Cardiorespiratory and Neuroprotective Effects of Caffeine in Neonate Animal Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pharmacokinetic Characteristics of Caffeine in Neonate Animals
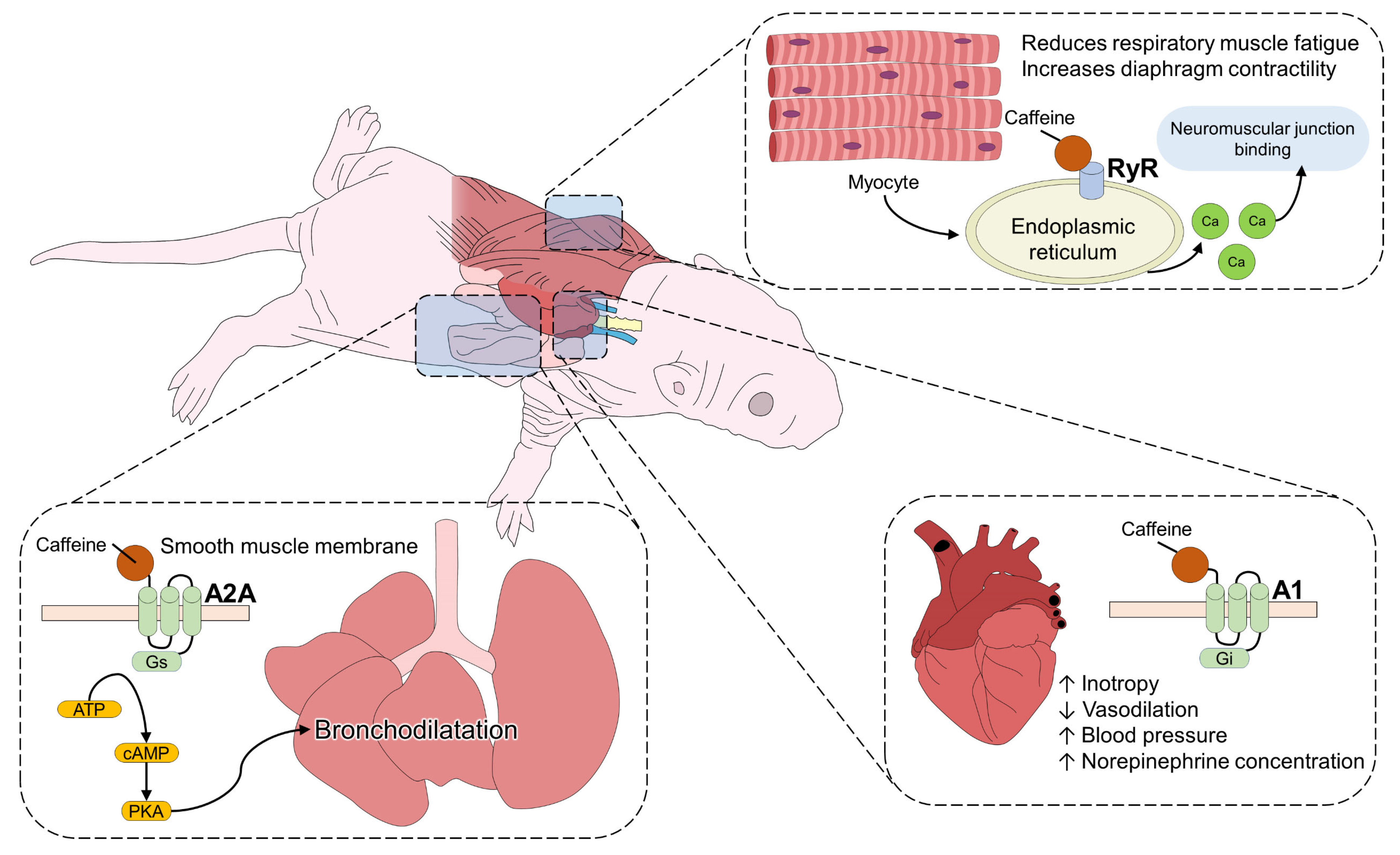
3. Caffeine Pharmacodynamics in Neonates
4. Stimulating Effect on the Respiratory Tract of the Newborn
5. Positive Inotropic Effect of Caffeine
6. Caffeine in Neuroprotection
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M. VI. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar] [PubMed]
- Aranda, J.V.; Beharry, K.D. Pharmacokinetics, pharmacodynamics and metabolism of caffeine in newborns. Semin. Fetal Neonatal Med. 2020, 25, 101183. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; DeVane, C.L. A primer on caffeine pharmacology and its drug interactions in clinical psychopharmacology. Psychopharmacol. Bull. 2001, 35, 30–48. [Google Scholar] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Herlenius, E.; Adén, U.; Tang, L.Q.; Lagercrantz, H. Perinatal respiratory control and its modulation by adenosine and caffeine in the rat. Pediatr. Res. 2002, 51, 4–12. [Google Scholar] [CrossRef]
- Pelchovitz, D.J.; Goldberger, J.J. Caffeine and cardiac arrhythmias: A review of the evidence. Am. J. Med. 2011, 124, 284–289. [Google Scholar] [CrossRef]
- Hsieh, E.M.; Hornik, C.P.; Clark, R.H.; Laughon, M.M.; Benjamin, D.K.; Smith, P.B. Medication use in the neonatal intensive care unit. Am. J. Perinatol. 2014, 31, 811–821. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef]
- Fredholm, B.B. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol. Toxicol. 1995, 76, 93–101. [Google Scholar] [CrossRef]
- Beyer, L.A.; Hixon, M.L. Review of animal studies on the cardiovascular effects of caffeine. Food Chem. Toxicol. 2018, 118, 566–571. [Google Scholar] [CrossRef]
- Blanchard, J.; Sawers, S.J.A. The absolute bioavailability of caffeine in man. Eur. J. Clin. Pharmacol. 1983, 24, 93–98. [Google Scholar] [CrossRef]
- Natarajan, G.; Botica, M.-L.; Thomas, R.; Aranda, J.V. Therapeutic Drug Monitoring for Caffeine in Preterm Neonates: An Unnecessary Exercise? Pediatrics 2007, 119, 936–940. [Google Scholar] [CrossRef]
- Menozzi, A.; Mazzoni, C.; Serventi, P.; Zanardelli, P.; Bertini, S. Pharmacokinetics of oral caffeine in sows: A pilot study. Large Anim. Rev. 2015, 21, 207–210. [Google Scholar]
- Lee, T.C.; Charles, B.; Steer, P.; Flenady, V.; Shearman, A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin. Pharmacol. Ther. 1997, 61, 628–640. [Google Scholar] [CrossRef]
- Noh, K.; Oh, D.G.; Nepal, M.R.; Jeong, K.S.; Choi, Y.; Kang, M.J.; Kang, W.; Jeong, H.G.; Jeong, T.C. Pharmacokinetic interaction of chrysin with caffeine in rats. Biomol. Ther. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Jiritano, L.; Bortolotti, A.; Gaspari, F.; Bonati, M. Caffeine disposition after oral administration to pregnant rats. Xenobiotica 1985, 15, 1045–1051. [Google Scholar] [CrossRef]
- Kimmel, C.A.; Kimmel, G.L.; White, C.G.; Grafton, T.F.; Young, J.F.; Nelson, C.J. Blood flow changes and conceptual development in pregnant rats in response to caffeine. Toxicol. Sci. 1984, 4, 240–247. [Google Scholar] [CrossRef]
