The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Molecular Analysis
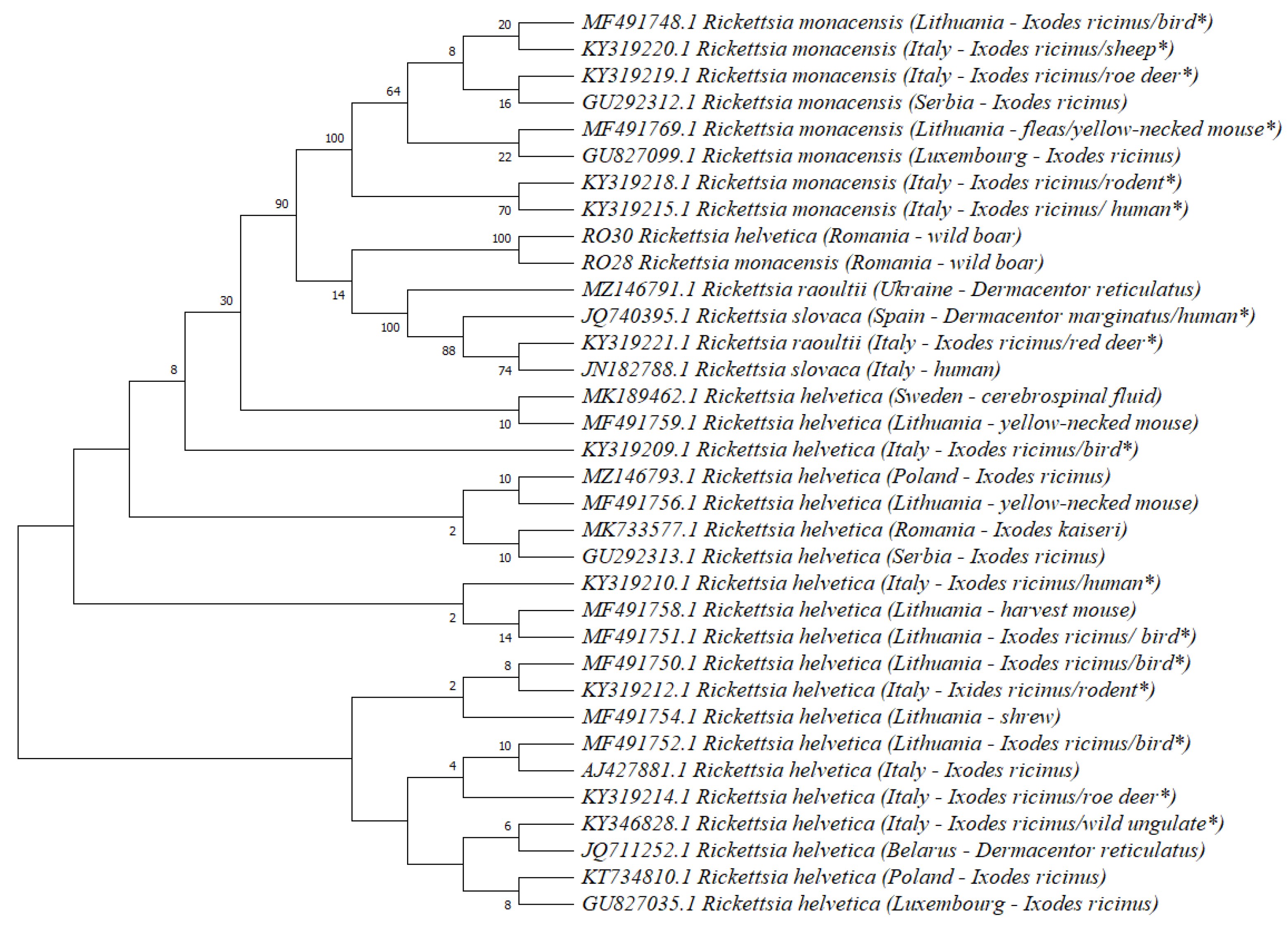
2.3. Phylogenetical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild ungulate overabundance in Europe: Contexts, causes, monitoring and management recommendations. Mammal Rev. 2020, 51, 95–108. [Google Scholar] [CrossRef]
- Markov, N.; Pankova, N.; Morelle, K. Where winter rules: Modeling wild boar distribution in its north-eastern range. Sci. Total Environ. 2019, 687, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Şelaru, N. Manual Pentru Examenul de Vânător; Editura Cynegis: Bucharest, Romania, 2001. (In Romanian) [Google Scholar]
- Contreras, R.C. Urban Wild Boar: Drivers of Presence, Phenotypic Responses and Health Concerns. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Merrill, M.M.; Boughton, R.K.; Lord, C.C.; Sayler, K.A.; Wight, B.; Anderson, W.M.; Wisely, S.M. Wild pigs as sentinels for hard ticks: A case study from south-central Florida. IJP Parasites Wildl. 2018, 7, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Szekeres, S.; Horváth, G.; Takács, N.; Bekő, K.; Kontschán, J.; Gyuranecz, M.; Tóth, B.; Sándor, A.D.; Juháasz, A.; et al. Diversity of tick species and associated pathogens on peri-urban wild boars—First report of the zoonotic Babesia cf. crassa from Hungary. Ticks Tick Borne Dis. 2022, 13, 101936. [Google Scholar] [CrossRef]
- Millán, J.; Proboste, T.; Fernández de Mera, I.G.; Chirife, A.D.; Fuente, J.; Altet, L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human–wildlife interface. Ticks Tick. Borne Dis. 2015, 7, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I.; Toledo, A. Emerging tick borne pathogens of public health importance : A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa. In A Guide to Species Identification; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–9. [Google Scholar]
- Ogden, N.H.; Beard, C.B.; Ginsberg, H.S.; Tsao, J.I. Special collection: Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit : Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Taussat, M.V.; Castro, L.V.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Faria, A.S.; Paiva-Cardoso, M.N.; Nunes, M.; Carreira, T.; Vale-Gonçalves, H.M.; Coelho, C.; Vieira-Pinto, M.; Vieira, M.L. First Detection of Borrelia burgdorferi sensu lato DNA in Serum of the Wild Boar (Sus scrofa) in Northern Portugal by Nested-PCR. EcoHealth 2015, 12, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Cecconi, G.; Sgorbini, M.; Cerri, D. Zoonotic tick-borne bacteria among wild boars (Sus scrofa) in Central Italy. Asian Pac. J. Trop. Dis. 2017, 7, 141–143. [Google Scholar] [CrossRef]
- Hrazdilová, K.; Lesiczka, P.M.; Bardoň, J.; Vyroubalová, Š.; Šimek, B.; Zurek, L.; Modrý, D. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick-Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef]
