Simple Summary
Pelteobagrus fulvidraco is a common freshwater fish, mainly distributed in China, Vietnam, Laos, and southeast Siberia of Russia. Its meat is delicious and has high nutritional value. The purpose of the study was to explore the suitable farming density of P. fulvidraco in integrated rice–fish farming. Our study revealed that the stocking density of P. fulvidraco in a paddy field should not exceed 250 g/m2. High stocking density inhibited growth performance and caused physiological response. These insights offer guidance for the selection of an appropriate stocking density for P. fulvidraco in integrated rice–fish farming systems.
Abstract
Pelteobagrus fulvidraco is a freshwater fish commonly raised in rice fields, yet the optimal stocking density for this species remains unknown. Therefore, this study aimed to investigate the appropriate stocking density of P. fulvidraco in integrated rice–fish farming systems. Three different stocking densities––low density (LD, 125 g/m2), middle density (MD, 187.5 g/m2), and high density (HD, 250 g/m2)––were set up to evaluate P. fulvidraco’s growth performance, stress indices, immune function, antioxidant status, and lipid metabolism after 90 days of farming. The results indicated that HD treatment had a detrimental effect on P. fulvidraco’s growth parameters. HD treatment led to an increase in cortisol (Cor) and lactate (La) levels, but a decrease in glucose (Glu) content in serum. After 90 days of farming, an immune response accompanied by the increase of complement 3 (C3), C4, and immunoglobulin M (IgM) was observed in the HD group. Meanwhile, HD treatment induced oxidative stress and altered antioxidative status evidenced by the levels of catalase (CAT), glutathione peroxidase (Gpx), glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC) in serum or liver. Additionally, the lipid metabolism-related genes including lipoprotein lipase (lpl), peroxisome proliferators-activated receptor (pparα), carnitine palmitoyltransferase-1 (cpt-1), and sterol regulatory element binding protein-1 (srebp-1) were markedly downregulated in the HD and/or MD group after 90 days of farming. In conclusion, this study contributes to a better understanding of P. fulvidraco’s response to different stocking densities in integrated rice–fish farming systems. We suggest that the appropriate stocking density for P. fulvidraco in these farming systems should be below 250 g/m2, considering both fish growth and physiological responses.
1. Introduction
Aquaculture is an increasingly important source of protein and plays a vital role in global food security [1]. In 2021, China’s aquaculture yield reached 53.94 million tons, accounting for approximately 60% of the world’s yield [2]. Various aquaculture models are used in China, including pond farming, cage farming, recirculating aquaculture, and integrated rice–fish farming [3]. To ensure sustainable aquaculture, it is necessary to improve the utilization rate of water and land resources as well as to reduce the environmental pollution [4]. Integrated rice–fish farming is an efficient ecological model that is in line with the development of green ecological agriculture, with significant economic, ecological, and social benefits [5,6]. This farming model greatly reduces environmental impacts and improves resource utilization [7,8]. In the system, fish feces improve soil fertility, which reduces the need for fertilizers [9], while plankton in rice fields provides a rich diet for fish [10]. Fish farming also helps to control weeds, pests, and diseases in rice fields [11]. Integrated rice–fish farming has been successfully practiced with species such as common carp (Cyprinus carpio) [12], largemouth bass (Micropterus salmoides) [13], tilapia (Oreochromis niloticus) [14], crayfish (Pmcambarus clarkii) [15], and Chinese mitten crab (Eriocheir sinensis) [16]. It has become a modern agricultural production model that utilizes an ecological cycle and achieves high quality and efficiency [10].
Stocking density is a pivotal factor affecting growth performance, physiological function, behavior, and welfare in aquatic animals [17]. In aquaculture practice, in order to increase productivity and profits, aquafarms often resort to increasing stocking density. However, an excessively high stocking density may result in negative consequences and decrease economic benefits from aquaculture [18]. A previous study has shown that high stocking densities (10.92 g/m2) can lead to decreased survival rate, weight, and specific growth rate of weight in O. niloticus (18.2 ± 0.17 g, 4 months) in rice–fish farming systems [19]. A similar result was found for GIFT (genetically improved farmed tilapia) tilapia (Oreochromis niloticus) (24.18 g/m2, 12.09 g, 92 d) culturing in rice–fish systems [20]. Additionally, the growth and survival rates of carp (Cyprinus carpio) (74.96 g/m2, 100 g, 90 d) [21] and M. salmoides (120 g/m3, 40.63 ± 0.13 g, 90 d) [22] were negatively impacted under high stocking density culturing in rice–fish systems. Under high stocking density, increased competition for food and living space can also cause significant physiological stress [23], which requires more energy to maintain physiological balance and resist stress reaction [24]. Reports suggest that high stocking density causes oxidative stress that leads to malondialdehyde (MDA) content increased in aquatic animals like crayfish (Cherax quadricarinatus) (107.19 g/m2, 14.29 ± 1.05 g, 90 d) culturing in aquariums [25]. Moreover, it has been observed that high stocking density can reduce the immunity of aquatic species, leading to higher rates of disease infection [26,27]. Therefore, selection of the appropriate stocking density for aquatic animals is crucial in aquaculture operations.
The yellow catfish (P. fulvidraco) is one of the most important cultivated freshwater fish species in China, with a production of 565,477 and 587,822 tons in 2020 [28] and 2021 [29], respectively. Previous studies on P. fulvidraco were limited to cage culture [30], recirculating aquaculture systems [31], and ponds [32], which revealed varying stocking densities for optimal growth. For example, the appropriate stocking density is 216–270 g/m3 (15–20 g/individual) in ponds [33,34], 2–3.5 kg/m2 (20–50 g/individual) in cages [35,36], and 1 kg/m3 (25 g/individual) in recirculating aquaculture systems [37]. Recently, researchers have compared growth performance and muscular quality of P. fulvidraco in ponds and paddy fields [38]. However, the suitable stocking density of P. fulvidraco in the integrated rice–fish farming system is still unclear. Therefore, this study aims to explore the suitable stocking density for P. fulvidraco in the integrated rice–fish farming system via comparing the differences of growth performance, physiological function, antioxidant status, and lipid metabolism. The results will provide crucial reference for determining stocking density of P. fulvidraco in rice field cultivation.
2. Materials and Methods
2.1. Fish and Experimental Design
The P. fulvidraco used in the study was provided by the farm of Freshwater Fisheries Research Center (Jingjiang, China). All fish were in good health and had an average body weight of 145.3 ± 5.19 g. The experiment was conducted at the same farm. According to the previous studies on the farming practice of Pelteobagrus fulvidraco in the integrated rice-fish farming systems in China [39,40], three stocking densities of P. fulvidraco were set: low density (LD, 125 g/m2, 344 individuals), middle density (MD, 187.5 g/m2, 516 individuals) and high density (HD, 250 g/m2, 688 individuals), with three repetitions per group. In the experiment, each replicate group consists of two zones, including a rice field (340 m2) and a canal refuge (1.2 m in depth, 60 m2). In the paddy, rice (Nangeng 5055) was planted at the start of July 2021, and harvested at the start of November. P. fulvidraco was stocked at the end of July 2021 and harvested at the end of October 2021. We added suitable water into the system to keep the stability of water volume when it decreased due to evaporation. In canal refuge, the aerator was installed to maintain the water oxygen level. During the experiment, commercial feed (HAID Group Co., Ltd., Guangzhou, China) was fed once daily, with the amount approximately 1% of their weight. The main nutrient composition of the diet included 42.0% crude protein, 5.0% crude fat, 3.0% crude fiber, 16.0% crude ash, and 0.80% total phosphorus. During the trial, the water’s dissolved oxygen was 4.4–5.0 mg/L, the pH was 7.41–8.05, and the temperature was 22.3–33.5 °C.
2.2. Samples Collection
For water quality analysis, we collected a water sample every 30 days according to the five-point sampling method [41]. Each repetition collected five samples. To evaluate the growth performance of P. fulvidraco, 90 fish in each group were caught randomly every 30 days to measure weight and body length.
At the end of the experiment, 36 fish were randomly caught from each group and anesthetized with 50 mg/L MS-222 (Fujian Shengyuan Aquatic Products Co., Ltd., Ningde, China). The blood was collected via caudal vein and centrifuged at 3600 r/min for 10 min to obtain serum. After blood sampling, the liver tissue was collected for biochemical analysis and gene expression. The blood and liver tissues from 4 fish were mixed to form one sample. All sample were stored at −80 °C. The liver was taken into a 1.5 mL centrifuge tube (0.1 g of the liver was added to 0.9 mL 0.86% physiological saline) to grind. Then, the homogenate was centrifuged at 3000 g for 10 min. Finally, the supernatant was collected to assay antioxidant parameters. The study was authorized by the Freshwater Fisheries Research Center (FFRC) of the Chinese Academy of Fishery Sciences (CAFS).
2.3. Water Quality Analysis
Total phosphorus (TP) was detected according to the ammonium molybdate spectrophotometric method [42]. Total nitrogen (TN) was tested according to the alkaline potassium persulfate digestion-UV spectrophotometry [43]. The Nessler’s reagent colorimetric method was selected for the determination of ammonium (NH4+-N) [44]. The spectrophotometric method was used to measure nitrite (NO2−-N) [45]. Nitrate (NO3−-N) was determined via the spectrophotometric method with phenol disulfonic acid [46].
2.4. Growth Parameters Analysis
The growth performance was assessed by analyzing weight gain (WG, %), specific growth rate of weight (SGR, %), condition factor (CF, g/cm3), and survival rate (SR, %). The specific calculation formulas are as follows:
where, W1 and W2 are initial body weight (g) and final body weight (g), L2 is the final body length (cm), and T is the total trial days (d).
WG = 100 × (W2 − W1)/W1
SGR = 100 × (lnW2 − lnW1)/T
CF = 100 × W2/L23
SR = 100 × final number/initial number
2.5. Serum Physiological Parameters Analysis
Serum physiological parameters, including total cholesterol (TC), triglyceride (TG), glucose (Glu), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c), were determined using automatic biochemical analyzer (BS-400, Mindray Biomedical, Shenzhen, China). The lactate (La) was measured by a commercial reagent kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Cortisol (Cor), heat shock protein 70 (HSP70), complement 3 (C3), complement 4 (C4), lysozyme (LZM), and immunoglobulin M (IgM) were determined using enzyme linked immunosorbent assay (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). The determination of all parameters was conducted according to the method described by the manufacturers.
2.6. Antioxidant Status Analysis
In order to assess the impact of stocking density on the antioxidant status of P. fulvidraco, a range of parameters were measured using commercially available kits. Specifically, the levels of catalase (CAT), glutathione peroxidase (Gpx), glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and total protein were measured in both the serum and liver of the fish. Commercial kits were used for each assay, including Beyotime Biotechnology (Shanghai, China) for CAT (References: S0051) and MDA (References: S0051) [47,48], Grace Biotechnology Co., Ltd. (Suzhou, China) for Gpx (References: S0051) [49], and Nanjing Jiancheng Bioengineering Institute (Nanjing, China) for GSH (References: S0051), SOD (References: A001-3), T-AOC (References: A015-3), and total protein (References: A045-4) [13,50]. The determination of all parameters was conducted according to the method described by the manufacturers.
2.7. Gene Expression Analysis
According to the manufacturer’s instructions, Trizol reagent (Takara Biomedical Technology Co., Ltd., Beijing, China) was used to extract total RNA from liver. The RNA purity and integrity were evaluated by calculating the ratio of OD260/OD280 (1.7–2.1) and detecting gel electrophoresis. The total RNA was then reverse transcribed into cDNA using a commercial kit (Takara, RR047A). Firstly, 1 μg total RNA was mixed into 2 μL 5× gDNA Eraser Buffer, 1 μL gDNA Eraser and appropriate amount RNase Free ddH2O to remove genome DNA under 42 °C 2 min (total volume 10 μL). Then, the mixture was mixed into 1 μL PrimeScript RT Enzyme Mix I, 1 μL RT Primer Mix, 4 μL 5× PrimeScript Buffer, and 4 μL RNase Free dH2O to perform reverse transcription using PCR system (ABI, Foster, CA, USA). The reverse transcription procedure consisted of the following parts: 37 °C for 15 min, and 85 °C for 5 s.