- Thorn, C.F.; Whirl-Carrillo, M.; Leeder, J.S.; Klein, T.E.; Altman, R.B. PharmGKB summary. Pharmacogenet. Genom. 2012, 22, 466–470. [Google Scholar] [CrossRef]
- Abdel-Hady, H. Caffeine therapy in preterm infants. World J. Clin. Pediatr. 2015, 4, 81. [Google Scholar] [CrossRef]
- Bienvenu, T.; Pons, G.; Rey, E.; Thiroux, G.; Olive, G. Caffeine metabolism in liver slices during postnatal development in rats. Drug Metab. Dispos. 1993, 21, 178–180. [Google Scholar]
- Le Guennec, J.C.; Billon, B.; Paré, C. Maturational changes of caffeine concentrations and disposition in infancy during maintenance therapy for apnea of prematurity: Influence of gestational age, hepatic disease, and breast-feeding. Pediatrics 1985, 76, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Habeeb, M.S. Caffeine modulates pharmacokinetic and pharmacodynamic profiles of pioglitazone in diabetic rats. Saudi Med. J. 2021, 42, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.S.B.; Lipshultz, E.S. Caffeine and clinical outcomes in premature neonates. Children 2019, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Pedata, F.; Dettori, I.; Coppi, E.; Melani, A.; Fusco, I.; Corradetti, R.; Pugliese, A.M. Purinergic signaling in brain ischemia. Neuropharmacology 2016, 104, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Rivkees, S.A. The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Brain Res. Dev. Brain Res. 1995, 89, 202–213. [Google Scholar] [CrossRef]
- Wojcik, W.J.; Neff, N.H. Differential location of adenosine A1 and A2 receptors in striatum. Neurosci. Lett. 1983, 41, 55–60. [Google Scholar] [CrossRef]
- Atik, A.; Harding, R.; De Matteo, R.; Kondos-Devcic, D.; Cheong, J.; Doyle, L.W.; Tolcos, M. Caffeine for apnea of prematurity: Effects on the developing brain. Neurotoxicology 2017, 58, 94–102. [Google Scholar] [CrossRef]
- Pardo Lozano, R.; Alvarez García, Y.; Barral Tafalla, D.; Farré Albaladejo, M. Cafeína: Un nutriente, un fármaco, o una droga de abuso. Adicciones 2007, 19, 225. [Google Scholar] [CrossRef]
- Fiani, B.; Zhu, L.; Musch, B.L.; Briceno, S.; Andel, R.; Sadeq, N.; Ansari, A.Z. The Neurophysiology of caffeine as a Central Nervous System stimulant and the resultant effects on cognitive function. Cureus 2021, 13, e15032. [Google Scholar] [CrossRef]
- Owolabi, J.O.; Olatunji, S.Y.; Olanrewaju, A.J. Caffeine and cannabis effects on vital neurotransmitters and enzymes in the brain tissue of juvenile experimental rats. Ann. Neurosci. 2017, 24, 65–73. [Google Scholar] [CrossRef]
- Persad, L.A.B. Energy drinks and the neurophysiological impact of caffeine. Front. Neurosci. 2011, 5, 116. [Google Scholar] [CrossRef]
- Kamp, T.J.; Hell, J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 2000, 87, 1095–1102. [Google Scholar] [CrossRef]
- Dias, R.B.; Rombo, D.M.; Ribeiro, J.A.; Henley, J.M.; Sebastião, A.M. Adenosine: Setting the stage for plasticity. Trends Neurosci. 2013, 36, 248–257. [Google Scholar] [CrossRef]
- Friel, D.D.; Tsien, R.W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurons modulates effects of Ca2+ entry on [Ca2+]i. J. Physiol. 1992, 450, 217–246. [Google Scholar] [CrossRef]
