Genetic Parameter Estimates of Growth Curve and Feed Efficiency Traits in Japanese Quail
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Basic Statistics
3.2. Heritability Estimates
3.3. Genetic and Phenotypic Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emmerson, D. Breeding objectives and selection strategies for broiler production. In Poultry Breeding, Genetics and Biotechnology; CAB International: Wallingford, UK, 2003; pp. 113–126. [Google Scholar]
- Tixier-Boichard, M.; Leenstra, F.; Flock, D.K.; Hocking, P.M.; Weigend, S. A century of poultry genetics. Worlds Poult. Sci. J. 2012, 68, 307–321. [Google Scholar] [CrossRef]
- Narinc, D.; Aksoy, T.; Kaplan, S. Effects of multi-trait selection on phenotypic and genetic changes in Japanese quail (Coturnix coturnix japonica). J. Poult. Sci. 2016, 53, 103–110. [Google Scholar] [CrossRef]
- Wolc, A. Understanding genomic selection in poultry breeding. Worlds Poult. Sci. J. 2014, 70, 309–314. [Google Scholar] [CrossRef]
- Wolc, A.; Kranis, A.; Arango, J.; Settar, P.; Fulton, J.E.; O’Sullivan, N.P.; Avendano, A.; Watson, K.A.; Hickey, J.M.; de los Campos, G.; et al. Implementation of genomic selection in the poultry industry. Anim. Front. 2016, 6, 23–31. [Google Scholar] [CrossRef]
- Marks, H.L. Long-term selection for body weight in Japanese quail under different environments. Poult. Sci. 1996, 75, 1198–1203. [Google Scholar] [CrossRef]
- Minvielle, F.; Monvoisin, J.L.; Costa, J.; Maeda, Y. Long-term egg production and heterosis in quail lines after within-line or reciprocal recurrent selection for high early egg production. Br. Poult. Sci. 2000, 41, 150–157. [Google Scholar] [CrossRef]
- Varkoohi, S.; Babak, M.M.S.; Pakdel, A.; Javaremi, A.N.; Zaghari, M.; Kause, A. Response to selection for feed conversion ratio in Japanese quail. Poult. Sci. 2010, 89, 1590–1598. [Google Scholar] [CrossRef]
- Minvielle, F. What are quail good for in a chicken-focused world? World’s Poult. Sci. J. 2009, 65, 601–608. [Google Scholar] [CrossRef]
- Akbas, Y.; Yaylak, E. Heritability estimates of growth curve parameters and genetic correlations between the growth curve parameters and weights at different age of Japanese quail. Arch. Geflugelkd. 2000, 64, 141–146. [Google Scholar]
- Aksit, M.; Oguz, I.; Akbas, Y.; Altan, Ö.; Özdoğan, M. Genetic variation of feed traits and relationships to some meat production traits in Japanese quail (Coturnix cot. japonica). Arch. Geflugelkd. 2003, 67, 76–82. [Google Scholar]