To detect the expression of the target gene, a commercial kit (Takara, RR820A) was used for quantitative real-time PCR (qPCR) amplification on a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The CFX Maestro software was used for data collection, analysis, and visualization. The PCR reaction solution consisted of 2 μL cDNA, 12.5 μL TB Green Premix Ex Taq II, 1 μL PCR forward and reverse primers (10 μM), and 8.5 μL RNase Free ddH2O. The PCR thermal cycling conditions were as follows: an initial denaturation step of 95 °C for 30 s, followed by 40 cycles of denaturation (95 °C, 5 s), and annealing and extension (60 °C, 30 s), followed by melt curve (65–95 °C) at the end of the last cycle. The specific primers used in qPCR are shown in Table 1.
The mathematical model Bestkeeper [51] was used to compare the stability of reference genes (β-actin, gapdh and 18s rRNA) [52,53,54]. According to Ct values (Table S2), we calculated the correlation coefficient (r), standard deviation (SD), and coefficient of variation (CV) of the reference genes under different groups. A reference gene with high r value and low SD and CV values is considered to be more stable. Moreover, when SD > 1, it indicates that the reference gene is unstable. The analysis results showed that β-actin is more stable compared with 18s rRNA and gapdh under different densities (Table S3). Therefore, the β-actin was chosen as a reference gene, and the relative expression levels of the target gene were calculated by 2−ΔΔCt method [55].

Table 1.
Gene-specific qPCR primers.
Table 1.
Gene-specific qPCR primers.
| Gene | F: Primer Sequence (5′-3′) | R: Primer Sequence (5′-3′) |
|---|---|---|
| β-actin [52] | GTACCACCATGTACCCTGGC | GTGCCTTTCATTCAGCCACC |
| lpl [56] | GACCAGAGAGATGATGCCGT | TAGCTTAGCTGGCTCTTGCTG |
| pparα [57] | CGAGGATGGGATGCTGGTG | CGTCTGGGTGGTTCGTCTGC |
| cpt-1 [57] | ATTTGAAGAAGCACCCAGAGTATGT | CCCTTTTATGGACGGAGACAGA |
| srebp-1 [57] | CTGGGTCATCGCTTCTTTGTG | TCCTTCGTTGGAGCTTTTGTCT |
| gapdh [53] | CACTGCCACCCAGAAGACA | AGGGACACGGAAAGCCAT |
| 18s rRNA [54] | CCTGAGAAACGGCTACCACATCC | AGCAACTTTAATATACGCTATTGGAG |
2.8. Statistical Analysis
The data were analyzed using SPSS 25.0, and the results were expressed as mean ± standard deviation (SD). The Shapiro–Wilk test and Levene test were used to evaluate the normal distribution and variance homogeneity of experimental data, respectively. The difference among different stocking densities was analyzed by one-way ANOVA with LSD post hoc test. It was considered statistically significant if the p-value < 0.05.
3. Results
3.1. Change of Water Quality Parameters
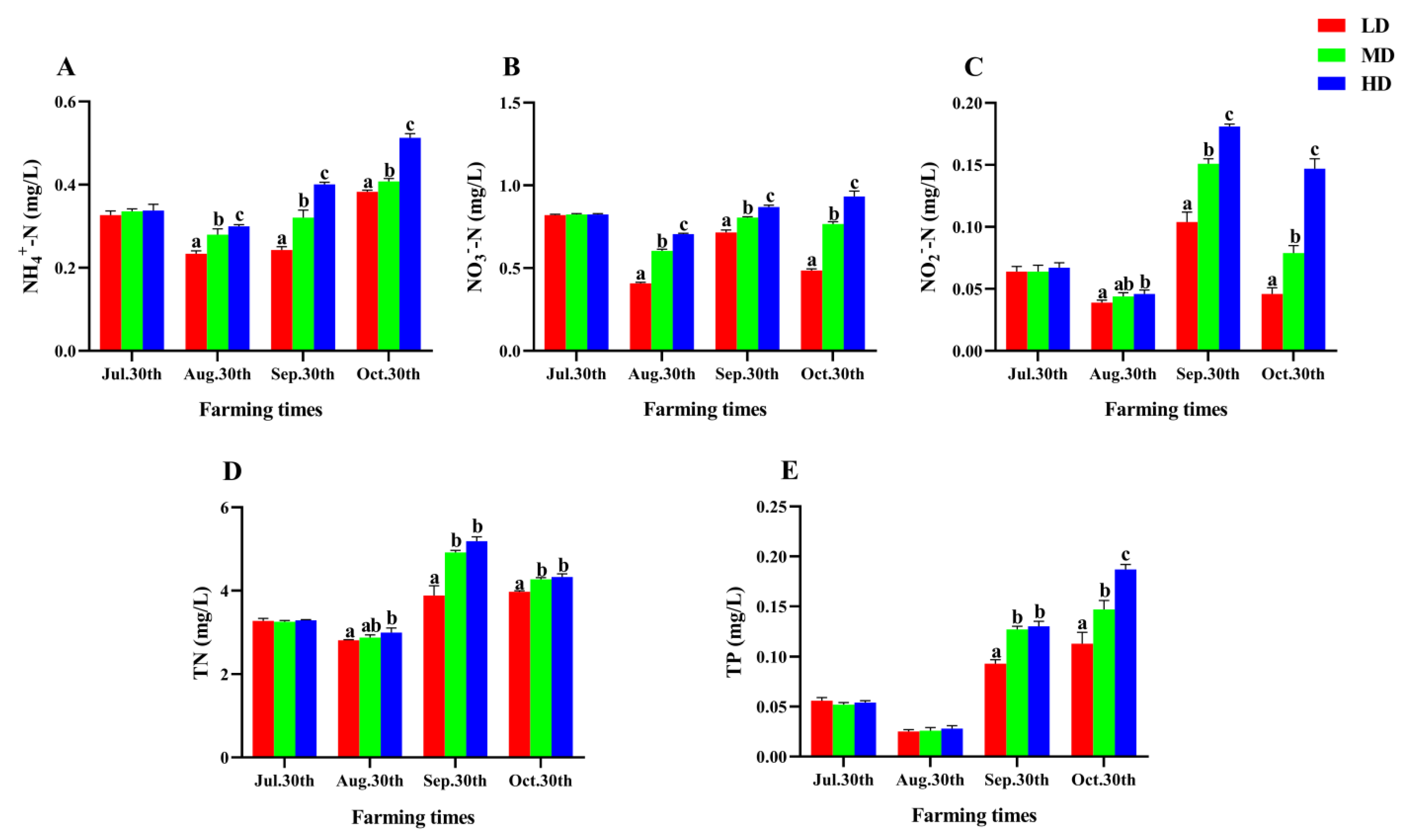
The change of water quality parameters in each group was shown in Figure 1. Throughout the experiment, the levels of NH4+-N, NO3−-N, NO2−-N, TN, and TP exhibited varying changes from 30 July to 30 October. The contents of NO2−-N and TN reached the maximum on 30 September, while the contents of NH4+-N and TP gradually increased after August 30 and reached the maximum on October 30. In addition, all nutrient contents in the HD group were significantly higher than those in the LD group after 90 days of farming (p < 0.05, Figure 1).
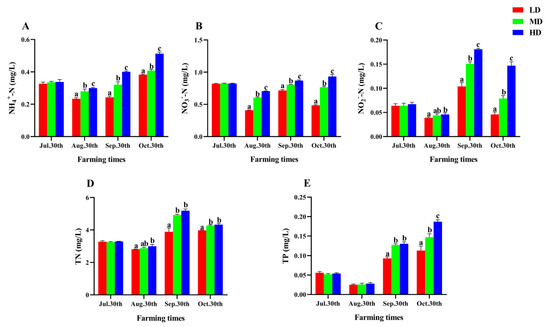
Figure 1.
Water quality parameters of P. fulvidraco reared at different densities in an integrated rice–fish farming system. NH4+-N (A), NO3−N (B), NO2−N (C), TN (D), and TP (E). Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
3.2. Change of Growth Performance
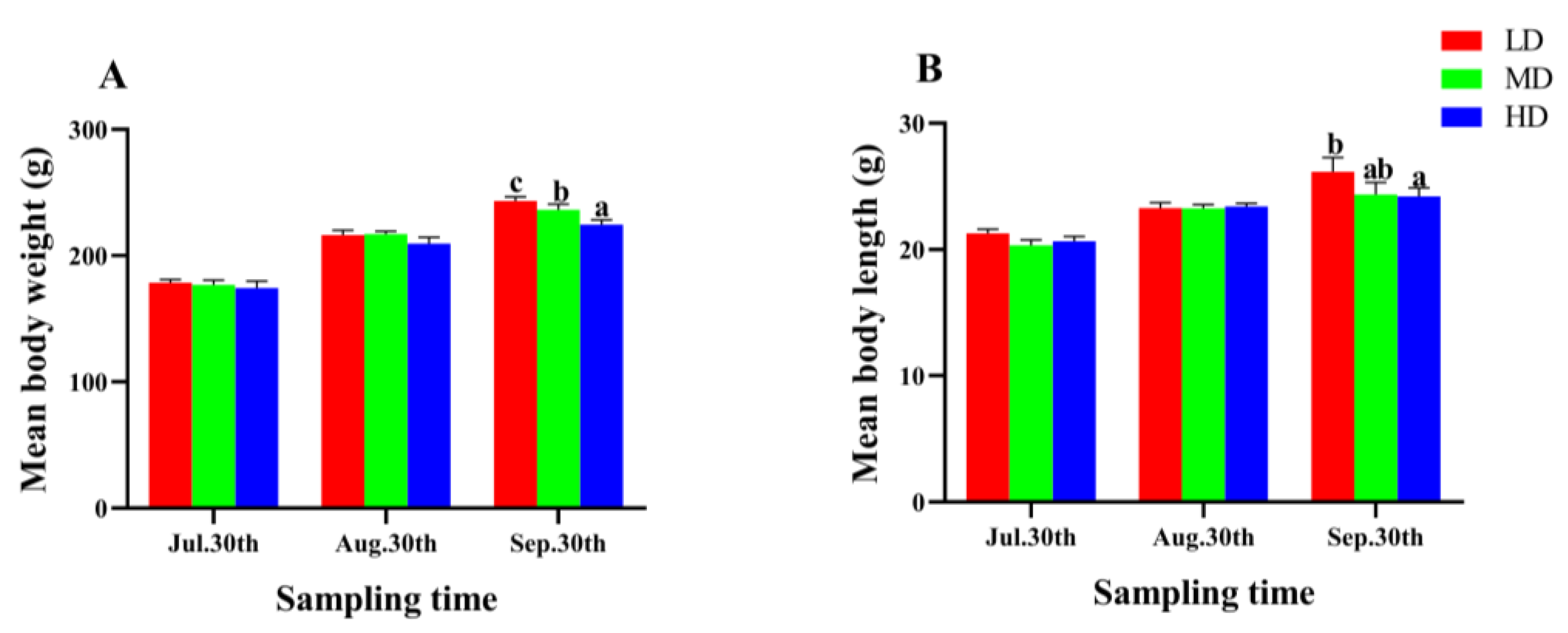
After 90 days of fish farming, the WG and SGR of P. fulvidraco were significantly lower in the HD group compared to the MD and LD groups (p < 0.05, Table 2). However, the CF and SR showed no significant difference among different groups (p > 0.05, Table 2). As the farming time increased, the mean body length and weight of P. fulvidraco in each group presented an upward trend (Figure 2), and the two parameters in the LD group were significantly higher than that in the HD group at the end of the trial (p < 0.05, Figure 2).