- Aronson, R.S.; Cranefield, P.F.; Wit, A.L. The effects of caffeine and ryanodine on the electrical activity of the canine coronary sinus. J. Physiol. 1985, 368, 593–610. [Google Scholar] [CrossRef]
- Kong, H.; Jones, P.P.; Koop, A.; Zhang, L.; Duff, H.J.; Chen, S.R.W. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem. J. 2008, 414, 441–452. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Effect of caffeine on the neuromuscular system—Potential as an ergogenic aid. Appl. Physiol. Nutr. Metab. 2008, 33, 1284–1289. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Caffeine and creatine use in sport. Ann. Nutr. Metab. 2011, 57, 1–8. [Google Scholar] [CrossRef]
- Sarbjit-Singh, S.S.; Matthews, H.R.; Huang, C.L.-H. Ryanodine receptor modulation by caffeine challenge modifies Na+ current properties in intact murine skeletal muscle fibers. Sci. Rep. 2020, 10, 2199. [Google Scholar] [CrossRef]
- Stark, A.; Smith, P.B.; Hornik, C.P.; Zimmerman, K.O.; Hornik, C.D.; Pradeep, S.; Clark, R.H.; Benjamin, D.K.; Laughon, M.; Greenberg, R.G. Medication Use in the Neonatal Intensive Care Unit and Changes from 2010 to 2018. J. Pediatr. 2022, 240, 66–71.e4. [Google Scholar] [CrossRef] [PubMed]
- Oñatibia-Astibia, A.; Martínez-Pinilla, E.; Franco, R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir. Med. 2016, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Smart, D.J.; Steer, P.A. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst. Rev. 2010, 20, CD000273. [Google Scholar] [CrossRef]
- Sawynok, J.; Yaksh, T.L. Caffeine as an analgesic adjuvant: A review of pharmacology and mechanisms of action. Pharmacol. Rev. 1993, 45, 43–85. [Google Scholar] [PubMed]
- Yoder, B.; Thomson, M.; Coalson, J. Lung function in immature baboons with respiratory distress syndrome receiving early caffeine therapy: A pilot study. Acta Paediatr. 2007, 94, 92–98. [Google Scholar] [CrossRef]
- Crossley, K.J.; Allison, B.J.; Polglase, G.R.; Morley, C.J.; Harding, R.; Davis, P.G.; Moss, T.J.M.; Hooper, S.B. Effects of caffeine on renal and pulmonary function in preterm newborn lambs. Pediatr. Res. 2012, 72, 19–25. [Google Scholar] [CrossRef]
- Trippenbach, T.; Zinman, R.; Milic-Emili, J. Caffeine effect on breathing pattern and vagal reflexes in newborn rabbits. Respir. Physiol. 1980, 40, 211–225. [Google Scholar] [CrossRef]
- Julien, C.A.; Joseph, V.; Bairam, A. Caffeine reduces apnea frequency and enhances ventilatory long-term facilitation in rat pups raised in chronic intermittent hypoxia. Pediatr. Res. 2010, 68, 105–111. [Google Scholar] [CrossRef]
- Montandon, G.; Bairam, A.; Kinkead, R. Long-term consequences of neonatal caffeine on ventilation, occurrence of apneas, and hypercapnic chemoreflex in male and female rats. Pediatr. Res. 2006, 59, 519–524. [Google Scholar] [CrossRef]
- Montandon, G.; Kinkead, R.; Bairam, A. Disruption of adenosinergic modulation of ventilation at rest and during hypercapnia by neonatal caffeine in young rats: Role of adenosine A1 and A2A receptors. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R1621–R1631. [Google Scholar] [CrossRef]
