrat. Recl. Trav. Chim. Pays-Bas. 1973, 92, 915–928. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.; Kim, D.D.; Lee, S.; Lee, B.; Lee, Y.; Kim, S.; Kwon, S.; Noh, M.; Lee, M.O.; et al. Bioactive Lipids and Their Derivatives in Biomedical Applications. Biomol. Ther. 2021, 29, 465–482. [Google Scholar] [CrossRef]
- Mooli, R.G.R.; Ramakrishnan, S.K. Emerging Role of Hepatic Ketogenesis in Fatty Liver Disease. Front. Physiol. 2022, 13, 946474. [Google Scholar] [CrossRef]
- Adijanto, J.; Du, J.; Moffat, C.; Seifert, E.L.; Hurle, J.B.; Philp, N.J. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J. Biol. Chem. 2014, 289, 20570–20582. [Google Scholar] [CrossRef]
- Wohlt, J.E.; Clark, J.H.; Derrig, R.G.; Davis, C.L. Valine, leucine, and isoleucine metabolism by lactating bovine mammary tissue. J. Dairy Sci. 1977, 60, 1875–1882. [Google Scholar] [CrossRef]
- Trayhurn, P.; Van Heyningen, R. The metabolism of glutamate, aspartate and alanine in the bovine lens. Biochem. J. 1971, 124, 72–73. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxid. Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- Van den Beld, A.W.; Kaufman, J.M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; van der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef] [PubMed]

| No. | Chemical Name | CAS | Molecular Formula | RM1 | RM2 | RM3 | WM | BM |
|---|---|---|---|---|---|---|---|---|
| 1 | Palmitic acid | 57-10-3 | C16H32O2 | 4.5182 | 8.9303 | 11.9766 | 10.4605 | 2.8420 |
| 2 | Oleic acid | 112-80-1 | C18H34O2 | 2.9752 | 9.2118 | 5.2908 | 1.9322 | 0.9327 |
| 3 | Stearic acid | 57-11-4 | C18H36O2 | 1.6265 | 2.5967 | 3.0584 | 2.5001 | 1.1093 |
| 4 | 2-Hydroxypyridine | 142-08-5 | C5H5NO | 0.9613 | 0.9644 | 0.8798 | 1.0861 | 0.6629 |
| 5 | Alanine | 56-41-7 | C3H7NO2 | 0.8807 | 1.2418 | 1.5152 | 0.9043 | 0.6899 |
| 6 | Testosterone | 58-22-0 | C19H28O2 | 0.7483 | 0.2393 | 7.6711 × 10−9 | 9.8221 × 10−9 | 1.1148 × 10−8 |
| 7 | 4-Oxoproline | 2002-02-0 | C5H7NO3 | 0.6002 | 1.0628 | 1.3813 | 1.1985 | 2.1974 |
| 8 | Glycine | 56-40-6 | C2H5NO2 | 0.5406 | 0.8617 | 1.1840 | 2.6123 | 1.3521 |
| 9 | D-Arabitol | 488-82-4 | C5H12O5 | 0.5005 | 0.0125 | 0.0229 | 1.0005 | 1.1348 |
| 10 | Leucine | 61-90-5 | C6H13NO2 | 0.4116 | 0.5335 | 0.7207 | 0.1310 | 0.0668 |
| 11 | Glycerol | 56-81-5 | C3H8O3 | 0.3551 | 2.1961 | 3.1816 | 0.4295 | 0.7871 |
| 12 | D-Fucose | 2438-80-4 | C6H12O5 | 0.3512 | 0.2863 | 0.3478 | 0.2382 | 0.0343 |
| 13 | Glucose | 50-99-7 | C6H12O6 | 0.3226 | 0.2003 | 7.6712 × 10−9 | 9.8221 × 10−9 | 1.1148 × 10−8 |
| 14 | Valine | 72-18-4 | C5H11NO2 | 0.3089 | 0.5364 | 0.6613 | 0.4140 | 0.1031 |
| 15 | Canavanine | 543-38-4 | C5H12N4O3 | 0.2698 | 0.3700 | 0.1309 | 0.2788 | 0.4252 |
| 16 | Serine | 56-45-1 | C3H7NO3 | 0.2481 | 0.3993 | 0.5218 | 0.4868 | 0.1616 |
| 17 | 4-Androstene-3,17-dione | 63-05-8 | C19H26O2 | 0.2438 | 0.2540 | 0.2944 | 9.8221 × 10−9 | 1.1148 × 10−8 |
| 18 | Palmitoleic acid | 373-49-9 | C16H30O2 | 0.2320 | 2.9838 | 4.4162 | 0.0930 | 0.0528 |
| 19 | Isoleucine | 73-32-5 | C6H13NO2 | 0.2223 | 0.3772 | 0.5495 | 0.0727 | 0.0530 |
| 20 | Urea | 57-13-6 | CH4N2O | 0.2188 | 7.9282 × 10−9 | 0.8148 | 0.0423 | 0.0386 |
| 21 | 2,4-Diaminobutyric acid | 305-62-4 | C4H10N2O2 | 0.2176 | 0.2830 | 0.2657 | 0.5681 | 1.1148 × 10−8 |