Table 2.
Growth parameters of P. fulvidraco at different stocking densities after 90 days of farming.
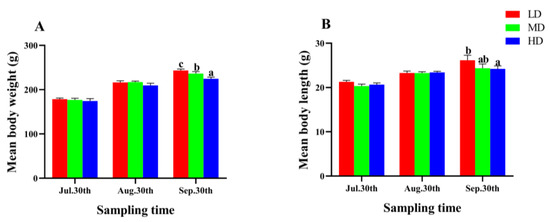
Figure 2.
Mean body weight (A) and mean body length (B) of P. fulvidraco reared at different densities in an integrated rice–fish farming system. Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
3.3. Change of Stress Indices
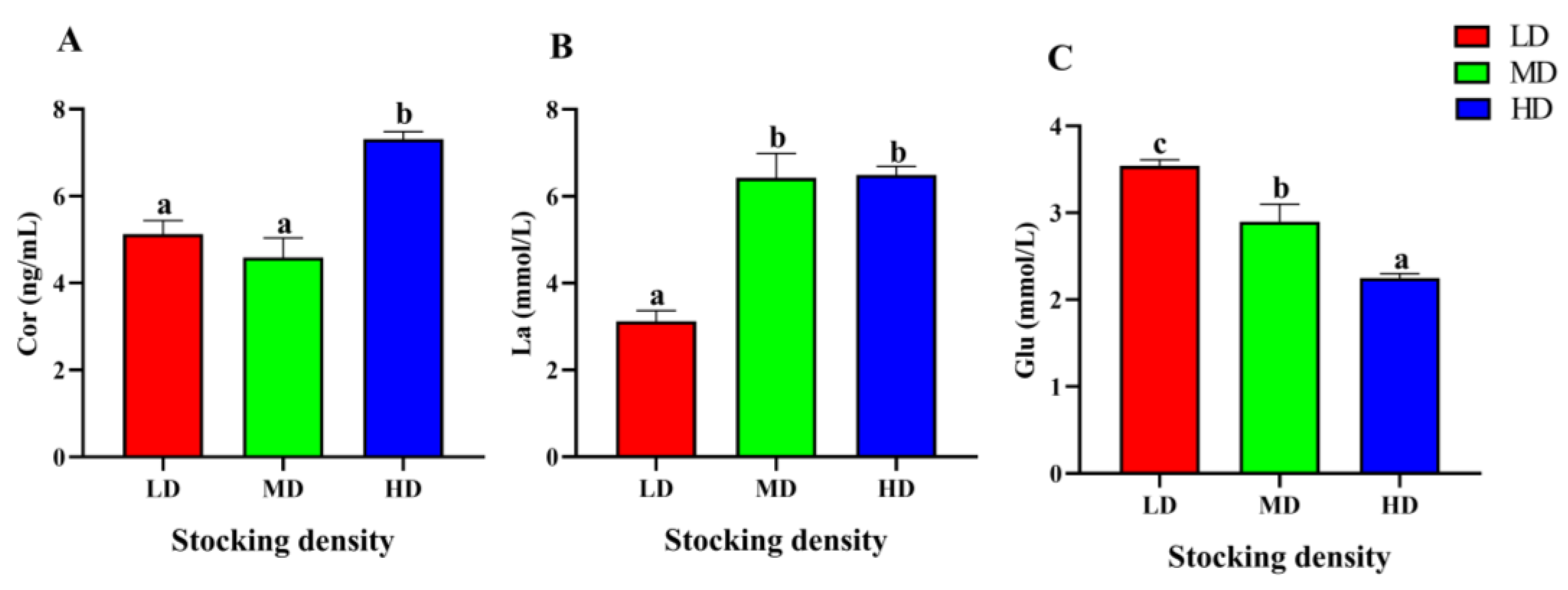
At the end of the trial, the serum stress parameters of P. fulvidraco under different stocking densities had significant differences (Figure 3). The content of Cor in the HD group was markedly higher than that in the LD and MD groups after 90 days of farming (p < 0.05). Compared with the LD group, the content of La in the MD and HD increased significantly (p < 0.05). In addition, with the increase of stocking density, the level of Glu in serum decreased significantly (p < 0.05).
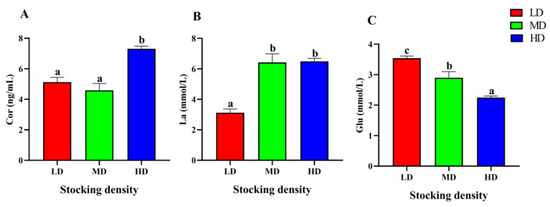
Figure 3.
Stress parameters of in the serum P. fulvidraco reared at different densities in an integrated rice–fish farming system. Cor (A), La (B), and Glu (C). Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
3.4. Change of Lipid Metabolism
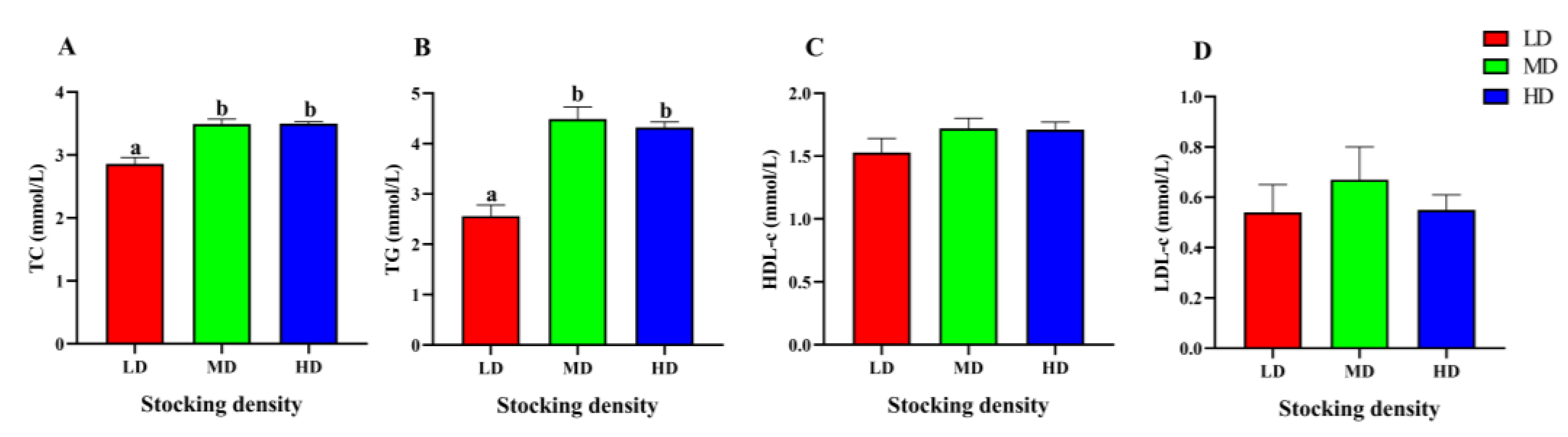
The changes of lipid metabolism parameters in the serum of P. fulvidraco under different densities were shown in Figure 4. Compared with the LD group, the content of TC and TG in the MD and HD groups increased significantly (p < 0.05). However, different stocking densities had no significant effect on the levels of TC, HDL-c, and LDL-c in serum (p > 0.05).
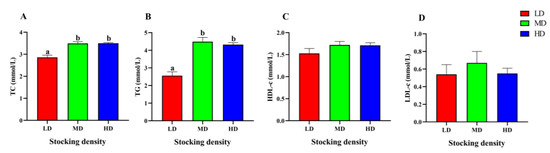
Figure 4.
Lipid metabolism parameters in the serum of P. fulvidraco reared at different densities in an integrated rice–fish farming system. TC (A), TG (B), HDL-c (C), and LDL-c (D). Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
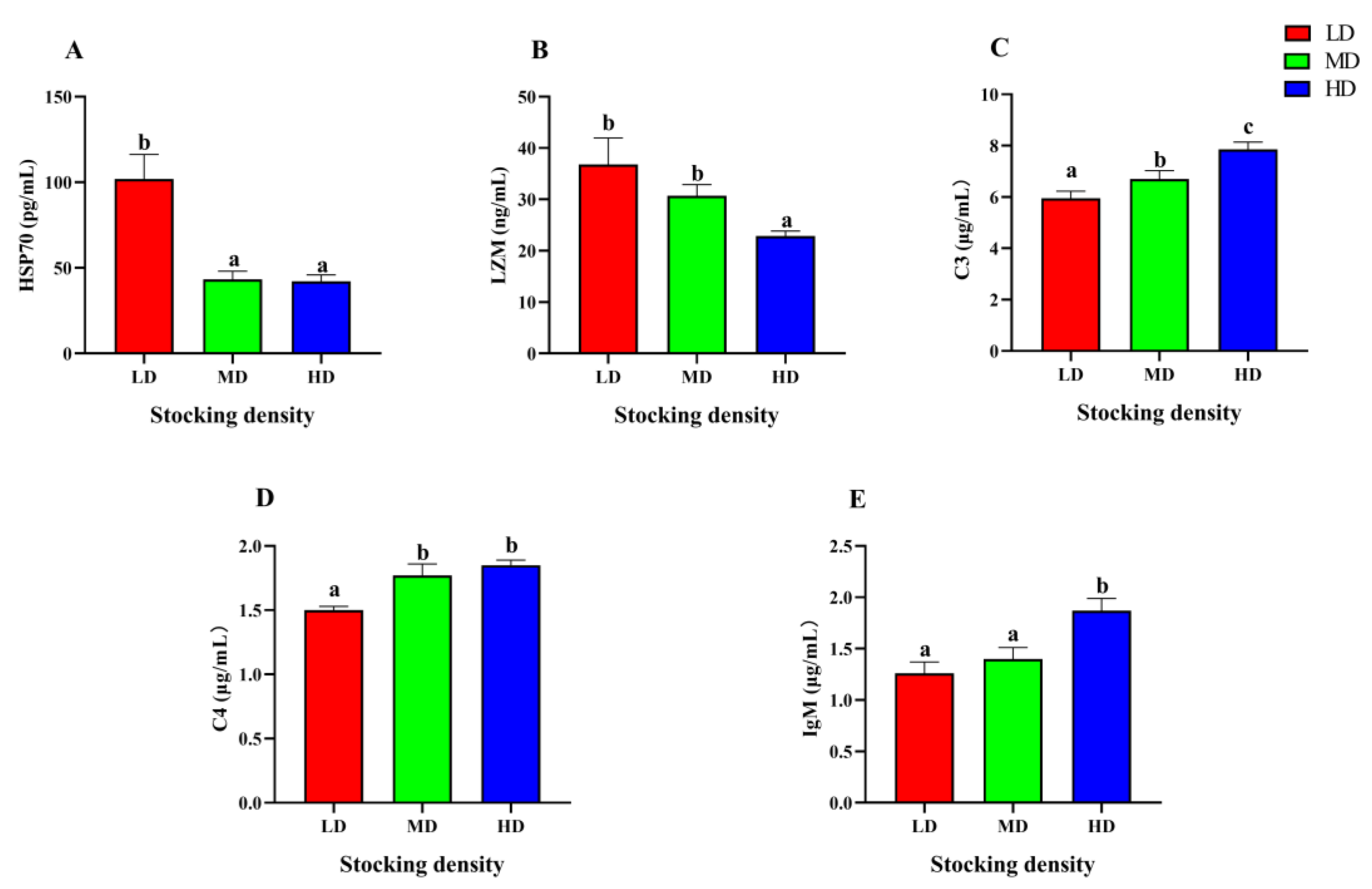
3.5. Change of Immune Function
After 90 days farming, significant differences were observed in the serum immune parameters of P. fulvidraco under different densities (Figure 5). The content of HSP70 in the MD and HD groups was significantly lower than that in the LD group, while the content of LZM in the HD group was significantly lower than that in the LD and MD groups (p < 0.05). As the stocking density increased, there was a corresponding increase in the levels of C3, C4, and IgM. Specifically, the HD group exhibited significantly higher levels of C3, C4, and IgM when compared to the LD group (p < 0.05).