- Narinc, D.; Aksoy, T.; Karaman, E.; Aygun, A.; Firat, M.Z.; Uslu, M.K. Japanese quail meat quality: Characteristics, heritabilities, and genetic correlations with some slaughter traits. Poult. Sci. 2013, 92, 1735–1744. [Google Scholar] [CrossRef]
- Narinc, D.; Karaman, E.; Aksoy, T.; Firat, M.Z. Genetic parameter estimates of growth curve and reproduction traits in Japanese quail. Poult. Sci. 2014, 93, 24–30. [Google Scholar] [CrossRef]
- Karabag, K.; Alkan, S.; Karsli, T.; Balcioglu, M.S. Genetic changes in growth curve parameters in Japanese quail lines divergently selected for body weight. Eur. Poult. Sci. 2017, 81, 10. [Google Scholar] [CrossRef]
- Prakash, A.; Saxena, V.K.; Singh, M.K. Genetic analysis of residual feed intake, feed conversion ratio and related growth parameters in broiler chicken: A review. Worlds Poult. Sci. J. 2020, 76, 304–317. [Google Scholar] [CrossRef]
- Skinner-Noble, D.; McKinney, L.; Teeter, R. Predicting effective caloric value of nonnutritive factors: III. Feed form affects broiler performance by modifying behavior patterns. Poult. Sci. 2005, 84, 403–411. [Google Scholar] [CrossRef]
- Dransfield, E.; Sosnicki, A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999, 78, 743–746. [Google Scholar] [CrossRef]
- Narinc, D.; Karaman, E.; Firat, M.Z.; Aksoy, T. Comparison of non-linear growth models to describe the growth in Japanese quail. J. Anim. Vet. Adv. 2010, 9, 1961–1966. [Google Scholar] [CrossRef]
- Akbas, Y.; Oguz, I. Growth curve parameters of lines of Japanese quail (Coturnix coturnix japonica), unselected and selected for four-week body weight. Arch. Fuer Gefluegelkunde 1998, 62, 104–109. [Google Scholar]
- Balcıoğlu, M.; Kızılkaya, K.; Yolcu, H.; Karabağ, K. Analysis of growth characteristics in short-term divergently selected Japanese quail. S. Afr. J. Anim. Sci. 2005, 35, 83–89. [Google Scholar]
- Zhang, Z. A note on wishart and inverse wishart priors for covariance matrix. J. Behav. Data Sci. 2021, 1, 119–126. [Google Scholar] [CrossRef]
- Hadfield, J.D. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Alkan, S.; Karabag, K.; Galic, A.; Karsli, T.; Balcioglu, M.S. Determination of body weight and some carcass traits in Japanese quails (Coturnix coturnix japonica) of different lines. Kafkas Univ. Vet. Fak. Derg. 2010, 16, 277–280. [Google Scholar]