- Montandon, G.; Bairam, A.; Kinkead, R. Neonatal caffeine induces sex-specific developmental plasticity of the hypoxic respiratory chemoreflex in adult rats. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R922–R934. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Davis, P.G. Prophylactic methylxanthines for endotracheal extubation in preterm infants. Cochrane Database Syst. Rev. 2010, CD000139. [Google Scholar] [CrossRef]
- Bhatia, J. Current options in the management of apnea of prematurity. Clin. Pediatr. 2000, 39, 327–336. [Google Scholar] [CrossRef]
- Kreutzer, K.; Bassler, D. Caffeine for apnea of prematurity: A neonatal success story. Neonatology 2014, 105, 332–336. [Google Scholar] [CrossRef]
- Skouroliakou, M.; Bacopoulou, F.; Markantonis, S.L. Caffeine versus theophylline for apnea of prematurity: A randomised controlled trial. J. Paediatr. Child Health 2009, 45, 587–592. [Google Scholar] [CrossRef]
- Miao, Y.; Zhou, Y.; Zhao, S.; Liu, W.; Wang, A.; Zhang, Y.; Li, Y.; Jiang, H. Comparative efficacy and safety of caffeine citrate and aminophylline in treating apnea of prematurity: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0274882. [Google Scholar] [CrossRef]
- Teng, R.-J.; Jing, X.; Michalkiewicz, T.; Afolayan, A.J.; Wu, T.-J.; Konduri, G.G. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L586–L598. [Google Scholar] [CrossRef]
- Onland, W.; Cools, F.; Kroon, A.; Rademaker, K.; Merkus, M.P.; Dijk, P.H.; van Straaten, H.L.; Te Pas, A.B.; Mohns, T.; Bruneel, E.; et al. Effect of Hydrocortisone Therapy Initiated 7 to 14 Days After Birth on Mortality or Bronchopulmonary Dysplasia Among Very Preterm Infants Receiving Mechanical Ventilation. JAMA 2019, 321, 354. [Google Scholar] [CrossRef]
- Tian, C.; Li, D.; Fu, J. Molecular Mechanism of Caffeine in Preventing Bronchopulmonary Dysplasia in Premature Infants. Front. Pediatr. 2022, 10, 902437. [Google Scholar] [CrossRef]
- Balany, J.; Bhandari, V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front. Med. 2015, 2, 90. [Google Scholar] [CrossRef]
- Weichelt, U.; Cay, R.; Schmitz, T.; Strauss, E.; Sifringer, M.; Bührer, C.; Endesfelder, S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 2013, 41, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Dayanim, S.; Lopez, B.; Maisonet, T.M.; Grewal, S.; Londhe, V.A. Caffeine induces alveolar apoptosis in the hyperoxia-exposed developing mouse lung. Pediatr. Res. 2014, 75, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I.; Roman, C. The Effects of Caffeine on the Preterm Sheep Ductus Arteriosus. Pediatr. Res. 2007, 62, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Voskoboinik, A.; Kalman, J.M.; Kistler, P.M. Caffeine and arrhythmias: Time to grind the data. JACC Clin. Electrophysiol. 2018, 4, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Howlett, R.A.; Kelley, K.M.; Grassi, B.; Gladden, L.B.; Hogan, M.C. Caffeine administration results in greater tension development in previously fatigued canine muscle in situ. Exp. Physiol. 2005, 90, 873–879. [Google Scholar] [CrossRef]
- Rasmussen, C.A.F.; Sutko, J.L.; Barry, W.H. Effects of ryanodine and caffeine on contractility, membrane voltage, and calcium exchange in cultured heart cells. Circ. Res. 1987, 60, 495–504. [Google Scholar] [CrossRef]