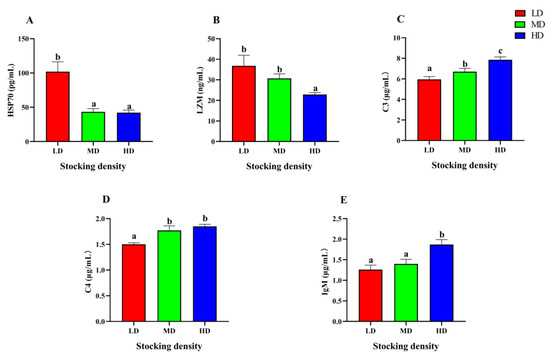
Figure 5.
Immune parameters in the serum of P. fulvidraco reared at different densities in an integrated rice–fish farming system. HSP70 (A), LZM (B), C3 (C), C4 (D), and IgM (E). Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
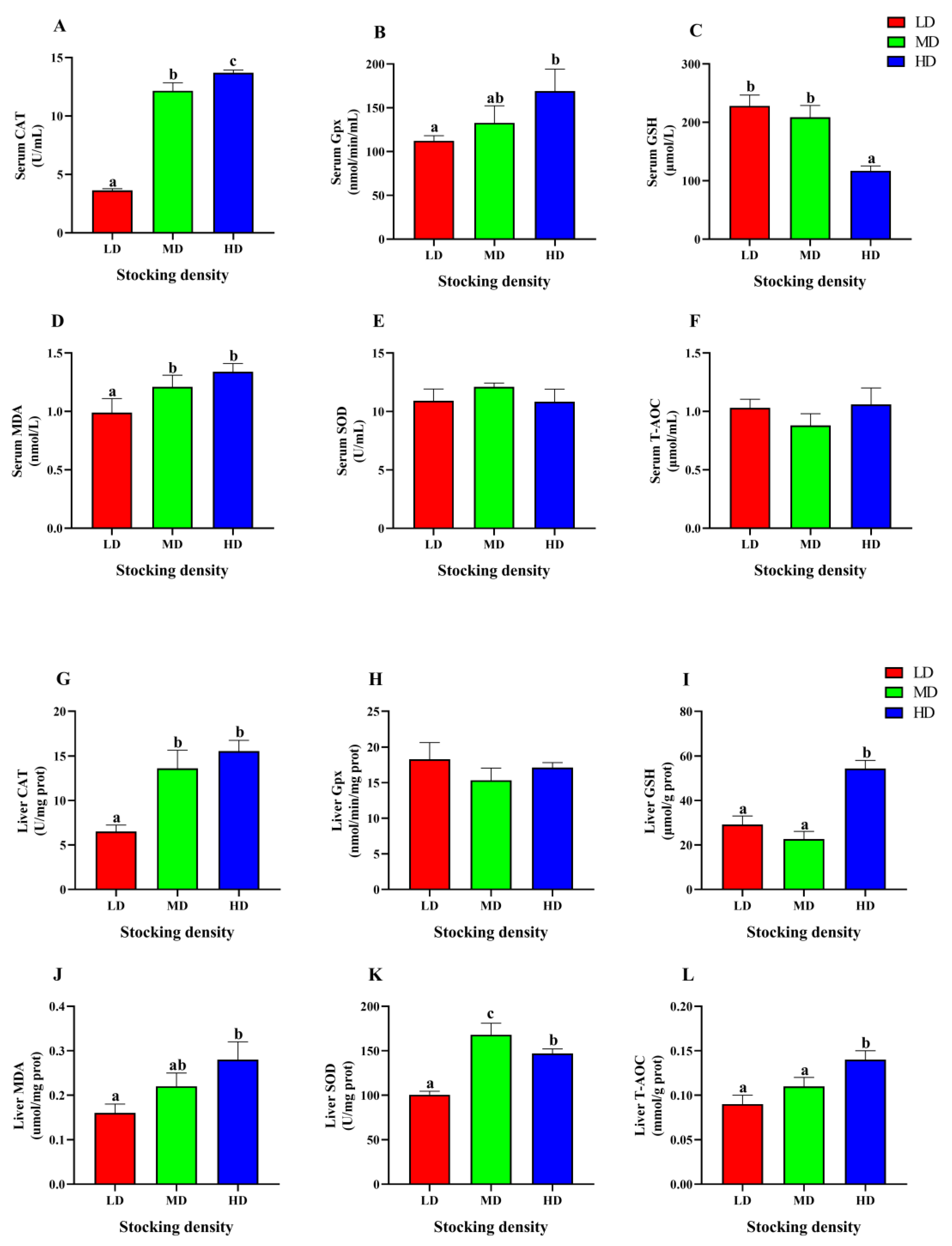
3.6. Change of Antioxidant Status
In the serum, the HD group showed significantly higher activities of CAT and Gpx, as well as MDA content, compared to the LD group (p < 0.05, Figure 6A,B,D). However, the GSH content was markedly decreased in the HD group compared to the LD group (p < 0.05, Figure 6C). In addition, there was no significant difference observed in SOD and T-AOC levels among the different groups (p > 0.05, Figure 6E,F).
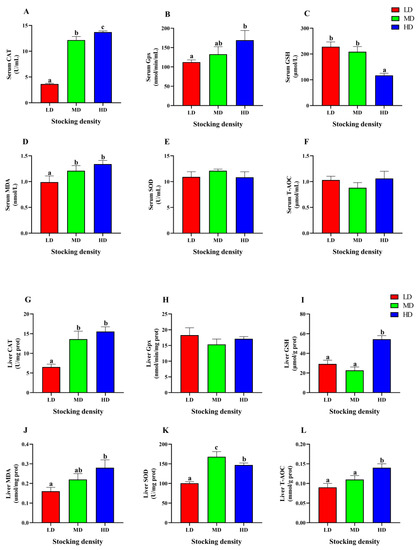
Figure 6.
Antioxidative parameters in serum (A–F) and liver (G–L) of P. fulvidraco reared at different densities in an integrated rice-fish farming system. Serum CAT (A), Serum Gpx (B), Serum GSH (C), Serum MDA (D), Serum SOD (E), Serum T-AOC (F), Liver CAT (G), Liver Gpx (H), Liver GSH (I), Liver MDA (J), Liver SOD (K) and Liver T-AOC (L). Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
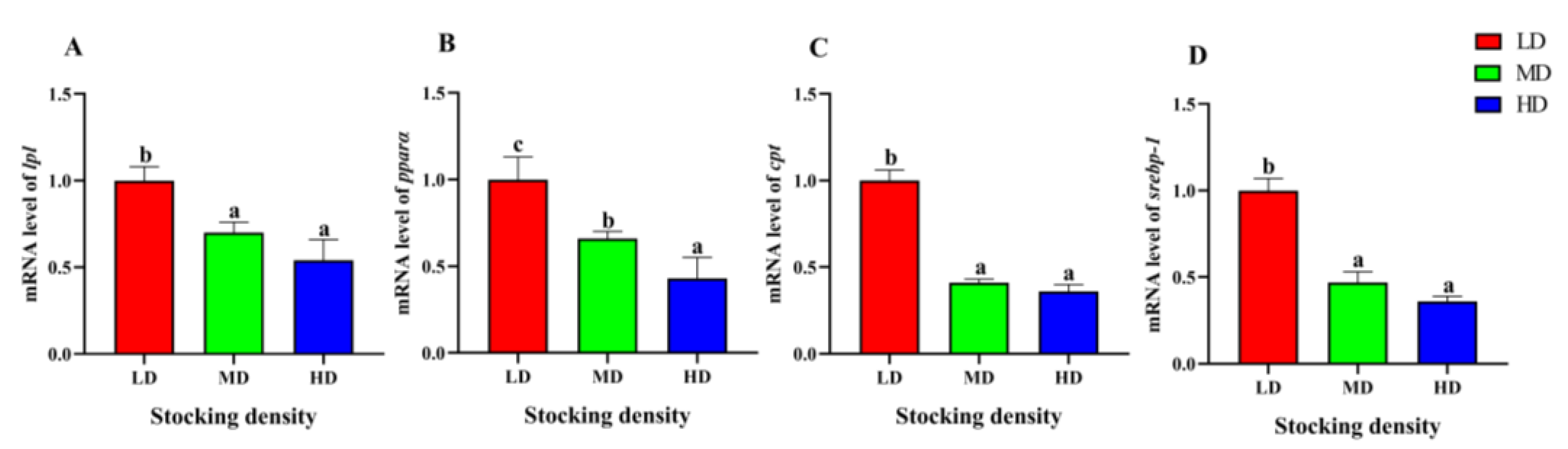
3.7. Change of Liver Gene Expression
The relative gene expression of lpl and pparα in the HD and MD groups were significantly lower than that in the LD group in the liver (p < 0.05, Figure 7). Similarly, the relative gene expression of cpt and srebp-1 in the MD and HD groups was significantly lower than that in the LD group (p < 0.05, Figure 7).
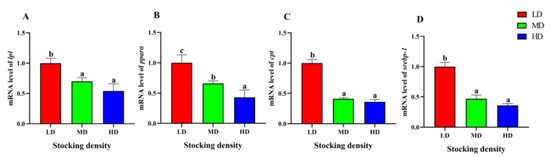
Figure 7.
Gene expression in the liver of P. fulvidraco reared at different densities in an integrated rice–fish farming system. lpl (A), pparα (B), cpt (C), and srebp-1 (D). Values are presented as means ± SD (n = 3). Different letters as superscripts indicate significant differences among the different groups (p < 0.05). LD, low stocking density; MD, medium stocking density; HD, high stocking density.
4. Discussion
4.1. Effect of Stocking Density on Water Quality
Water quality is a crucial factor in the success of aquaculture. Poor water quality can lead to increased stress in aquatic animals, resulting in reduced survival and growth rates [58,59]. The relationship between stocking density and water quality parameters in various aquaculture systems has been widely reported. Studies have found that stocking density has a significant impact on water quality, and that the levels of water quality parameters gradually increase with increasing stocking density in pond farming, which could be responsible for the growth inhibition of C. idella (2570 ± 102.96 g, HD: 15 kg/m3, 120 d) and M. amblycephala (95.47 ± 6.30 g, HD: 10 kg/m3, 120 d) in ponds [60]. However, Liu et al. (2022) have shown that the stocking density does not impact water quality in the integrated rice–fish farming system [61], which is because rice can absorb nutrients and improve water quality [62]. In our study, we found that the water quality parameters, including TP, TN, NH4+-N, NO3−-N, and NO2−-N, were significantly higher in the HD and MD groups than the LD group after 90 days of farming. We attribute this to the increased dissolution of excrement and feed in the water under high stocking density.
The absorption capacity of rice to N and P is different at different rice growth stages [10]. During the tillering stage, rice displays a strong ability to assimilate N and P, effectively reducing the N and P load in water and soil [63,64,65]. Our study revealed that rice demands a substantial amount of N and P during the tillering stage to support its growth, resulting in a decrease in N and P content (30th August). However, during non-irrigation periods, the absorption and purification capacity of rice attenuates, leading to an upsurge in N and P concentration in water. Notably, paddy fields represent a confined ecosystem, with its small water volume highly susceptible to temperature and precipitation fluctuations [66,67]. Consequently, nutrient concentrations displayed a fluctuating trend during the experiment.