- Foomani, N.N.; Zerehdaran, S.; Azari, M.A.; Lotfi, E. Genetic parameters for feed efficiency and body weight traits in Japanese quail. Br. Poult. Sci. 2014, 55, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.Y.; Hafez, A.S.A.; Emam, A.M.; Abdelmoniem, A.M.; ElSafty, S.A. Feathering rate impact on growth and slaughter traits of Japanese quail. J. Agric. Sci. 2018, 156, 942–948. [Google Scholar] [CrossRef]
- Fathi, M.M.; Al-Homidan, I.; Ebeid, T.A.; Galal, A.; Abou-Emera, O.K. Assessment of residual feed intake and its relevant measurements in two varieties of Japanese quails (Coturnixcoturnix japonica) under high environmental temperature. Animals 2019, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, E.; Zerehdaran, S.; Azari, M.A. Genetic evaluation of carcass composition and fat deposition in Japanese quail. Poult. Sci. 2011, 90, 2202–2208. [Google Scholar] [CrossRef]
- Karaman, E.; Firat, M.Z.; Narinc, D. Single-trait Bayesian analysis of some growth traits in Japanese quail. Braz. J. Poult. Sci. 2014, 16, 51–56. [Google Scholar] [CrossRef]
- Shafik, B.M.; Kamel, E.R.; Mamdouh, M.; Elrafaay, S.; Nassan, M.A.; El-Bahy, S.M.; El-Tarabany, M.S.; Manaa, E.A. Performance, blood lipid profile, and the expression of growth hormone receptor (GHR) and insulin-like growth factor-1 (IGF-1) genes in purebred and crossbred quail lines. Animals 2022, 12, 1245. [Google Scholar] [CrossRef]
- Altan, O.; Oguz, I.; Akbas, Y.; Aksit, M. Genetic variability of residual feed consumption (RFC) and its relationships with some production traits and fear response in Japanese quail hens (Cotumix cot. japonica). Arch. Geflugelkd. 2004, 68, 223–229. [Google Scholar]
- Narinc, D.; Aksoy, T.; Karaman, E. Genetic parameters of growth curve parameters and weekly body weights in Japanese quails (Coturnix coturnix japonica). J. Anim. Vet. Adv. 2010, 9, 501–507. [Google Scholar] [CrossRef]
- Beiki, H.; Pakdel, A.; Moradi-Shahrbabak, M.; Mehrban, H. Evaluation of growth functions on Japanese quail lines. J. Poult. Sci. 2013, 50, 20–27. [Google Scholar] [CrossRef]
- Hyankova, L.; Knížetová, H.; Dědková, L.; Hort, J. Divergent selection for shape of growth curve in Japanese quail. 1. Responses in growth parameters and food conversion. Br. Poult. Sci. 2001, 42, 583–589. [Google Scholar] [CrossRef]