- Selmi, M.; Martello, E.; Bertolotti, L.; Bisanzio, D.; Tomassone, L. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus ticks collected on wild boars in Tuscany, Italy. J. Med. Entomol. 2009, 46, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; Wielinga, P.R.; Fonville, M.; Reusken, C.; Brandenburg, A.H.; Borgsteede, F.; Gaasenbeek, C.; Van der Giessen, J.W. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasites Vector 2009, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Giglio, G.; Ramassa, E.; Nobili, F.; Rossi, L.; Tomassone, L. Dermacentor marginatus and Dermacentor reticulatus, and their infection by SFG Rickettsiae and Francisella -Like Endosymbionts, in mountain and periurban habitats of Northwestern Italy. Vet. Sci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Sgroi, G.; Iatta, R.; Lia, R.; Alessio, N.D.; Manoj, R.; Veneziano, V.; Otranto, D. Spotted fever group Rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound Emerg. Dis. 2020, 68, 2111–2120. [Google Scholar] [CrossRef]
- Zeroual, F.; Leulmi, H.; Bitam, I.; Benakhla, A. Molecular evidence of Rickettsia slovaca in spleen of wild boars in northeastern Algeria. New Microbes New Infect. 2018, 24, 17–20. [Google Scholar] [CrossRef] [PubMed]
- CastilloüContreras, R.; Magen, L.; Birtles, R.; Varela-Castro, L.; Hall, J.L.; Conejero, C.; López-Olvera, J.R. Ticks on wild boar in the metropolitan area of Barcelona (Spain) are infected with spotted fever group rickettsiae. Transbound Emerg. Dis. 2022, 69, 82–95. [Google Scholar]
- Grassi, L.; Menandro, M.L.; Cassini, R.; Mondin, A.; Pasotto, D.; Grillini, M.; Rocca, G.; Drigo, M. High Prevalence of tick-borne zoonotic Rickettsia slovaca in ticks from wild boars, northeastern Italy. Animals 2022, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, A.; Lopez-Claessens, S.; Castella, J.; Sanfeliu, I.; Anton, E.; Segura-Porta, F. The role of wild boar (Sus scrofa) in the eco-epidemiology of R. slovaca in northeastern Spain. Vector-Borne Zoo Dis. 2007, 7, 59–64. [Google Scholar] [CrossRef]
- Motoi, Y.; Asano, M.; Inokuma, H.; Ando, S.; Kawabata, H.; Takano, A. Detection of Rickettsia tamurae DNA in ticks and wild boar (Sus scrofa leucomystax) skins in Shimane Prefecture, Japan. J. Vet. Med. Sci. 2012, 75, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kmetiuk, L.B.; Krawczak, F.S.; Machado, F.P.; Paploski, I.A.D.; Martins, T.F.; Teider-Junior, P.I.; Serpa, M.C.A.; Barbieri, A.R.; Bach, R.V.; Barros-Filho, I.R.; et al. Ticks and serosurvey of anti- Rickettsia spp. antibodies in wild boars (Sus scrofa), hunting dogs and hunters of Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007405. [Google Scholar] [CrossRef] [PubMed]
- Kmetiuk, L.B.; Martins, T.F.; Bach, R.V.W.; Martins, C.M. Risk factors associated with ticks and Rickettsia spp. exposure in wild boars (Sus scrofa), hunting dogs, and hunters of Brazil. Vet World 2021, 14, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Genov, P.V. A review of the cranial characteristics of the Wild Boar (Susscrofa Linnaeus 1758), with systematic conclusions. Mammal Rev. 1999, 29, 205–234. [Google Scholar] [CrossRef]