4.2. Effect of Stocking Density on Growth Performance
It was reported that the stocking density is one of the critical factors of fish growth performance [68]. A previous study showed that SGR, FCR, and protein efficiency ratio (PER) of spotted sea bass (Labeo bata) (203.27 ± 26.55 g) were negatively impacted by high stocking densities (618 g/m3, 14 d), which led to decreased growth performance [69]. Debnath et al. (2016) found that Indian butter catfish (Ompok bimaculatus) (0.83 ± 0.02 g), reared at lower density (3.32 g/m2, 8 months) demonstrated significant improvements in body length, weight, and survival rates compared to those reared at higher density (4.98 g/m2, 8 months) [70]. Similarly, in this study, the body weight, body length, SGR, and WG in the HD group were significantly lower than that in the LD group after 90 days of farming, indicating that high stocking density inhibited the growth of P. fulvidraco. This effect is likely due to increased competition for food and living space caused by increased social interaction among fish. It was reported that increased social interaction caused significant differences in individual size [71,72,73]. Wickins et al. (2010) reported that the growth performance of the juvenile European eel (Anguilla anguilla) (0.26 g) in an aquarium was significantly improved after it was removed from high stocking density (247 g/m3, 86 d) and isolated [74]. Although higher stocking density is often seen as a way to improve the utilization of water and land resources, it may not necessarily lead to increased production [75]. Research on juvenile turbot (Scophthalmus maximus) raised in PVC tanks (8.62 ± 0.06 g), for example, suggests that high stocking densities (1.8 kg/m3, 45 d) can prolong growth periods and reduce yield by decreasing SGR [76]. Given the potential economic risks, there is a need for further study and attention to optimal stocking densities in aquaculture activities [77].
4.3. Effect of Stocking Density on Stress Response
High stocking density is a common stressor that disrupts physiological functions [78]. Cor is a well-known indicator of stress response [79,80] and is produced through hypothalamus-pituitary-interrenal tissues in fish under stress [81]. It has been reported that a large amount of energy was generated to resist external stress, when Cor acts on carbohydrate metabolism, proteolysis, and lipid oxidation [80,82]. However, prolonged elevation of Cor levels can cause serious cell damage [83]. La is also a crucial biomarker for stress response in aquatic animals [84]. For the juvenile flounder (Paralichthys orbignyanus) raised in tanks, the serum Cor was significantly increased at high stocking density (7.8 kg/m3, 15 d) [85]. Similar results were observed in gilthead sea bream (Sparus aurata) (89.5 ± 21.1 g, 70–90 kg/m3, 45 d) [86] and Channel catfish (Ictalurus punctatus) (40.6 ± 2.23 g, 12.18 kg/m3, 60 d) [87] in the high-density group. Our study on P. fulvidraco also revealed increased Cor level in the HD group and increased La level in the MD and HD groups after 90 days of farming, highlighting the chronic stress caused by inappropriate stocking density.
Glucose has been widely recognized as a common indicator for assessing the level of stress [88]. It has been observed that the elevation of cortisol content facilitates metabolic stimulation, increases gluconeogenesis, and promotes the accumulation of glucose [89]. Previous findings have indicated that acute crowding stress (70 kg/m3, 3 h) can result in hyperglycemia in C. carpio (70.69 ± 8.11 g) raised in cages [90]. However, in this study, serum Glu content of P. fulvidraco decreased significantly with the increase of stocking density. One possible explanation for this phenomenon is that high stocking density intensifies the competition for food and living space amongst individuals, thereby leading to strenuous physical activity and increased glucose consumption.
4.4. Effect of Stocking Density on Lipid Metabolism
Lipid metabolism is a physiological process that is often altered during periods of stress, as the body expends extra energy. Blood lipid levels, such as TC, TG, HDL-c, and LDL-c are commonly used as parameters for evaluating lipid metabolism [91]. In M. salmoides (9.71 ± 3.75 g) raised in ponds, high stocking density (1.94 kg/m3, 180 d) led to a significant increase in the TG level, but the TC level did not exhibit any significant differences among different stocking densities, suggesting that high stocking density may accelerate lipid metabolism [92]. In a previous study on I. punctatus (40.6 ± 2.1 g, 12.18 kg/m3, 60 d) raised in tanks, the TC, TG, and HDL-c levels in the high-density group were significantly higher than those in the low-density group, while the LDL-c level in the medium density group were significantly higher than those in the low-density group, likely due to increased energy demands at high stocking densities [89]. Our study also revealed a marked increase in TC and TG levels in both the HD and MD groups, indicating that lipid metabolism may be enhanced to produce more TC and TG for energy consumption under high stocking density conditions.
We further found that the expression of four pivotal genes associated with lipid metabolism including lipoprotein lipase (lpl), peroxisome proliferators-activated receptor (pparα), carnitine palmitoyltransferase-1 (cpt-1), and sterol regulatory element binding protein-1 (srebp-1) in the liver of P. fulvidraco were significantly altered under different stocking densities. LPL is a crucial enzyme involved in the process of lipid deposition and metabolism [93,94], while PPARα plays a major role in stimulating lipid catabolism and maintaining cholesterol homeostasis [95,96]. Cpt-1 is the rate-limiting enzyme affecting fatty acid β-oxidation [97], and SREBP-1, in conjunction with PPARα, participates in regulating the synthesis of unsaturated fatty acids [98,99]. Previous studies have shown that under high stocking density, the mRNA level of lpl was reduced in amur sturgeon (Acipenser schrenckii) (225.5 ± 32.3 g, 11.0 kg/m3, 70 d) and C. idella (98.48 ± 6.00 g, 14.9 kg/m3, 70 d), indicating that lipid synthesis was inhibited [100,101]. The mRNA level of pparα in the liver of A. schrenckii increased significantly with the increase of stocking density, indicating that the lipid utilization ability was enhanced under high stocking density, resulting in a decrease in lipid accumulation [100]. Contrary result was found in the research on M. salmoides, indicating a disturbance in lipid metabolism under high density stress [13]. Several studies showed that the expression of cpt-1 and srebp-1 in fish can easily be influenced by stress. For instance, the mRNA levels of cpt-1 was inhibited significantly in M. salmoides (10.87 ± 0.17 g, 60 d) exposed to hypoxia stress [102] and in tilapia exposed to tetracycline for a long time [103]. Similarly, srebp-1 expression was down-regulated in the liver of S. maximus (197 ± 3.68 g, 48 h) subjected to low salinity stress [104]. In this study, the HD and MD treatments induced a down-regulation of lpl, pparα, cpt-1, and/or srebp-1, indicating that stress caused by high stocking condition may disrupt lipid metabolism in the liver.
4.5. Effect of Stocking Density on Immune Performance
The immune system of fish is a vital defense mechanism against disease. Immune response is a common phenomenon that helps fish cope with adverse stimuli. LZM, C3, C4, IgM, and HSP70 are well studied immune parameters in fish [105]. LZM is involved in gram-positive cell wall lysis [106], while C3 is considered an intersection between nonspecific and adaptive immunity, activating the complement through the classical, alternative, and lectin pathways [107]. C4 plays a role in the neutralization of viruses via classical complement activation [108,109], whereas IgM is the first immunoglobulin found in fish and is important in acquired immunity [110]. HSP70 is an inducible heat shock protein that mitigates cellular damage by repairing and degrading denatured proteins [111,112]. As a chaperone molecule, HSP70 plays important roles in metabolism and cell survival [113]. Numerous studies have confirmed that stocking density affects immune parameters. Specifically, the level of LZM significantly decreased with increasing stocking density in aquatic animals, such as turbot (Scophthalmus maximus) [114] and Chinese sturgeon (Acipenser sinensis) [115]. The effect of stocking density on C3 and C4 content varies between species. For instance, C3 content was observed to increase significantly under high stocking density of I. punctatus [87], while it decreased significantly with increasing stocking density of Russian sturgeon (Acipenser gueldenstacdti) [116]. A recent study showed that the expression of hsp70 in the liver of I. punctatus was markedly reduced while the level of IgM increased significantly under high stocking density [87]. Our study found that the levels of C3 and C4 in the HD and MD groups, and IgM in the HD group were significantly higher than that in the LD group after 90 days of farming, indicating that the high stocking density induced P. fulvidraco to respond to external stimulation by activating immune system. Meanwhile, the levels of LZM in the HD group and HSP70 in the MD and HD groups were markedly lower compared with the LD group. We speculated that the LZM was consumed and HSP70 synthesis was inhibited under an unsuitable stocking density. In addition, the decrease of HSP70 may affect cell proliferation [117].
4.6. Effect of Stocking Density on Antioxidative Status
Numerous studies have demonstrated that multiple stressors can cause an intracellular redox imbalance, which induces oxidative stress in fish [118]. Long term or severe oxidative stress may disturb the antioxidant defense system and lead to cell damage [119]. High stocking density has been shown to decrease levels of antioxidant parameters, such as SOD, CAT, Gpx, GSH, and T-AOC, thereby damaging the antioxidant defense system in several fish species, including M. salmoides (4.50 ± 0.23 g, 0.6 kg/m3, 60 d) [120] raised in ponds and M. amblycephala (25.76 ± 2.25 g, 3.09 kg/m3, 6 weeks) raised in cages [121]. Conversely, some studies suggest that high stocking density can lead to an increase in antioxidant levels in golden pompano (Trachinotus ovatus) (9.75 ± 0.11 g, 2.93 kg/m3, 70 d) raised in cages [122], jaw characin (Salminus brasiliensis) (5.67 g, 1.70 kg/m3, 80 d) raised in ponds [123], and large yellow croaker (Larimichthys crocea) (89.06 ± 11.70 g, 34.2 kg/m3, 72 h) raised in aquariums [124]. These findings indicated that more antioxidant enzymes were produced to eliminate oxygen free radicals under high density stress [125]. In this study, the HD and MD groups showed a marked increase in the levels of T-AOC, GSH, CAT, and/or SOD in the liver after 90 days of farming, which indicated that the antioxidant defense system was activated to maintain ROS balance under a high stocking density.
MDA is the final product of lipid peroxidation, and it can combine with free amino acids to cause crosslink polymerization of macromolecules, including proteins and nucleic acids, leading to cellular injury [126,127]. Under normal physiological status, the content of MDA in fish is low [128]. However, exposure to stressors like high stocking density can significantly increase MDA content [120,121]. Refaey et al. have demonstrated that the MDA content of I. punctatus reared in high density increased markedly compared to low density conditions [87]. Moreover, S. maximus (185.4 g) raised in recirculating aquaculture system also displayed a marked increase in MDA formation at high-density group (19.1 kg/m2, 120 d) [129]. Similarly, in this study, chronic stress induced by high stocking density caused increased lipid peroxidation, leading to a significant rise in MDA content in the serum and liver of fish after 90 days of farming.
5. Conclusions
Our findings indicated that the P. fulvidraco is a suitable species for the integrated rice–fish farming system. However, we observed that growth parameters were reduced when the stocking density of P. fulvidraco exceeded 250 g/m2 in the integrated rice–fish farming system. In addition, although the MD group did not show differences in growth compared to LD, some evidence of suffering stress was found, which indicated that this density seems to be inappropriate for longer rearing periods (higher than 90 days). Our data also revealed that MD and/or HD treatments resulted in increased physiological stress, which was evidenced by alterations in lipid metabolism, immune function, and antioxidant status. Consequently, P. fulvidraco required additional energy to maintain a balance in its physiological state. These results suggested that the growth inhibition of P. fulvidraco in high density stocking conditions may be attributed to a degradation of water quality and changes in physiological status due to increased interspecific interaction. These insights offer guidance for selection of an appropriate stocking density for P. fulvidraco in integrated rice–fish farming systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13111721/s1, Table S1: Gene-specific qPCR primers. Table S2: Ct values of the three reference genes. Table S3. Bestkeeper analysis of reference genes from liver in Pelteobagrus fulvidraco. Refs. [51,52,53,54] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, writing—original draft preparation, software, W.D.; methodology, investigation, R.J.; validation, formal analysis, Y.H. and Y.D.; resources, data curation, writing-review and editing, B.L.; visualization, supervision, project administration, funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by earmarked fund for CARS (CARS-45), and Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2020TD60).