- Aggrey, S.E.; Karnuah, A.B.; Sebastian, B.; Anthony, N.B. Genetic properties of feed efficiency parameters in meat-type chickens. Genet. Sel. Evol. 2010, 42, 25. [Google Scholar] [CrossRef]
- Vali, N.; Edriss, M.; Rahmani, H. Genetic parameters of body and some carcass traits in two quail strains. Int. J. Poult. Sci. 2005, 4, 296–300. [Google Scholar]
- Narinç, D.; Aksoy, T. Effects of mass selection based on phenotype and early feed restriction on the performance and carcass characteristics in Japanese quails. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 425–430. [Google Scholar]
- Waldmann, P.; Ericsson, T. Comparison of REML and Gibbs sampling estimates of multi-trait genetic parameters in Scots pine. Theor. Appl. Genet. 2006, 112, 1441–1451. [Google Scholar] [CrossRef]
- Narinc, D.; Aydemir, E. Genetic parameter estimates of chick quality, growth, and carcass characteristics in Japanese quail. J. Hell. Vet. Med. Soc. 2021, 72, 3363–3370. [Google Scholar] [CrossRef]
- Taroco, G.; Gaya, L.G.; Mota, L.F.M.; Souza, K.A.R.; Lima, H.J.D.; Silva, M.A. Heritability and genotype-environment interactions for growth curve parameters in meat-type quail fed different threonine:lysine ratios from hatching to 21 d of age. Poult. Sci. 2019, 98, 69–73. [Google Scholar] [CrossRef]
- Caetano, G.D.; Mota, R.R.; da Silva, D.A.; de Oliveira, H.R.; Viana, J.M.S.; de Siqueira, O.; Freitas, P.H.F.; Silva, F.F.E. Bayesian estimation of genetic parameters for individual feed conversion and body weight gain in meat quail. Livest. Sci. 2017, 200, 76–79. [Google Scholar] [CrossRef]
- Mignon-Grasteau, S.; Piles, M.; Varona, L.; De Rochambeau, H.; Poivey, J.; Blasco, A.; Beaumont, C. Genetic analysis of growth curve parameters for male and female chickens resulting from selection on shape of growth curve. J. Anim. Sci. 2000, 78, 2515–2524. [Google Scholar] [CrossRef]
- Saghi, R.; Saghi, D. Estimation of heritability, phenotypic and genetic correlations for growth curve characteristics of Japanese quail. J. Anim. Sci. Res. 2022, 31, 113–126. [Google Scholar]
- Akbas, Y.; Takma, C.; Yaylak, E. Genetic parameters for quail body weights using a random regression model. S. Afr. J. Anim. Sci. 2004, 34, 104–109. [Google Scholar] [CrossRef]
- Alkan, S.; Karslı, T.; Galiç, A.; Karabağ, K.; Balcıoğlu, M. Japon bıldırcınlarında (Coturnix coturnix japonica) canlı ağırlığa ait genetik parametrelerin şansa bağlı regresyon modeli kullanılarak tahmin edilmesi. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 935–939. [Google Scholar]