- Miller, M.S.; Friedman, W.F.; Wetzel, G.T. Caffeine-induced contractions in developing rabbit heart. Pediatr. Res. 1997, 42, 287–292. [Google Scholar] [CrossRef]
- Herrmann-Frank, A.; Lüttgau, H.; George Stephenson, D. Caffeine and excitation–contraction coupling in skeletal muscle: A stimulating story. J. Muscle Res. Cell. Motil. 1999, 20, 223–236. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Miranda-Cortés, A.; Ibarra-Ríos, D.; Casas-Alvarado, A.; Mora-Medina, P.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Avalos, I. Caffeine: Cardiorespiratory effects and tissue protection in animal models. Exp. Anim. 2021, 70, 431–439. [Google Scholar] [CrossRef]
- Robertson, S.M.; Friend, M.A.; Doran, G.S.; Edwards, S. Caffeine supplementation of ewes during lambing may increase lamb survival. Animal 2018, 12, 376–382. [Google Scholar] [CrossRef]
- Ishida, S.; Ito, M.; Takahashi, N.; Fujino, T.; Akimitsu, T.; Saikawa, T. Caffeine Induces Ventricular Tachyarrhythmias Possibly Due to Triggered Activity in Rabbits in Vivo. Jpn. Circ. J. 1996, 60, 157–165. [Google Scholar] [CrossRef]
- Mehta, A.; Jain, A.C.; Mehta, M.C.; Billie, M. Caffeine and cardiac arrhythmias. An experimental study in dogs with review of literature. Acta Cardiol. 1997, 52, 273–283. [Google Scholar]
- Tofovic, S.P.; Kost, C.K.; Jackson, E.K.; Bastacky, S.I. Long-term caffeine consumption exacerbates renal failure in obese, diabetic, ZSF1 (fa-facp) rats. Kidney Int. 2002, 61, 1433–1444. [Google Scholar] [CrossRef]
- Awaad, A.S.; Soliman, G.A.; Al-Outhman, M.R.; Al-Shdoukhi, I.F.; Al-Nafisah, R.S.; Al-Shamery, J.; Al-Samkhan, R.; Baqer, M.; Al-Jaber, N.A. The Effect of Four Coffee types on Normotensive rats and Normal/Hypertensive Human Volunteers. Phyther. Res. 2011, 25, 803–808. [Google Scholar] [CrossRef]
- Rashid, A.; Hines, M.; Scherlag, B.J.; Yamanashi, W.S.; Lovallo, W. The effects of caffeine on the inducibility of atrial fibrillation. J. Electrocardiol. 2006, 39, 421–425. [Google Scholar] [CrossRef]
- Ferraz, G.C.; Teixeira-Neto, A.R.; Mataqueiro, M.I.; Lacerda-Neto, J.C.; Queiroz-Neto, A. Effects of intravenous administration of caffeine on physiologic variables in exercising horses. Am. J. Vet. Res. 2008, 69, 1670–1675. [Google Scholar] [CrossRef]
- DeGraves, F.J.; Ruffin, D.C.; Duran, S.H.; Spano, J.S.; Whatley, E.M.; Schumacher, J.; Riddell, M.G. Pharmacokinetics of caffeine in lactating dairy cows. Am. J. Vet. Res. 1995, 56, 619–622. [Google Scholar]
- Van der Veeken, L.; Grönlund, S.; Gerdtsson, E.; Holmqvist, B.; Deprest, J.; Ley, D.; Bruschettini, M. Long-term neurological effects of neonatal caffeine treatment in a rabbit model of preterm birth. Pediatr. Res. 2020, 87, 1011–1018. [Google Scholar] [CrossRef]
- Endesfelder, S.; Strauß, E.; Bendix, I.; Schmitz, T.; Bührer, C. Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats. Oxid. Med. Cell. Longev. 2020, 2020, 3840124. [Google Scholar] [CrossRef]