Institutional Review Board Statement
All animals in this study were approved by the Animal Care and Use Ethics Committee of the Freshwater Fisheries (2020TD60, 18-06-2021), and all procedures were performed according to Jiangsu Laboratory’s Animal Management Guidelines (014000319/2008-00079).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, A.L.; Chin, J.Y.; Mohd Harun, M.H.Z.; Low, S.C. Environmental impacts and imperative technologies towards sustainable treatment of aquaculture wastewater: A review. J. Water Process Eng. 2022, 46, 102553. [Google Scholar] [CrossRef]
- Wang, S.; Hu, H.; Xiong, X.; Gao, Z. Promotion of genetic improvement to world aquaculture development. J. Fish. China 2023, 47, 27–38. [Google Scholar]
- Cao, J.; Sang, F. Thinking on Theory, Model and Evaluation Method of Aquaculture Green Development. Ecol. Econ. 2020, 36, 101–106. [Google Scholar]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, H.; Xia, Y.; Gong, W.; Li, Z.; Yu, E.; Tian, J.; Wang, G.; Xie, J. Evaluating ecological mechanisms and optimization strategy of rice–fish co–culture system by ecosystem approach. Aquaculture 2022, 560, 738561. [Google Scholar] [CrossRef]
- Haroon, A.K.Y.; Pittman, K.A. Rice-fish culture: Feeding, growth and yield of two size classes of Puntius gonionotus Bleeker and Oreochromis spp. in Bangladesh. Aquaculture 1997, 154, 261–281. [Google Scholar] [CrossRef]
- Liu, D.; Tang, R.; Xie, J.; Tian, J.; Shi, R.; Zhang, K. Valuation of ecosystem services of rice–fish coculture systems in Ruyuan County, China. Ecosyst. Serv. 2020, 41, 101054. [Google Scholar] [CrossRef]
- Dwiyana, E.; Mendoza, T.C. Comparative Productivity, Profitability and Efficiency of Rice Monoculture and Rice-Fish Culture Systems. J. Sustain. Agric. 2006, 29, 145–166. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.; Ren, W.; Guo, L.; Wu, M.; Tang, J.; Chen, X. Effects of fish on field resource utilization and rice growth in rice-fish coculture. Chin. J. Appl. Ecol. 2017, 28, 299–307. [Google Scholar] [CrossRef]
- Guan, W.; Liu, K.; Shi, W.; Xuan, F.; Wang, W. Scientific paradigm of integrated farming of rice and fish. Acta Ecol. Sin. 2020, 40, 5451–5464. [Google Scholar]
- Vromant, N.; Chau, N. Overall effect of rice biomass and fish on the aquatic ecology of experimental rice plots. Agric. Ecosyst. Environ. 2005, 111, 153–165. [Google Scholar] [CrossRef]
- Ye, Y.; Ren, W.; Zhang, S.; Zhao, L.; Tang, J.; Hu, L.; Chen, X. Genetic Diversity of Fish in Aquaculture and of Common Carp (Cyprinus carpio) in Traditional Rice–Fish Coculture. Agriculture 2022, 12, 997. [Google Scholar] [CrossRef]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Redox Status and Lipid Metabolism of Micropterus salmoides in Integrated Rice-Fish Farming Systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Mridha, M.A.R.; Shah, A.K.M.A.; Nahiduzzaman, M.; Uddin, M.S. Performance of mono-sex tilapia (Oreochromis niloticus) in rice field with different ditch size. Aquac. Res. 2015, 46, 1891–1901. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Chen, S.; Guo, Y.; Cao, C. Certified rice–crayfish as an alternative farming modality in waterlogged land in the Jianghan Plain region of China. Agron. J. 2021, 113, 4568–4580. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, R.; Zhang, D.; Lei, X.; Wang, S.; Wan, J.; Liu, H.; Chen, Y.; Zhao, Y.; Wang, G.; et al. Effects of different stocking densities on growth performance, nutritional quality and economic benefit of juvenile female Chinese mitten crab (Eriocheir sinensis) in rice-crab culture systems. Aquaculture 2022, 553, 738111. [Google Scholar] [CrossRef]
- Liu, B.; Jia, R.; Zhao, K.; Wang, G.; Lei, J.; Huang, B. Stocking density effects on growth and stress response of juvenile turbot (Scophthalmus maximus) reared in land-based recirculating aquaculture system. Acta Oceanol. Sin. 2016, 36, 31–38. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Peng, X.; Yang, Q.; Xu, Q.; Dou, Z.; Gao, H. Effects of Stocking Density on Rice Yield, Rice Quality and Ecological Environment in the Coculture of Rice and Aquatic (poultry) Animals. China Rice 2023, 29, 55–59. [Google Scholar]
- Mridha, M.A.R.; Hossain, M.A.; Azad Shah, A.K.M.; Uddin, M.S.; Nahiduzzaman, M. Effects of Stocking Density on Production and Economics of All-Male Tilapia (Oreochromis niloticus) Culture in a Rain Fed Rice-Fish Ecosystem. J. Appl. Aquac. 2014, 26, 60–70. [Google Scholar] [CrossRef]
- Hazrat Ali, M.; Mateo, L.G.; Aragon, M.L. Effect of stocking density on growth and yield of GIFT tilapia under rice-fish production system. Bangladesh J. Fish 2006, 10, 35–39. [Google Scholar]
- Zhou, X.; Xiang, X.; Zhang, W.; Wang, L.; Peng, Y.; Qi, L. Study on the Growth Performance of Rice Flower Fish with Different Stocking Density. Contemp. Aquac. 2023, 48, 78–79. [Google Scholar]
- Zeng, T.; Zhao, H.; Long, S.; Yang, T.; Li, L.; Liu, Y.; Peng, T.; Hou, Z.; Fan, D.; Xiong, Y.; et al. Effects of Fry Release Density on Growth and Economic Benefit of Rice and Fish in Main Rice growing Areas in Guizhou. Guizhou Agric. Sci. 2020, 48, 102–106. [Google Scholar]
- Yuan, Q.; Qian, J.; Ren, Y.; Zhang, T.; Li, Z.; Liu, J. Effects of stocking density and water temperature on survival and growth of the juvenile Chinese mitten crab, Eriocheir sinensis, reared under laboratory conditions. Aquaculture 2018, 495, 631–636. [Google Scholar] [CrossRef]
- Song, Z.; Wen, H.; Li, J.; Ni, M.; Zhang, M.; Bu, Y.; Ren, Y.; Ding, H.; Lai, C.; Liu, C. The influence of stocking density on the growth performance of juvenile Russian sturgeon( Acipenser gueldenstaedti) in flowing water cultivation. J. Fish. China 2014, 38, 835–842. [Google Scholar]
- Dong, Y.; Jia, R.; Hou, Y.; Diao, W.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, mitophagy, endocytosis and metabolism of Cherax quadricarinatus in integrated rice–crayfish farming systems. Front. Physiol. 2022, 13, 1040712. [Google Scholar] [CrossRef]
- Shourbela, R.M.; El-Hawarry, W.N.; Elfadadny, M.R.; Dawood, M.A.O. Oregano essential oil enhanced the growth performance, immunity, and antioxidative status of Nile tilapia (Oreochromis niloticus) reared under intensive systems. Aquaculture 2021, 542, 736868. [Google Scholar] [CrossRef]
- Apún-Molina, J.P.; Robles-Romo, A.; Alvarez-Ruiz, P.; Santamaria-Miranda, A.; Arjona, O.; Racotta, I.S. Influence of stocking density and exposure to white spot syndrome virus in biological performance, metabolic, immune, and bioenergetics response of whiteleg shrimp Litopenaeus vannamei. Aquaculture 2017, 479, 528–537. [Google Scholar] [CrossRef]
- Bureau of Fisheries of the Ministry of Agriculture and Rural Affairs. 2021 China Fishery Statistical Yearbook; National Bureau of Statistics of China, Ed.; China Statistics Press: Beijing, China, 2021. [Google Scholar]
- Bureau of Fisheries of the Ministry of Agriculture and Rural Affairs. 2022 China Fishery Statistical Yearbook; National Bureau of Statistics of China, Ed.; China Statistics Press: Beijing, China, 2022. [Google Scholar]
- Chen, W.; Wang, Y.; Han, D.; Han, D.; Zhu, X.; Xie, S.; Long, F.; Jia, J.; Hu, Q. Effects of dietary supplementation with filamentous microalgae (Oedocladium sp. or Tribonema ultriculosum) on growth performance, fillet fatty acid composition, skin pigmentation, and immune response of yellow catfish (Pelteobagrus fulvidraco). J. World Aquac. Soc. 2021, 52, 1273–1289. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, G.; Nie, Z.; Shao, N.; Li, Q.; Xu, P. Growth Performance of Bluntnose Black Bream, Channel Catfish, Yellow Catfish, and Largemouth Bass Reared in the In-Pond Raceway Recirculating Culture System. N. Am. J. Aquac. 2019, 81, 153–159. [Google Scholar] [CrossRef]
- Griffin, M.J.; Ware, C.; Rosser, T.G.; Woodyard, E.T.; Mischke, C.C.; Byars, T.S.; Wise, D.J. Monoculture of ♀ channel (Ictalurus punctatus) × ♂ blue (I. furcatus) hybrid catfish mitigates proliferative gill disease caused by Henneguya ictaluri (Cnidaria: Myxobolidae) in catfish aquaculture ponds. J. World Aquac. Soc. 2020, 51, 729–739. [Google Scholar] [CrossRef]
- Bao, H.; Fang, Y.; Jiang, L. Experiment on the suitable stocking density for healthy culture of Pelteobagrus fulvidraco in pond. Mod. Agric. Sci. Technol. 2013, 9, 259–260. [Google Scholar]
- Mao, G.; Tang, Y. Pond aquaculture techniques for Pelteobagrus fulvidraco. Fish. Guidetobe Rich 2021, 4, 55–57. [Google Scholar]
- Chen, T. Artificial propagation and cage culture technology of Pelteobagrus fulvidraco. Anim. Breed. Feed 2017, 10, 43–44. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, H. Techniques for cage culture of Pelteobagrus fulvidraco. Mod. Agric. Sci. Technol. 2010, 12, 294–296. [Google Scholar]
- Yao, Q.; Yan, S.; Guo, Q.; Hu, B.; Lin, Q. Effect of Stocking Density on Growth Performance and Parameters of Pelteobagrus vachelli Juveniles. Fujian J. Agric. Sci. 2018, 33, 670–675. [Google Scholar] [CrossRef]
- LU, M.; Gan, H.; Chen, T.; Lu, X.; Ruan, Z.; Zhu, J.; Huang, G.; Li, J.; Ma, H. Comparison of Growth Performance and Muscle Quality of Yellow Catfish (Pseudobagrus vachelli) Cultured in Rice Fields and Ponds. Chin. J. Fish. 2022, 35, 75–81. [Google Scholar]
- Ji, L.; Zhen, G.; Wang, Y.; Tang, Y. Techniques for raising Pelteobagrus fulvidraco in rice fields. Jiangxi Fish. Sci. Technol. 2013, 133, 35–36. [Google Scholar]
- Wang, W. Six techniques for healthy culture of Pelteobagrus fulvidraco. New Ctry. 2019, 5, 28–29. [Google Scholar]
- Zhang, K.; Wang, G.; Gong, W.; Yu, E.-M.; Li, Z.-F.; Xia, Y.; Tian, J.; Xie, J. Study on environment of zero-water exchange culture pond of Ctenopharyngodon idella, Hypothalmichthys nobilis and Carassius auratus. Freshw. Fish. 2022, 43, 188–198. [Google Scholar] [CrossRef]
- Huang, F.; Tang, Q.; Liang, P.; Xiao, L. Improvement of the ammonium molybdate spectrophotometric method for phosphorus monitoring in freshwater. J. Lake Sci. 2016, 28, 1404–1410. [Google Scholar]
- Smart, M.M.; Rada, R.G.; Donnermeyer, G.N. Determination of total nitrogen in sediments and plants using persulfate digestion. An evaluation and comparison with the Kjeldahl procedure. Water Res. 1983, 17, 1207–1211. [Google Scholar] [CrossRef]