- Kaplan, S.; Narinc, D.; Gurcan, E.K. Genetic parameter estimates of weekly body weight and Richard’s growth curve in Japanese quail. Eur. Poult. Sci. 2016, 80, 10. [Google Scholar] [CrossRef]
- Pakdel, A.; Van Arendonk, J.A.M.; Vereijken, A.L.J.; Bovenhuis, H. Genetic parameters of ascites-related traits in broilers: Correlations with feed efficiency and carcase traits. Br. Poult. Sci. 2005, 46, 43–53. [Google Scholar] [CrossRef]
- Chambers, J.R.; Lin, C.Y. Age-constant versus weight-constant feed consumptions and efficiency in broiler-chickens. Poult. Sci. 1988, 67, 565–576. [Google Scholar] [CrossRef]
- Koerhuis, A.; Hill, W. Predicted response in food conversion ratio for growth by selection on the ratio or on linear component traits, in a (sequential) selection programme. Br. Poult. Sci. 1996, 37, 317–327. [Google Scholar] [CrossRef]

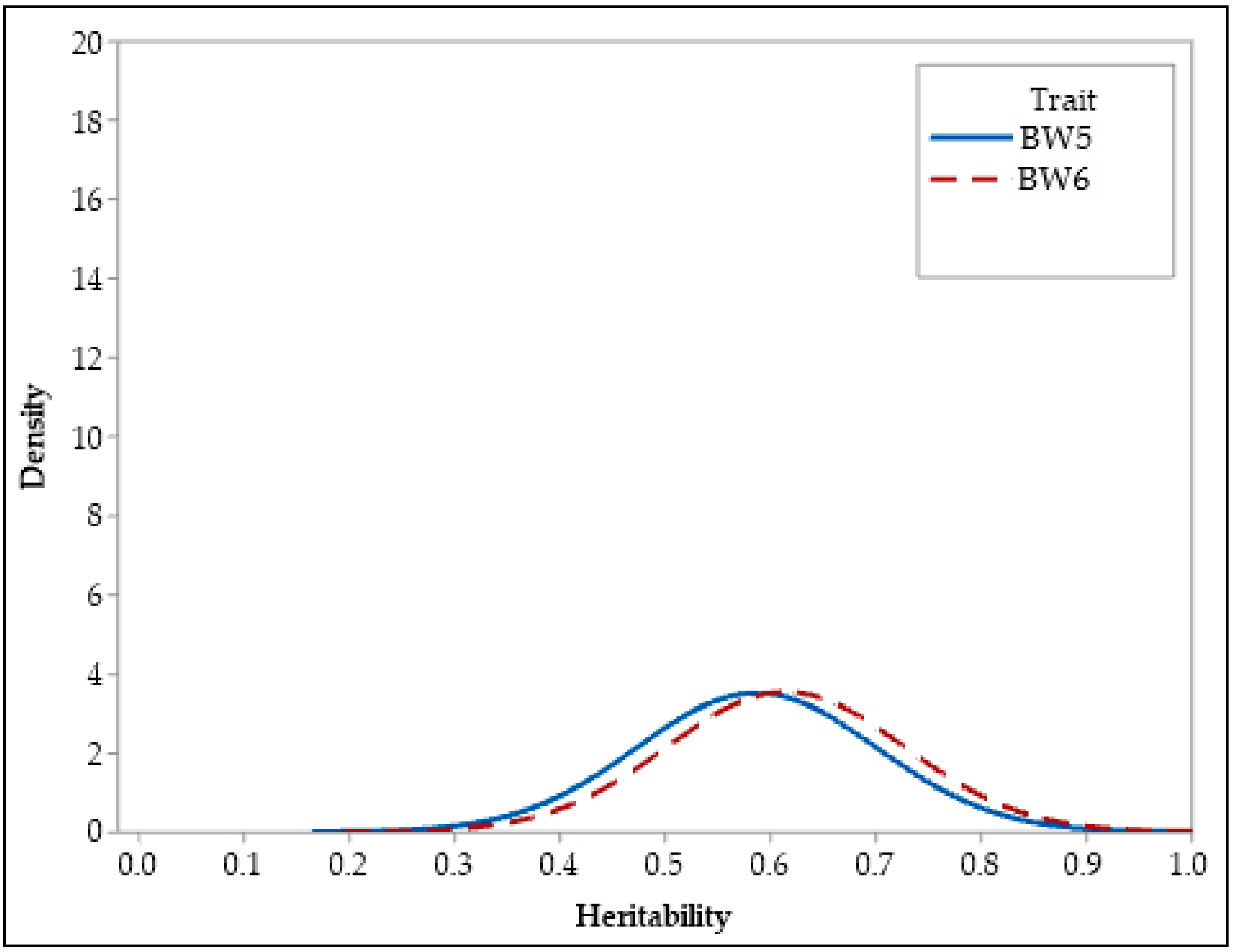

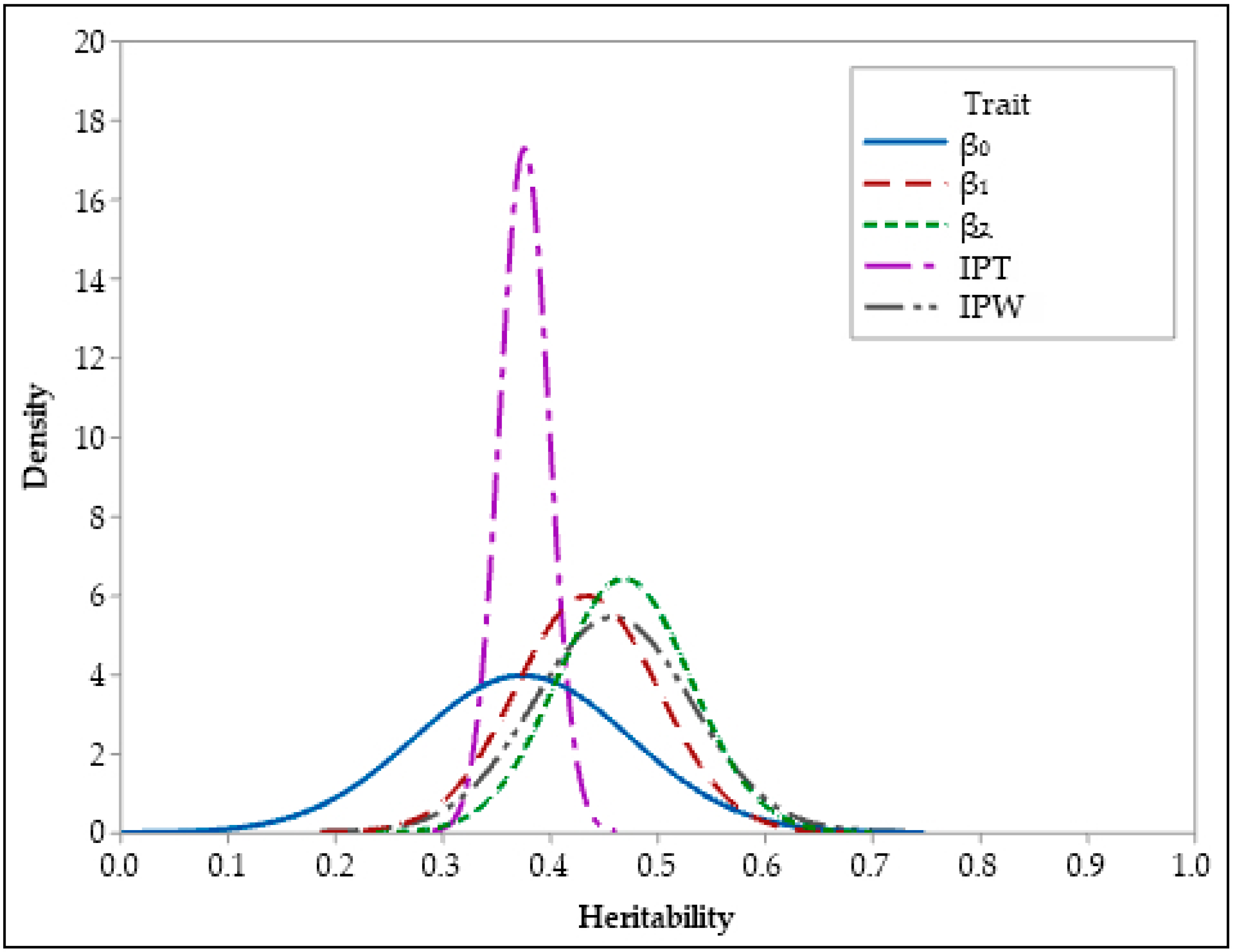
| Trait | Mean | Standard Deviation | Coefficient of Variance (%) | Minimum | Maximum | Sex Effect 2 |
|---|---|---|---|---|---|---|
| BW5 | 185.84 | 19.23 | 10.35 | 134.89 | 242.90 | 0.001 |
| BW6 | 204.54 | 20.48 | 10.01 | 150.22 | 277.83 | 0.001 |
| FI5 | 449.77 | 51.89 | 11.54 | 291.59 | 614.51 | 0.001 |
| FI6 | 621.48 | 77.74 | 12.51 | 354.52 | 822.28 | 0.001 |
| FCE5 | 0.409 | 0.0576 | 14.09 | 0.254 | 0.608 | 0.078 |
| FCE6 | 0.328 | 0.0507 | 15.44 | 0.211 | 0.466 | 0.126 |
| β0 | 249.91 | 36.64 | 14.66 | 171.08 | 338.44 | 0.003 |
| β1 | 3.31 | 0.23 | 6.84 | 2.77 | 3.99 | 0.125 |
| β2 | 0.069 | 0.010 | 13.89 | 0.040 | 0.096 | 0.245 |
| IPT | 17.60 | 2.58 | 14.68 | 12.01 | 31.61 | 0.001 |
| IPW | 91.94 | 13.48 | 14.66 | 62.94 | 124.50 | 0.003 |
| Trait 1 | Mean | Median | SD 2 | MCSE 3 | BCI 4 2.5 | BCI 97.5 | HPDI 5 2.5 | HPDI 97.5 |
|---|---|---|---|---|---|---|---|---|
| BW5 | 0.59 | 0.57 | 0.11 | 0.001 | 0.45 | 0.73 | 0.46 | 0.73 |
| BW6 | 0.61 | 0.60 | 0.11 | 0.001 | 0.48 | 0.75 | 0.47 | 0.75 |
| FI5 | 0.36 | 0.35 | 0.09 | 0.001 | 0.26 | 0.48 | 0.27 | 0.49 |
| FI6 | 0.23 | 0.23 | 0.05 | 0.001 | 0.11 | 0.32 | 0.12 | 0.35 |
| FCE5 | 0.37 | 0.37 | 0.02 | 0.001 | 0.25 | 0.49 | 0.29 | 0.51 |
| FCE6 | 0.26 | 0.26 | 0.05 | 0.002 | 0.14 | 0.38 | 0.18 | 0.37 |