- Winerdal, M.M.E.; Urmaliya, V.; Winerdal, M.M.E.; Fredholm, B.B.; Winqvist, O.; Ådén, U. Single dose caffeine protects the neonatal mouse brain against hypoxia ischemia. PLoS ONE 2017, 12, e0170545. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-García, D.; Mota-Rojas, D.; Miranda-Cortés, A.E.; Mora-Medina, P.; Hernández-Avalos, I.; Casas-Alvarado, A.; Olmos-Hernández, A.; Martínez-Burnes, J. Neurobehavioral and neuroprotector effects of caffeine in animal models. J. Anim. Behav. Biometeorol. 2020, 8, 298–307. [Google Scholar] [CrossRef]
- Dobson, N.R.; Hunt, C.E. Pharmacology review. Caffeine use in neonates: Indications, pharmacokinetics, clinical effects, outcomes. Neoreviews 2013, 14, e540–e550. [Google Scholar] [CrossRef]
- Alves-Martinez, P.; Atienza-Navarro, I.; Vargas-Soria, M.; Carranza-Naval, M.J.; Infante-Garcia, C.; Benavente-Fernandez, I.; Del Marco, A.; Lubian-Lopez, S.; Garcia-Alloza, M. Caffeine Restores Neuronal Damage and Inflammatory Response in a Model of Intraventricular Hemorrhage of the Preterm Newborn. Front. Cell Dev. Biol. 2022, 10, 908045. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Xue, X.; Fu, J. Encephalopathy in preterm infants: Advances in neuroprotection with caffeine. Front. Pediatr. 2021, 9, 724161. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef]
- Bruschettini, M.; Moreira, A.; Pizarro, A.B.; Mustafa, S.; Romantsik, O. The effects of caffeine following hypoxic-ischemic encephalopathy: A systematic review of animal studies. Brain Res. 2022, 1790, 147990. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Li, D.; Xue, X.; Fu, J. Proteomic analysis of the effects of caffeine in a neonatal rat model of hypoxic-ischemic white matter damage. CNS Neurosci. Ther. 2022, 28, 1019–1032. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Xue, X.; Fu, J. Caffeine treatment started before injury reduces hypoxic–ischemic white-matter damage in neonatal rats by regulating phenotypic microglia polarization. Pediatr. Res. 2022, 92, 1543–1554. [Google Scholar] [CrossRef]
- Sun, H.; Gonzalez, F.; McQuillen, P.S. Caffeine restores background EEG activity independent of infarct reduction after neonatal hypoxic ischemic brain injury. Dev. Neurosci. 2020, 42, 72–82. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kind, K.; Hebart, M.L.; van Wettere, W.H.E.J. Caffeine supplementation at birth, but not 8 to 12 h post-birth, increased 24 h pre-weaning mortality in piglets. Animal 2020, 14, 1529–1535. [Google Scholar] [CrossRef]
- Jarratt, L.; James, S.E.; Kirkwood, R.N.; Nowland, T.L. Effects of caffeine and glucose supplementation at birth on piglet pre-weaning growth, thermoregulation, and survival. Animals 2023, 13, 435. [Google Scholar] [CrossRef]
- Murdock, N.J.; Weaver, A.C.; Kelly, J.M.; Kleemann, D.O.; van Wettere, W.H.E.J.; Swinbourne, A.M. Supplementing pregnant Merino ewes with caffeine to improve neonatal lamb thermoregulation and viability. Anim. Reprod. Sci. 2021, 226, 106715. [Google Scholar] [CrossRef]
- Dearlove, B.A.; Kind, K.L.; Gatford, K.L.; van Wettere, W.H.E.J. Oral caffeine administered during late gestation increases gestation length and piglet temperature in naturally farrowing sows. Anim. Reprod. Sci. 2018, 198, 160–166. [Google Scholar] [CrossRef]