- Wu, H.Z.; Cao, A. Preparation and Adding Methods of Nessler’s Reagent Having Effects on Determination of Water Quality Ammonia Nitrogen. Adv. Mater. Res. 2013, 726–731, 1362–1366. [Google Scholar] [CrossRef]
- Lo, H.S.; Lo, K.W.; Yeung, C.F.; Wong, C.Y. Rapid visual and spectrophotometric nitrite detection by cyclometalated ruthenium complex. Anal. Chim. Acta 2017, 990, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.A. Accurate Determination of Soil Nitrates by Phenol Disulfonic Acid Method. J. Ind. Eng. Chem. 2002, 11, 213–218. [Google Scholar] [CrossRef]
- Kate, K.; Waters, S.M.; Kelly, A.K.; Wylie, A.R.; Kenny, D.A. Effect of feed restriction and subsequent re-alimentation on hormones and genes of the somatotropic axis in cattle. Physiol. Genom. 2015, 47, 264–273. [Google Scholar]
- Fang, H.; Wu, Y.; Guo, J.; Rong, J.; Ma, L.; Zhao, Z.; Zuo, D.; Peng, S. T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis 2012, 17, 895–907. [Google Scholar] [CrossRef]
- Mou, F.; Yang, J.; Liu, C.; Liu, C.; Liu, M.; Chen, J.; Zu, Y.; Wang, J. Effects of sulfur on the characteristics of sulfur-containing compounds and Pb accumulation in Arabis alpina L. var. parviflora Franch. J. Agro Environ. Sci. 2021, 40, 1851–1859. [Google Scholar]
- Liu, J.; Liu, H.; Fan, Y.; Tang, X.; Wang, X.; Wang, Y.; Gai, C.; Haibin, Y. Effects of acute nitrite stress on oxidative stress, energy metabolism and osmotic regulation of Penaeus monodon. J. Fish. China 2023, 47, 049604. [Google Scholar]
- Aursnes, I.A.; Rishovd, A.L.; Karlsen, H.E.; Gjøen, T. Validation of reference genes for quantitative RT-qPCR studies of gene expression in Atlantic cod (Gadus morhua l.) during temperature stres. BMC Res. Notes 2011, 4, 104. [Google Scholar] [CrossRef]
- Guo, J.; Pu, Y.; Zhong, L.; Wang, K.; Duan, X.; Chen, D. Lead impaired immune function and tissue integrity in yellow catfish (Peltobargus fulvidraco) by mediating oxidative stress, inflammatory response and apoptosis. Ecotoxicol. Environ. Saf. 2021, 226, 112857. [Google Scholar] [CrossRef]
- Song, Y.F.; Luo, Z.; Huang, C.; Chen, Q.L.; Pan, Y.X.; Xu, Y.H. Endoplasmic Reticulum Stress-Related Genes in Yellow Catfish Pelteobagrus fulvidraco: Molecular Characterization, Tissue Expression, and Expression Responses to Dietary Copper Deficiency and Excess. G3 2015, 5, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Luo, Z.; Shi, X.; Wu, K.; Zhuo, M.Q.; Song, Y.F.; Hu, W. Dietary methimazole-induced hypothyroidism reduces hepatic lipid deposition by down-regulating lipogenesis and up-regulating lipolysis in Pelteobagrus fulvidraco. Gen. Comp. Endocrinol. 2015, 217–218, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using RealTime Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, G.; Wang, H.; Mo, W.; Huang, Y.; Cao, J.; Li, P. Effects of dietary sodium butyrate on growth, digestive enzymes, body composition and nutrient retention-related gene expression of juvenile yellow catfish (Pelteobagrus fulvidraco). Anim. Nutr. 2021, 7, 539–547. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Li, M.; Xie, Y.; Qian, Y.; Wang, R. Effects of Starvation and Re-Feeding on Enzyme Activities and Gene Expression Involved in Liver Lipid Metabolism of Yellow Catfish (Pelteobagrus fulvidraco) under Ammonia Nitrogen Stress. Chin. Anim. Nutr. 2021, 33, 436–447. [Google Scholar]
- Lima, P.C.M.; Silva, A.E.M.; Silva, D.A.; Silva, S.M.B.C.; Brito, L.O.; Gálvez, A.O. Effect of stocking density of Crassostrea sp. in a multitrophic biofloc system with Litopenaeus vannamei in nursery. Aquaculture 2021, 530, 735913. [Google Scholar] [CrossRef]
- Nga, B.T.; Lürling, M.; Peeters, E.T.H.M.; Roijackers, R.; Scheffer, M.; Nghia, T.T. Chemical and physical effects of crowding on growth and survival of Penaeus monodon Fabricius post-larvae. Aquaculture 2005, 246, 455–465. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; He, X.; Tang, R.; Li, D. An in-pond tank culture system for high-intensive fish production: Effect of stocking density on growth of grass carp (Ctenopharyngodon idella Valenciennes, 1844) and blunt snout bream(Megalobrama amblycephala Yih, 1955). Aquaculture 2022, 549, 737808. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Luo, L.; Wang, S.; Zhang, R.; Guo, K.; Bai, Q.; Li, H.; Li, M. Effects of different crab stocking density on production performance and environmental factors under integrated cultivation of rice crab in the cold area. Freshw. Fish. 2022, 52, 89–97. [Google Scholar] [CrossRef]
- Feng, J.; Li, F.; Zhou, X.; Xu, C.; Fang, F. Nutrient removal ability and economical benefit of a rice-fish co-culture system in aquaculture pond. Ecol. Eng. 2016, 94, 315–319. [Google Scholar] [CrossRef]
- Mabaya, G.; Unami, K.; Yoshioka, H.; Takeuchi, J.; Fujihara, M. Robust optimal diversion of agricultural drainage water from tea plantations to paddy fields during rice growing seasons and non-rice growing seasons. Paddy Water Environ. 2015, 14, 247–258. [Google Scholar] [CrossRef]
- Nakasone, H. Runoff water quality characteristics in a small agriculture watershed. Paddy Water Environ. 2003, 1, 183–188. [Google Scholar] [CrossRef]
- Xiao, X.; Zhu, W.; Xiao, L.; Liu, C.; Deng, Y.; Wang, J. Effects of Water and Fertilizer Management on Root Characteristics and Nitrogen, Phosphorous and Potassium Uptakes of Rice at Tillering Stage. Chin. J. Soil Sci. 2016, 47, 903–908. [Google Scholar] [CrossRef]
- Yoon, K.S.; Cho, J.Y.; Choi, J.K.; Son, J.G. Water management and N, P losses from paddy fields in southern Korea. J. Am. Water Resour. Assoc. 2006, 42, 1205–1216. [Google Scholar] [CrossRef]
- Head, M.A.; Oleszkiewicz, J.A. Bioaugmentation with nitrifying bacteria acclimated to different temperatures. J. Environ. Eng. 2005, 131, 1046–1051. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Taheri Mirghaed, A.; Ghelichpour, M. Effects of dietary tryptophan levels and fish stocking density on immunological and antioxidant responses and bactericidal activity against Aeromonas hydrophilain rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2020, 51, 1455–1463. [Google Scholar] [CrossRef]
- Karnatak, G.; Das, B.K.; Mishal, P.; Tayung, T.; Kumari, S.; Sarkar, U.K.; Das, A.K.; Ali, Y. Impact of stocking density on growth, feed utilization and survival of cage reared minor carp, Labeo bata (Hamilton, 1822) in Maithon reservoir, India. Aquaculture 2021, 532, 736078. [Google Scholar] [CrossRef]
- Debnath, C.; Dube, K.; Saharan, N.; Tiwari, V.K.; Datta, M.; Sahoo, L.; Yadav, G.S.; Das, P. Growth and production of endangered Indian butter catfish, Ompok bimaculatus (Bloch) at different stocking densities in earthen ponds. Aquac. Res. 2016, 47, 3265–3275. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Nicolaiewsky, S.; Souza, S.M.G.D.; Lulhier, F. The effects of stocking density and social interaction on acute stress response in nile tilapia Oreochromis niloticus (L.) ngerlings. Aquac. Res. 1999, 30, 887–892. [Google Scholar] [CrossRef]
- Jobling, M. Physiological and social constraints on growth of fish with special reference to Arctic charr, Salvelinus alpinus L. Aquaculture 1985, 44, 83–90. [Google Scholar] [CrossRef]
- Yamagishi, H.; Maruyama, T.; Mashiko, K. Social relation in a small experimental population of Odontobutis obscurus (Temminck et Schlegel) as related to individual growth and food intake. Oecologia 1974, 17, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Wickins, J.F. Growth variability in individually confined elvers, Anguilla anguilla (L.). J. Fish Biol. 1985, 27, 469–478. [Google Scholar] [CrossRef]
- Wu, X.; Ma, H.; Gao, S.; Chen, Y.; Lin, S. Physiological Responses of Hybrid Snakehead (Channa maculata × C. argus) to Different Stocking Densities. Fish. Sci. 2017, 36, 557–562. [Google Scholar] [CrossRef]
- Irwin, S.; O’Halloran, J.; FitzGerald, R. Stocking density, growth and growth variation in juvenile turbot, Scophthalmus maximus (Rafinesque). Aquaculture 1999, 178, 78–88. [Google Scholar] [CrossRef]
- Nhan, D.T.; Tu, N.P.C.; Tu, N.V. Comparison of growth performance, survival rate and economic efficiency of Asian seabass (Lates calcarifer) intensively cultured in earthen ponds with high densities. Aquaculture 2022, 554, 738151. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Xie, C. Effect of stocking density on growth and serum concentrations of thyroid hormones and cortisol in Amur sturgeon, Acipenser schrenckii. Fish Physiol. Biochem. 2012, 38, 511–520. [Google Scholar] [CrossRef]
- Tolussi, C.E.; Hilsdorf, A.W.S.; Caneppele, D.; Moreira, R.G. The effects of stocking density in physiological parameters and growth of the endangered teleost species piabanha, Brycon insignis (Steindachner, 1877). Aquaculture 2010, 310, 221–228. [Google Scholar] [CrossRef]
- Cao, Y.; Li, E.; Chen, L.; Long, L.; Cui, C.; Du, Z.; Sun, S.; Li, M. Effects of Stocking density on growth, physiological and immune responses in Juvenile Russian Sturgeon. Acta Hydrobiol. Sin. 2014, 38, 968–974. [Google Scholar]
- Wang, X.; Fang, X.; Peng, S.; Wang, Q.; Shi, Z. Impact of abrupt salinity changes on activitiy of metabolic enzymes, antioxidant enzymes and cortisol content in serum and liver of Lateolabrax maculatus. Mar. Fish. 2021, 43, 340–349. [Google Scholar] [CrossRef]
- Vijayan, M.M.; Leatherland, J.F. High stocking density affects cortisol secretion and tissue distribution in brook charr, Salvelinus fontinalis. Endocrinology 1990, 124, 311–318. [Google Scholar] [CrossRef]
- Ran, F.; Jin, W.; Huang, S.; Liu, C.; Li, Z.; Li, C. Research progress on the effects of salinity change on fish. J. Northwest A F Univ. Nat. Sci. Ed. 2020, 48, 10–18. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Xu, Y.; Sun, Y.; Song, L.; Tian, B.; Liu, T. Effects of stocking density on the growth performance, physiological response and intestinal microbiota of juvenile Echiura worms (Urechis unicinctus). Aquac. Res. 2020, 51, 3983–3992. [Google Scholar] [CrossRef]
- Bolasina, S.N. Stress response of juvenile flounder (Paralichthys orbignyanus, Valenciennes 1839), to acute and chronic stressors. Aquaculture 2011, 313, 140–143. [Google Scholar] [CrossRef]
- Gorshkov, S.; Gorshkova, G.; Ron, B. Physiological Stress Responses in Strains of the Gilthead Sea Bream (Sparus aurata). Isr. J. Aquac. Bamidgeh 2010, 62, 1–8. [Google Scholar]