| 22 | Proline | 147-85-3 | C5H9NO2 | 0.1821 | 0.2990 | 0.4460 | 0.3287 | 1.1148 × 10−8 |
| 23 | Atropine | 51-55-8 | C17H23NO3 | 0.1617 | 0.2891 | 0.4272 | 9.8221 × 10−9 | 0.0071 |
| 24 | 6-Hydroxy caproic acid trimer | 1191-25-9 | C6H12O3 | 0.1520 | 0.2782 | 0.4126 | 0.3575 | 0.1047 |
| 25 | Arachidic acid | 506-30-9 | C20H40O2 | 0.1407 | 0.3384 | 0.5872 | 0.2763 | 0.7918 |
| 26 | Behenic acid | 112-85-6 | C22H44O2 | 0.1088 | 0.1580 | 0.2217 | 3.7522 | 2.5273 |
| 27 | Monoolein | 111-03-5 | C21H40O4 | 0.0475 | 0.4066 | 0.6111 | 0.0028 | 0.0362 |
| 28 | Lignoceric acid | 557-59-5 | C24H48O2 | 0.0391 | 0.0642 | 0.0950 | 1.1968 | 0.7426 |
| 29 | Succinic acid | 110-15-6 | C4H6O4 | 0.0291 | 0.1264 | 0.1512 | 7.2419 | 9.2730 |
| 30 | 1-Monopalmitin | 542-44-9 | C19H38O4 | 0.0123 | 0.2774 | 0.6080 | 0.0465 | 1.1148 × 10−8 |
| 31 | 4-Hydroxyphenylacetic acid | 156-38-7 | C8H8O3 | 0.0084 | 0.0599 | 0.0637 | 0.5719 | 1.0516 |
| 32 | Carbobenzyloxy-L-leucine degr | 2018-66-8 | C14H19NO4 | 8.1136 × 10−9 | 0.0425 | 0.0216 | 0.0434 | 15.2327 |
| 33 | Hippuric acid | 495-69-2 | C9H9NO3 | 8.1136 × 10−9 | 0.0989 | 0.0024 | 9.8221 × 10−9 | 5.4626 |
| 34 | Threo-beta-hyrdoxyaspartate | 7298-99-9 | C4H7NO5 | 8.1136 × 10−9 | 0.0009 | 7.6712 × 10−9 | 0.0021 | 3.2056 |
| 35 | Hypoxanthine | 68-94-0 | C5H4N4O | 8.1136 × 10−9 | 7.9282 × 10−9 | 0.0019 | 9.8221 × 10−9 | 2.1271 |
| 36 | Methyl trans-cinnamate | 103-26-4 | C10H10O2 | 8.1136 × 10−9 | 7.9282 × 10−9 | 0.0005 | 9.8221 × 10−9 | 1.6237 |
| 37 | Biuret | 108-19-0 | C2H5N3O2 | 8.1136 × 10−9 | 7.9282 × 10−9 | 7.6712 × 10−9 | 9.8221 × 10−9 | 1.2075 |
| 38 | 1-Octanal | 124-13-0 | C8H16O | 8.1136 × 10−9 | 7.9282 × 10−9 | 7.6712 × 10−9 | 9.8221 × 10−9 | 0.7773 |
| 39 | Thymidine | 50-89-5 | C10H14N2O5 | 8.1136 × 10−9 | 7.9282 × 10−9 | 7.6712 × 10−9 | 1.3371 | 0.5811 |
| 40 | 1-Hexadecanol | 36653-82-4 | C16H34O | 8.1136 × 10−9 | 0.0127 | 0.0329 | 9.8164 | 0.3257 |
| 41 | 5-Aminovaleric acid | 660-88-8 | C5H11NO2 | 8.1136 × 10−9 | 7.9282 × 10−9 | 7.6712 × 10−9 | 1.4447 | 1.1148 × 10−8 |
| 42 | Putrescine | 110-60-1 | C4H12N2 | 8.1136 × 10−9 | 0.0039 | 0.0087 | 1.2105 | 1.1148 × 10−8 |
| 43 | Octadecanol | 112-92-5 | C18H38O | 8.1136 × 10−9 | 7.9282 × 10−9 | 7.6712 × 10−9 | 1.0077 | 1.1148 × 10−8 |
| 44 | Glucoheptonic acid | 23351-51-1 | C7H14O8 | 8.1136 × 10−9 | 7.9282 × 10−9 | 7.6712 × 10−9 | 0.6318 | 1.1148 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zeng, D.; Zhao, G.; Zhang, C.; Feng, X.; Zheng, C.; Li, D.; Zhang, M.; Jie, H. Correlation Analysis between Muskrat (Ondatra zibethicus) Musk and Traditional Musk. Animals 2023, 13, 1678. https://doi.org/10.3390/ani13101678
Shi X, Zeng D, Zhao G, Zhang C, Feng X, Zheng C, Li D, Zhang M, Jie H. Correlation Analysis between Muskrat (Ondatra zibethicus) Musk and Traditional Musk. Animals. 2023; 13(10):1678. https://doi.org/10.3390/ani13101678
Chicago/Turabian StyleShi, Xin, Dejun Zeng, Guijun Zhao, Chenglu Zhang, Xiaolan Feng, Chengli Zheng, Diyan Li, Ming Zhang, and Hang Jie. 2023. "Correlation Analysis between Muskrat (Ondatra zibethicus) Musk and Traditional Musk" Animals 13, no. 10: 1678. https://doi.org/10.3390/ani13101678