- Albarella, U.; Dobney, K.; Rowley-Conwy, P. Size and shape of the Eurasian wild boar (Sus scrofa), with a view to the reconstruction of its Holocene history. Environ. Archaeol. 2009, 14, 103–136. [Google Scholar] [CrossRef]
- Markov, N.; Economov, A.; Hjeljord, O.; Rolandsen, C.M.; Bergqvist, G.; Danilov, P.; Senchik, A. The wild boar Sus scrofa in northern Eurasia: A review of range expansion history, current distribution, factors affecting the northern distributional limit, and management strategies. Mammal Rev. 2022, 52, 10–12. [Google Scholar] [CrossRef]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Zobba, R.; Chessa, B.; Addis, M.F.; Sparagano, O.; Pinna Parpaglia, M.L.; Cubeddu, T.; Pintori, G.; Pittau, M. Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl. Environ. Microbiol. 2005, 71, 6418–6422. [Google Scholar] [CrossRef]
- Hornok, S.; Szőke, K.; Kováts, D.; Estók, P.; Görfö, T.; Boldogh, S.A.; Takács, N.; Kontschán, J.; Földvári, G.; Barti, L.; et al. DNA of Piroplasms of ruminants and dogs in Ixodid bat ticks. PLoS ONE 2016, 11, e0167735. [Google Scholar] [CrossRef]
- Coipan, E.C.; Fonville, M.; Tijsse-Klasen, E.; van der Giessen, J.W.; Takken, W.; Sprong, H. Geodemographic analysis of Borrelia burgdorferi sensu lato using the 5S-23S rDNA spacer region. Infect. Genet. Evol. 2013, 17, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Siarkou, V.I.; Mylonakis, M.E.; Bourtzi-Hatzopoulou, E.; Koutinas, A.F. Sequence and phylogenetic analysis of the 16S rRNA gene of Ehrlichia canis strains in dogs with clinical monocytic ehrlichiosis. Vet. Microbiol. 2007, 125, 304–312. [Google Scholar] [CrossRef]
- Leitner, M.; Yitzhaki, S.; Rzotkiewicz, S.; Keysary, A. Polymerase chain reaction-based diagnosis of Mediterranean spotted fever in serum and tissue samples. Am. J. Trop. Med. Hyg. 2002, 67, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; Leeuwen, A.D.V.; Hengeveld, P.; Heylen, D.; Heyman, P.; Maanen, C.V.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vector 2014, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Parreira, R.; Nunes, M.; Casadinho, A.; Vieira, M.L.; Campino, L.; Maia, C. Molecular detection of tick-borne bacteria and protozoa in cervids and wild boars from Portugal. Parasites Vectors 2016, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Guccione, C.; Colomba, C.; Iaria, C.; Cascio, A. Rickettsiales in the WHO European Region: An update from a One Health perspective. Parasites Vectors. 2023, 16, 41. [Google Scholar] [CrossRef]
- Mihalca, A.D.; Dumitrache, M.O.; Magdaş, C.; Gherman, C.M.; Domşa, C.; Mircean, V.; Sándor, A.D. Synopsis of the hard ticks (Acari: Ixodidae) of Romania with update on host associations and geographical distribution. Exp. Appl. Acaro 2012, 58, 183–206. [Google Scholar] [CrossRef]