| β0 | 0.37 | 0.36 | 0.10 | 0.001 | 0.25 | 0.49 | 0.28 | 0.51 |
| β1 | 0.43 | 0.43 | 0.07 | 0.001 | 0.31 | 0.55 | 0.33 | 0.55 |
| β2 | 0.47 | 0.47 | 0.06 | 0.001 | 0.36 | 0.58 | 0.36 | 0.61 |
| IPT | 0.37 | 0.37 | 0.02 | 0.001 | 0.27 | 0.50 | 0.26 | 0.49 |
| IPW | 0.38 | 0.37 | 0.10 | 0.001 | 0.27 | 0.48 | 0.29 | 0.50 |
| BW5 | BW6 | FI5 | FI6 | FCE5 | FCE6 | β0 | β1 | β2 | IPT | IPW | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BW5 | 0.90 * | 0.40 * | 0.33 * | 0.67 * | 0.49 * | 0.52 * | −0.11 | −0.13 | 0.18 | 0.53 * | |
| BW6 | 0.92 (0.01) 2 | 0.28 * | 0.43 * | 0.59 * | 0.75 * | 0.76 * | −0.09 | −0.18 | 0.12 | 0.75 * | |
| FI5 | 0.49 (0.01) | 0.34 (0.01) | 0.68 * | −0.49 * | −0.29 * | 0.08 | −0.02 | −0.02 | 0.08 | 0.09 | |
| FI6 | 0.35 (0.11) | 0.48 (0.01) | 0.75 (0.07) | −0.29 * | −0.44 * | 0.09 | −0.01 | −0.01 | 0.10 | 0.08 | |
| FCE5 | 0.71 (0.07) | 0.61 (0.08) | −0.53 (0.08) | −0.32 (0.05) | 0.78 * | 0.29 * | 0.19 | −0.09 | 0.15 | 0.29 | |
| FCE6 | 0.52 (0.05) | 0.83 (0.03) | −0.45 (0.03) | −0.58 (0.02) | 0.83 (0.06) | 0.27 * | 0.06 | −0.23 | 0.11 | 0.28 | |
| β0 | 0.58 (0.01) | 0.82 (0.01) | 0.16 (0.05) | 0.12 (0.06) | 0.34 (0.08) | 0.28 (0.02) | −0.34 * | −0.64 * | 0.66 * | 0.99 * | |
| β1 | −0.17 (0.02) | −0.17 (0.03) | 0.06 (0.05) | 0.21 (0.04) | 0.20 (0.05) | 0.12 (0.02) | −0.37 (0.01) | 0.51 * | 0.71 * | −0.34 * | |
| β2 | −0.16 (0.02) | −0.19 (0.04) | 0.13 (0.01) | 0.11 (0.07) | 0.12 (0.06) | 0.14 (0.04) | −0.70 (0.02) | 0.66 (0.01) | −0.89 * | −0.64 * | |
| IPT | 0.22 (0.04) | 0.20 (0.03) | 0.12 (0.04) | 0.14 (0.04) | 0.17 (0.01) | 0.12 (0.06) | 0.69 (0.07) | 0.79 (0.01) | −0.95 (0.01) | 0.66 * | |
| IPW | 0.58 (0.01) | 0.82 (0.01) | 0.15 (0.08) | 0.22 (0.06) | 0.34 (0.03) | 0.29 (0.03) | 0.99 (0.01) | −0.36 (0.06) | −0.71 (0.01) | 0.70 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya Başar, E.; Narinç, D. Genetic Parameter Estimates of Growth Curve and Feed Efficiency Traits in Japanese Quail. Animals 2023, 13, 1765. https://doi.org/10.3390/ani13111765
Kaya Başar E, Narinç D. Genetic Parameter Estimates of Growth Curve and Feed Efficiency Traits in Japanese Quail. Animals. 2023; 13(11):1765. https://doi.org/10.3390/ani13111765
Chicago/Turabian StyleKaya Başar, Ebru, and Doğan Narinç. 2023. "Genetic Parameter Estimates of Growth Curve and Feed Efficiency Traits in Japanese Quail" Animals 13, no. 11: 1765. https://doi.org/10.3390/ani13111765