- Refaey, M.M.; Li, D.; Tian, X.; Onxayvieng, K.; Tang, R. Physiological responses of channel catfish (Ictalurus punctatus) reared at different stocking densities in a recirculating aquaculture system. Aquaculture 2022, 557, 738329. [Google Scholar] [CrossRef]
- Luo, G.; Liu, G.; Tan, H.-x. Effects of stocking density and food deprivation-related stress on the physiology and growth in adult Scortum barcoo (McCulloch & Waite). Aquac. Res. 2013, 44, 885–894. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Yu, M.; Fan, Q.; Cheng, P.; Zhang, L.; Liu, W.; Du, H. Effect of Acute Crowding Stress on Cortisol and Several Biochemical Indexes in Cyprinus carpio Serum. Freshw. Fish. 2008, 38, 20–24. [Google Scholar]
- Tian, J.-J.; Jin, Y.-Q.; Yu, E.-M.; Sun, J.-H.; Xia, Y.; Zhang, K.; Li, Z.-F.; Gong, W.-B.; Wang, G.-J.; Xie, J. Intestinal farnesoid X receptor mediates the effect of dietary berberine on lipid accumulation in grass carp (Ctenopharyngodon idella). Aquaculture 2022, 553, 738055. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, J.; Yang, J.; Liao, W.; Lu, J.; He, X. Captive density on growth performance and health status of largemouth bass (Micropterus Salmoides). Acta Ecol. Sin. 2022, 46, 671–678. [Google Scholar]
- Wang, A.; Han, G.; Qi, Z.; Lv, F.; Yu, Y.; Huang, J.; Wang, T.; Xu, P. Cloning of lipoprotein lipase (LPL) and the effects of dietary lipid levels on LPL expression in GIFT tilapia (Oreochromis niloticus). Aquac. Int. 2013, 21, 1219–1232. [Google Scholar] [CrossRef]
- Wang, A.; Yang, W.; Liu, F.; Wang, Z.; Cang, P.; Yin, X.; Yu, Y.; Qiao, G.; Ni, J. Cloning and characterization of lipoprotein lipase (LPL) and the effects of dietary lipid levels on the expression of LPL in the redlip mullet (Liza haematocheila). Aquac. Nutr. 2018, 24, 832–841. [Google Scholar] [CrossRef]
- Corcoran, J.; Winter, M.J.; Lange, A.; Cumming, R.; Owen, S.F.; Tyler, C.R. Effects of the lipid regulating drug clofibric acid on PPARalpha-regulated gene transcript levels in common carp (Cyprinus carpio) at pharmacological and environmental exposure levels. Aquat. Toxicol. 2015, 161, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Fan, H.; Peng, J.; Zhou, L.; Gan, L. Pleiotropic function of vitamin C on fatty acids in liver and muscle of juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2019, 512, 734352. [Google Scholar] [CrossRef]
- Liao, T.; Zhang, L.; Ding, L.; Li, X.; Wu, P.; Mei, W.; Zhang, N.; Wang, P.; Zhang, L. Mitigation mechanism of Xibining decoction on pain of KOA by regulating redox homeostasis of synoviocytes through CPT1 enzyme. Med. J. Chin. People’s Lib. Army 2022, 48, 49–57. [Google Scholar]
- Dong, X.; Xu, H.; Mai, K.; Xu, W.; Zhang, Y.; Ai, Q. Cloning and characterization of SREBP-1 and PPAR-alpha in Japanese seabass Lateolabrax japonicus, and their gene expressions in response to different dietary fatty acid profiles. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 180, 48–56. [Google Scholar] [CrossRef]
- Wu, W.; Cao, Z.; Fan, W.; Xu, J.; Wang, W.; Zhao, Y. Cloning and expression of SREBP-1 gene in Megalobrama amblycephala. J. Huazhong Agric. Univ. 2019, 38, 9–16. [Google Scholar] [CrossRef]
- Ren, Y.; Wen, H.; Li, Y.; Li, J.; He, F.; Ni, M. Effects of stocking density on lipid deposition and expression of lipid-related genes in Amur sturgeon (Acipenser schrenckii). Fish Physiol. Biochem. 2017, 43, 1707–1720. [Google Scholar] [CrossRef]
- Chen, J. Effect of Stocking Density on Lipid Accumulation and the Expression Level of Genes Related to Lipid Metabolish in Grass Carp (Ctenopharngodon idella). Master’s Degree Dissertation, Huazhong Agricultural University, Wuhan, China, 2017. [Google Scholar]
- Sun, J.L.; He, K.; Liu, Q.; Luo, J.; Wang, Y.; Zhang, D.M.; Liang, J.; Liao, L.; Yang, S.; Zhao, L.L. Inhibition of fatty acid oxidation induced by up-regulation of miR-124 and miR-205 during exposure of largemouth bass (Micropterus salmoides) to acute hypoxia. Aquaculture 2020, 529, 735679. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Wei, X.; Zhong, C.; Liu, X.; Luo, Z. Long-term environmental-related tetracycline exposure on growth performance, hepatic lipid metabolish and antioxidant responses in gift tilapia (Oreochromis niloticus). Acta Ecol. Sin. 2022, 46, 1642–1648. [Google Scholar]
- Liu, Z.; Ma, A.; Yuan, C.; Zhao, T.; Chang, H.; Zhang, J. Transcriptome analysis of liver lipid metabolism disorders of the turbot Scophthalmus maximus in response to low salinity stress. Aquaculture 2021, 534, 736273. [Google Scholar] [CrossRef]
- Yusuf, A.; Huang, X.; Chen, N.; Apraku, A.; Wang, W.; Cornel, A.; Rahman, M.M. Impact of dietary vitamin c on plasma metabolites, antioxidant capacity and innate immunocompetence in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2020, 17, 100383. [Google Scholar] [CrossRef]
- Arends, R. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J. Endocrinol. 1999, 163, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hawlisch, H.; Köhl, J. Complement and Toll-like receptors: Key regulators of adaptive immune responses. Mol. Immunol. 2006, 43, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yao, H. Determination and clinical significance of serum complement C4 and LDH in children with MPP. Mater. Child Health Care China 2022, 37, 3157–3160. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Mai, K.; Lu, K.; Liu, Y.; Ai, Q. The effects of valine level on plasma biochemical indexes, lipid content and gene expression involved in lipid metabolism in cobia (Rachycentron canadum). Acta Ecol. Sin. 2016, 40, 744–751. [Google Scholar]
- He, Y.; Wu, J.; Chen, M.; Huang, W.; Han, K.; Li, J.; Shi, X. Development and Characterization of Mouse Polyclonal Antibodies to Serum IgM in Large Yellow Croaker Larimichthys crocea. Chin. J. Fish. 2022, 35, 32–37. [Google Scholar]
- Aksu, Ö.; Benzer, F.; Erişir, M.; Can, E.; Kutluyer, F. Influence of Stock Density on Digestive Enzyme Activity (Trypsin), Heat Shock Protein 70 (HSP70), and Oxidative Stress Biomarkers of Narrow Clawed Crayfish, Astacus leptodactylus Eschscholtz, 1823 (Decapoda, Astacidae). Crustaceana 2016, 89, 1193–1202. [Google Scholar] [CrossRef]
- Zahedi, S.; Akbarzadeh, A.; Mehrzad, J.; Noori, A.; Harsij, M. Effect of stocking density on growth performance, plasma biochemistry and muscle gene expression in rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 498, 271–278. [Google Scholar] [CrossRef]
- Abarike, E.D.; Jian, J.; Tang, J.; Cai, J.; Sakyi, E.M.; Kuebutornye, F.K. A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100438. [Google Scholar] [CrossRef]
- Liu, B.; Fei, F.; Li, X.; Wang, X.; Huang, B. Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquac. Int. Aquac. Int. 2019, 27, 1599–1612. [Google Scholar] [CrossRef]
- Long, L.; Zhang, H.; Ni, Q.; Liu, H.; Wu, F.; Wang, X. Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wen, H.; Zhao, Y.; Li, J.; Lai, C.; Liu, C. Effects of Stocking Density on the Non-Specific Immune Functions of Juvenile Russian Sturgeon (Acipenser gueldenstacdti) in Flowing Water Cultivation. Guangxi Sci. 2017, 24, 389–395. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Chen, M. Effect of thermal stimulus on immune function and heat shock protein expression of scallop Chlamysfarreri. Period. Ocean Univ. China 2017, 47, 31–43. [Google Scholar] [CrossRef]
- Welker, T.L.; Congleton, J.L. Oxidative stress in juvenile chinook salmon, Oncorhynchus tshawytscha (Walbaum). Aquac. Res. 2004, 35, 881–887. [Google Scholar] [CrossRef]
- Adeli, M.M.; Kazempoor, R.; Shirazi, N.H. Effect of Probiotic Lactobacillus fermentum on Growth Performance, Bioaccumulation and Antioxidant Defenses of Zebrafish (Danio rerio) Under Cadmium Toxicity. Aquac. Stud. 2022, 23, AQUAST991. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Nie, Z.; Gao, J.; Sun, Y.; Shao, N.; Li, Q.; Hu, J.; Xu, P.; Xu, G. Effects of stocking density on growth, serum parameters, antioxidant status, liver and intestine histology and gene expression of largemouth bass (Micropterus salmoides) farmed in the in-pond raceway system. Aquac. Res. 2020, 51, 5228–5240. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zhu, J.; Ge, X.P.; Sun, S.M.; Su, Y.L.; Li, B.; Hou, Y.R.; Ren, M.C. Effects of stocking density on the growth performance, digestive enzyme activities, antioxidant resistance, and intestinal microflora of blunt snout bream (Megalobrama amblycephala) juveniles. Aquac. Res. 2018, 50, 236–246. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, L.; Liu, B.-S.; Guo, H.-Y.; Zhu, K.-C.; Zhang, N.; Jiang, S.-G.; Zhang, D.-C. Effects of stocking density on the growth performance, serum biochemistry, muscle composition and HSP70 gene expression of juvenile golden pompano Trachinotus ovatus (Linnaeus, 1758). Aquaculture 2020, 518, 734841. [Google Scholar] [CrossRef]
- Braun, N.; de Lima, R.L.; Baldisserotto, B.; Dafre, A.L.; de Oliveira Nuñer, A.P. Growth, biochemical and physiological responses of Salminus brasiliensis with different stocking densities and handling. Aquaculture 2010, 301, 22–30. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Sun, P.; Tang, B. Physiological response of Larimichthys crocea under different stocking densities and screening of stress sensitive biomarkers. Mar. Fish. 2021, 43, 327–339. [Google Scholar] [CrossRef]
- Storey, K.B. biological research = Revista brasileira de pesquisas medicas e biologicas. Braz. J. Med. 1996, 29, 1715. [Google Scholar]
- Zhong, L.; Hu, Y.; Hu, Y.; Li, J.; Tian, Y.; Chen, J.; Ai, Q.; Xiao, T. Effects of dietary tea polyphenols on growth, immunity and lipid metabolism of juvenile black carp Mylopharyngodon piceus. Aquac. Res. 2019, 51, 569–576. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, Y.; Hu, W.; Tang, H.; Li, H.; Li, X. Effects of sulfamonomethoxine sodium on SOD activitiy and MDA content in juveniles of Megalobrama pellegrini. Freshw. Fish. 2016, 46, 55–59. [Google Scholar] [CrossRef]
- Papadimitriou, E.; Loumbourdis, N.S. Exposure of the Frog Rana ridibunda to Copper: Impact on Two Biomarkers, Lipid Peroxidation, and Glutathione. Bull. Environ. Contam. Toxicol. 2002, 69, 885–891. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Feng, W.R.; Han, C.; Huang, B.; Lei, J.L. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish Shellfish. Immunol. 2016, 55, 131–139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).