- Ivan, T.; Matei, I.A.; Novac, C.Ș.; Kalmár, Z.; Borșan, S.D.; Panait, L.C.; Mihalca, A.D. Spotted Fever Group Rickettsia spp. diversity in ticks and the first report of Rickettsia hoogstraalii in Romania. Vet. Sci. 2022, 9, 343. [Google Scholar] [CrossRef]
- Raileanu, C.; Moutailler, S.; Porea, D.; Oslobanu, L.; Anita, D.; Anita, A.; Vayssier-Taussat, M.; Savuta, G. Molecular evidence of Rickettsia spp., Anaplasma phagocytophilum, and ‘Candidatus Neoehrlichia mikurensis’ in ticks from natural and urban habitats in eastern Romania. Vector-Borne Zoonotic Dis. 2018, 18, 343–349. [Google Scholar] [CrossRef]
- Borșan, S.D.; Ionică, A.M.; Galon, C.; Toma-Naic, A.; Peștean, C.; Sandor, A.D.; Moutailler, D.; Mihalca, A.D. High diversity, prevalence, and co-infection rates of tick-borne pathogens in ticks and wildlife hosts in an urban area in Romania. Front. Microbiol. 2021, 12, 645002. [Google Scholar] [CrossRef]
- Mǎrcuţan, I.D.; Kalmár, Z.; IonicǍ, A.M.; D’Amico, G.; Mihalca, A.D.; Vasile, C.; Sándor, A.D. Spotted fever group rickettsiae in ticks of migratory birds in Romania. Parasites Vector 2016, 9, 3–9. [Google Scholar] [CrossRef]
- Sándor, A.D.; Kalmár, Z.; Matei, I.; Ionică, A.M.; Mărcuțan, I.D. Urban Breeding Corvids as Disseminators of Ticks and tick-borne pathogens. Vector Borne Zoonotic Dis. 2017, 17, 152–154. [Google Scholar] [CrossRef]
- Matei, I.A.; Corduneanu, A.; Sándor, A.D.; Ionica, A.M.; Panait, L.; Kalmár, Z.; Ivan, T.; Papuc, I.; Bouari, C.; Fit, N.; et al. Rickettsia spp. in bats of Romania: High prevalence of Rickettsia monacensis in two insectivorous bat species. Parasites Vectors 2021, 14, 1–9. [Google Scholar]
- Nilsson, K.; Lindquist, O.; Påhlson, C. Association of Rickettsia helvetica with chronic perimy ocarditis in sudden cardiac death. Lancet 1999, 354, 1169–1173. [Google Scholar] [CrossRef]
- Fournier, P.; Allombert, C.; Supputamongkol, Y.; Caruso, G.; Brouqui, P.; Raoult, D. Aneruptive Fever Associated with Antibodies to Rickettsia helvetica in Europe and Thailand. J. Clin. Microbiol. 2004, 42, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Jado, I.; Oteo, J.A.; Aldámiz, M.; Gil, H.; Escudero, R.; García-amil, C.; Rodríguez-moreno, I.; Anda, P. Rickettsia monacensis and human disease, Spain. Emerg. Infect Dis. 2007, 13, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Boretti, F.S.; Perreten, A.; Meli, M.L.; Cattori, V.; Willi, B.; Wengi, N.; Honegger, H.; Hegglin, D.; Reusch, C.E.; Lutz, H.; et al. Molecular Investigations of Rickettsia helvetica Infection in Dogs, Foxes, Humans, and Ixodes ticks. App. Environ. Microbiol. 2009, 75, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Mancini, F.; Caddeo, A.; Ciervo, A.; Babudieri, S.; Maida, I.; Mura, M.S. Rickettsia monacensis as Cause of Mediterranean Spotted Fever – like Illness, Italy. Emerg. Infect. Dis. 2012, 18, 702–704. [Google Scholar] [CrossRef]