- Robertson, S.M.; Edwards, S.H.; Doran, G.S.; Friend, M.A. Maternal caffeine administration to ewes does not affect perinatal lamb survival. Anim. Reprod. Sci. 2021, 231, 106799. [Google Scholar] [CrossRef]
- Christensen, Z.P.; Freedman, E.G.; Foxe, J.J. Caffeine exposure in utero is associated with structural brain alterations and deleterious neurocognitive outcomes in 9–10 year old children. Neuropharmacology 2021, 186, 108479. [Google Scholar] [CrossRef]
- McLeod, R.; Rosenkrantz, T.; Fitch, R.H. Therapeutic Interventions in Rat Models of Preterm Hypoxic Ischemic Injury: Effects of Hypothermia, Caffeine, and the Influence of Sex. Life 2022, 12, 1514. [Google Scholar] [CrossRef]
- Guo, H.-L.; Long, J.-Y.; Hu, Y.-H.; Liu, Y.; He, X.; Li, L.; Xia, Y.; Ding, X.-S.; Chen, F.; Xu, J.; et al. Caffeine Therapy for Apnea of Prematurity: Role of the Circadian CLOCK Gene Polymorphism. Front. Pharmacol. 2022, 12, 724145. [Google Scholar] [CrossRef]
- Dai, H.-R.; Guo, H.-L.; Hu, Y.-H.; Xu, J.; Ding, X.-S.; Cheng, R.; Chen, F. Precision caffeine therapy for apnea of prematurity and circadian rhythms: New possibilities open up. Front. Pharmacol. 2022, 13, 1053210. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Annual rhythmicity and maturation of physiological parameters in goats. Res. Vet. Sci. 2007, 83, 239–243. [Google Scholar] [CrossRef]
- Piccione, G.; Assenza, A.; Fazio, F.; Giannetto, C.; Caola, G. Chronobiologic blood pressure assessment: Maturation of the daily rhythm in newborn foals. Biol. Res. 2008, 41, 51–57. [Google Scholar] [CrossRef] [PubMed]

| Species | Route Administrated | Dose | Reference |
|---|---|---|---|
| Adults | |||
| Wistar rats | Caffeine (oral/single, bolus) | 0.5, 15, or 45 mg/kg | [74] |
| Sprague Dawley rats | Instant coffee extract (oral/ single dose) | 250 or 500 mg/ kg | [75] |
| Mongrel dogs | Caffeine (IV) | 1, 3 and 5 mg/kg | [76] |
| Arabian horses | Caffeine (IV) | 5 mg/kg | [77] |
| Neonates | |||
| Lactating dairy cows | Caffeine (IV) | 2 mg/kg | [78] |
| Rabbit New Zealand White | Caffeine (PO) | loading dose 20 mg/kg 10 mg/kg daily | [79] |
| Rabbits New Zealand White | Caffeine (IP) | 10 mg/ kg daily | [47] |
| Wistar rats | Caffeine (IV) | 10 mg/kg | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Villanueva-García, D.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Lezama-García, K.; Miranda-Cortés, A.; Martínez-Burnes, J. Cardiorespiratory and Neuroprotective Effects of Caffeine in Neonate Animal Models. Animals 2023, 13, 1769. https://doi.org/10.3390/ani13111769
Mota-Rojas D, Villanueva-García D, Hernández-Ávalos I, Casas-Alvarado A, Domínguez-Oliva A, Lezama-García K, Miranda-Cortés A, Martínez-Burnes J. Cardiorespiratory and Neuroprotective Effects of Caffeine in Neonate Animal Models. Animals. 2023; 13(11):1769. https://doi.org/10.3390/ani13111769
Chicago/Turabian StyleMota-Rojas, Daniel, Dina Villanueva-García, Ismael Hernández-Ávalos, Alejandro Casas-Alvarado, Adriana Domínguez-Oliva, Karina Lezama-García, Agatha Miranda-Cortés, and Julio Martínez-Burnes. 2023. "Cardiorespiratory and Neuroprotective Effects of Caffeine in Neonate Animal Models" Animals 13, no. 11: 1769. https://doi.org/10.3390/ani13111769