- Kiss, T.; Cadar, D.; Krupaci, A.F.; Bordeanu, A.; Brudaşcǎ, G.F.; Mihalca, A.D.; Mircean, V.; Gliga, L.; Dumitrache, M.O.; Spînu, M. Serological reactivity to Borrelia burgdorferi sensu lato in dogs and horses from distinct areas in Romania. Vector-Borne Zoonotic Dis. 2011, 11, 1259–1262. [Google Scholar] [CrossRef]
- Michalik, J.; Stańczak, J.; Cieniuch, S.; Racewicz, M.; Sikora, B.; Dabert, M. Wild Boars as Hosts of Human- Pathogenic Anaplasma phagocytophilum Variants. Emerg. Infect. Dis. 2012, 18, 2094–2095. [Google Scholar] [CrossRef]
- Huhn, C.; Winter, C.; Wolfsperger, T.; Wüppenhorst, N.; Strašek Smrdel, K.; Skuballa, J.; Von Loewenich, F.D. Analysis of the population structure of Anaplasma phagocytophilum using multilocus sequence typing. PLoS ONE 2014, 9, e93725. [Google Scholar] [CrossRef] [PubMed]
- Smrdel, K.S.; Bidovec, A.; Malovrh, T.; Petrovec, M.; Duh, D.; Zupanc, T.A. Detection of Anaplasma phagocytophilum in wild boar in Slovenia. Clin. Microbiol. Infect. 2008, 15, 50–52. [Google Scholar] [CrossRef] [PubMed]

| Pathogen | Primer Name and Sequence | Gene | Reference |
|---|---|---|---|
| Anaplasma spp./ A. phagocytophilum | Ge3a: CACATGCAAGTCGAACGGATTATTC Ge10: TTCCGTTAAGAAGGATCTAATCTCC Ge2: AACGGATTATTCTTTATAGCTTGCT Ge9: GGCAGTATTAAAAGCAGCTCCAGG | 16S rRNA | [30] |
| A. phagocytophilum | EphplgroEL(569)F: ATGGTATGCAGTTTGATCGC EphplgroEL(1193)R: TCTACTCTGTCTTTGCGTTC EphgroEL(1142)R: TTGAGTACAGCAACACCACCGGAA | groEL | [31] |
| Babesia/Theileria spp., | BJ1: GTCTTGTAATTGGAATGATGG BN2: TAGTTTATGGTTAGGACTACG | 18S rRNA | [32] |
| Borrelia spp. | 5SCB: GAGTTCGCGGGAGAGTAGGTTATTGCC 23SN: TCAGGGTACTTAGATGGTTCACTTCC | IGS | [33] |
| Ehrlichia spp./ E. canis | ECC: AGAACGAACGCTGGCGGCAAGCC ECB: CGTATTACCGCGGCTGCTGGCA Canis: CAATTATTTATAGCCTCTGGCTATAGGA HE3: TATAGGTACCGTCATTATCTTCCCTAT | 16S rRNA | [34] |
| SFG Rickettsia spp. | 17DrikP3 GGAACACTTCTTGGCGGTG 17DrikP2 CATTGTCCGTCAGGTTGGCG 17DrikP4 GAGAGATGCTTATGGTAAGAC 17DrikP5 GAGAGATGCTTATGGTAAGAC | 17 kDA | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, I.A.; Kalmár, Z.; Balea, A.; Mihaiu, M.; Sándor, A.D.; Cocian, A.; Crăciun, S.; Bouari, C.; Briciu, V.T.; Fiț, N. The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence. Animals 2023, 13, 1743. https://doi.org/10.3390/ani13111743
Matei IA, Kalmár Z, Balea A, Mihaiu M, Sándor AD, Cocian A, Crăciun S, Bouari C, Briciu VT, Fiț N. The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence. Animals. 2023; 13(11):1743. https://doi.org/10.3390/ani13111743
Chicago/Turabian StyleMatei, Ioana Adriana, Zsuzsa Kalmár, Anamaria Balea, Marian Mihaiu, Attila D. Sándor, Adrian Cocian, Smaranda Crăciun, Cosmina Bouari, Violeta Tincuța Briciu, and Nicodim Fiț. 2023. "The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence" Animals 13, no. 11: 1743. https://doi.org/10.3390/ani13111743
APA StyleMatei, I. A., Kalmár, Z., Balea, A., Mihaiu, M., Sándor, A. D., Cocian, A., Crăciun, S., Bouari, C., Briciu, V. T., & Fiț, N. (2023). The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence. Animals, 13(11), 1743. https://doi.org/10.3390/ani13111743





