Effects of Climate Change on the Distribution of Threatened Fishing Bat Myotis pilosus in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Distribution Data
2.2. Environmental Variables
2.3. MaxEnt Procedures
2.4. Classification of Suitable Habitats
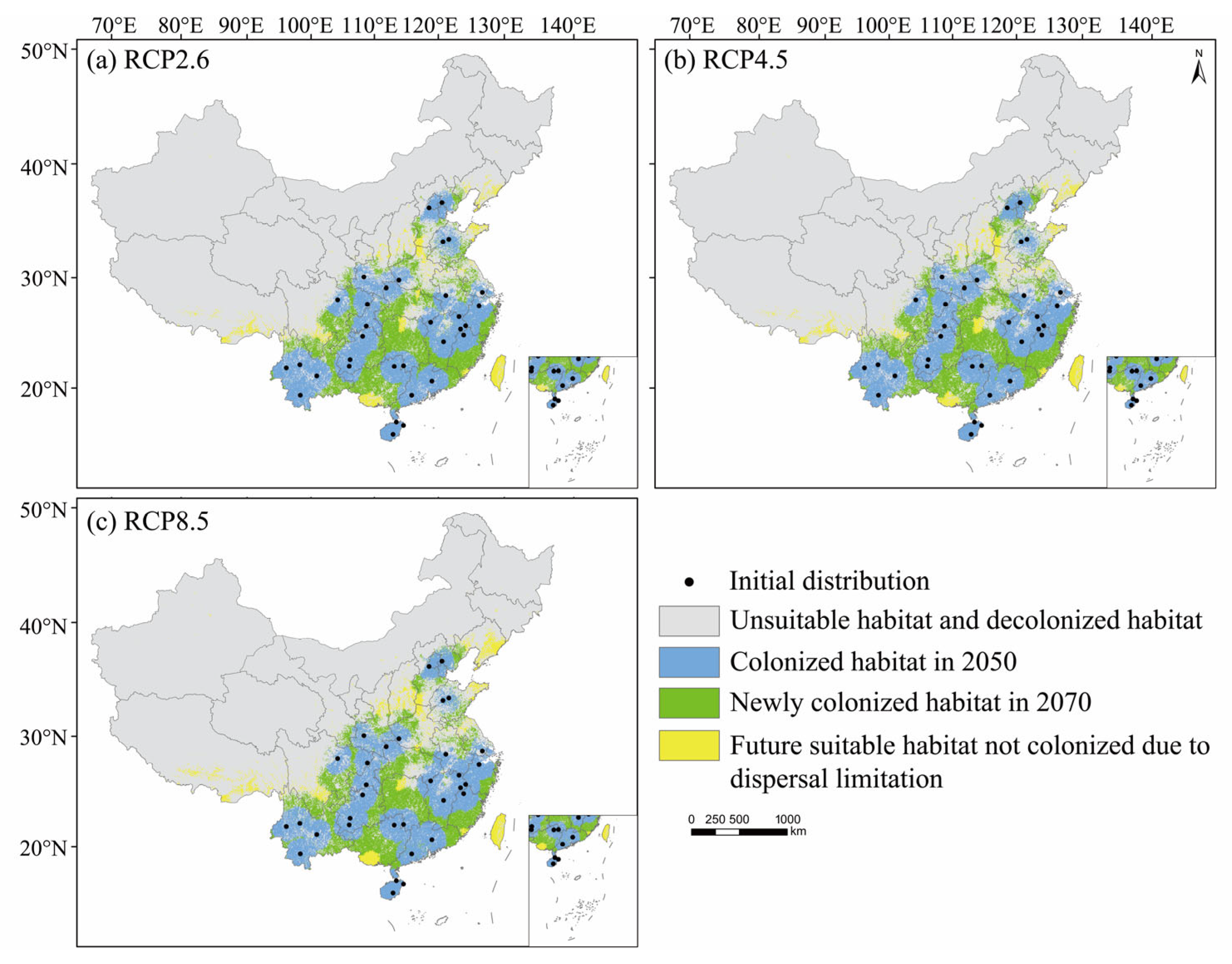
2.5. Dispersal Simulations
2.6. Identification of Climate Refugia
3. Results
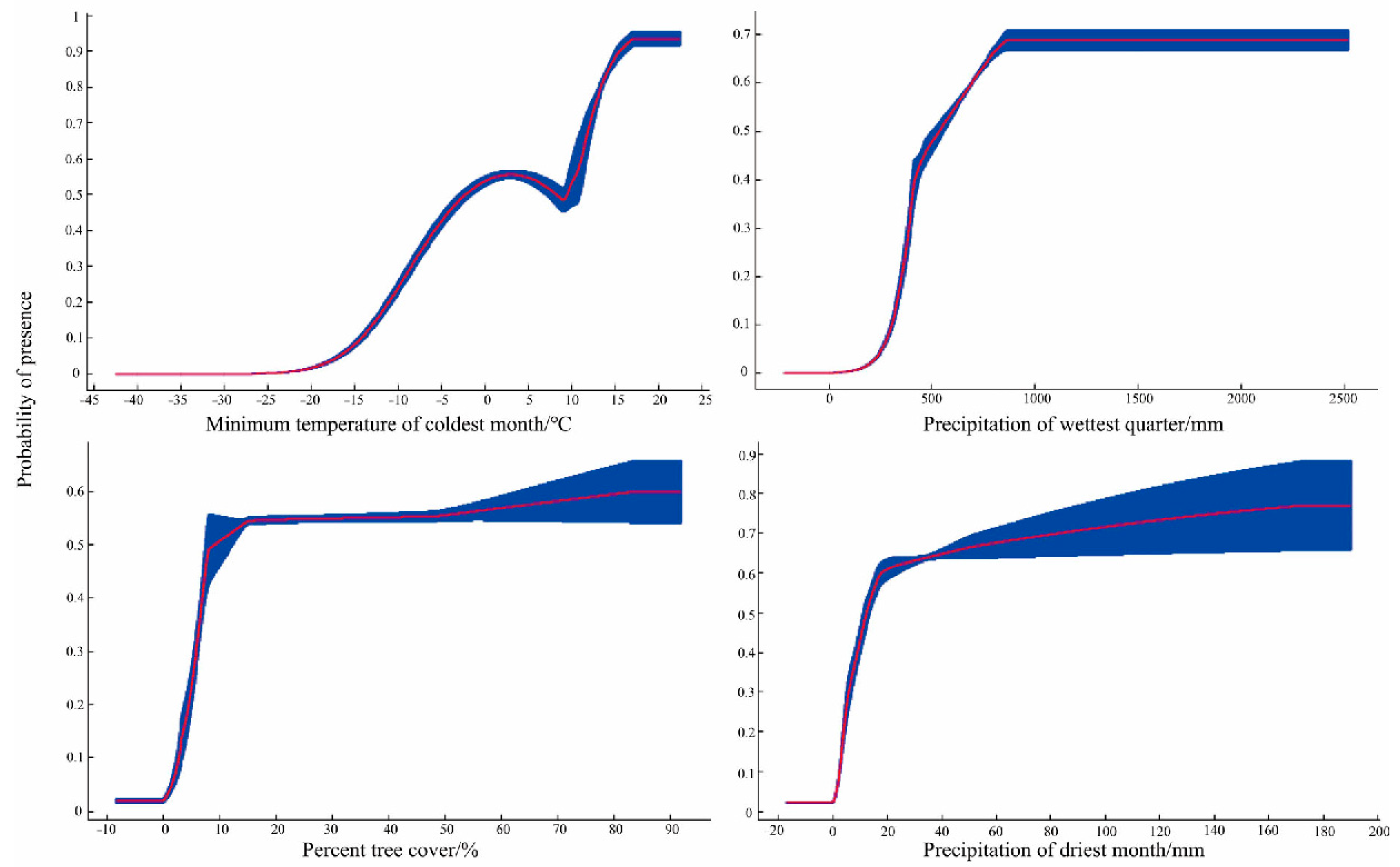
3.1. Model Verification and Environmental Variables
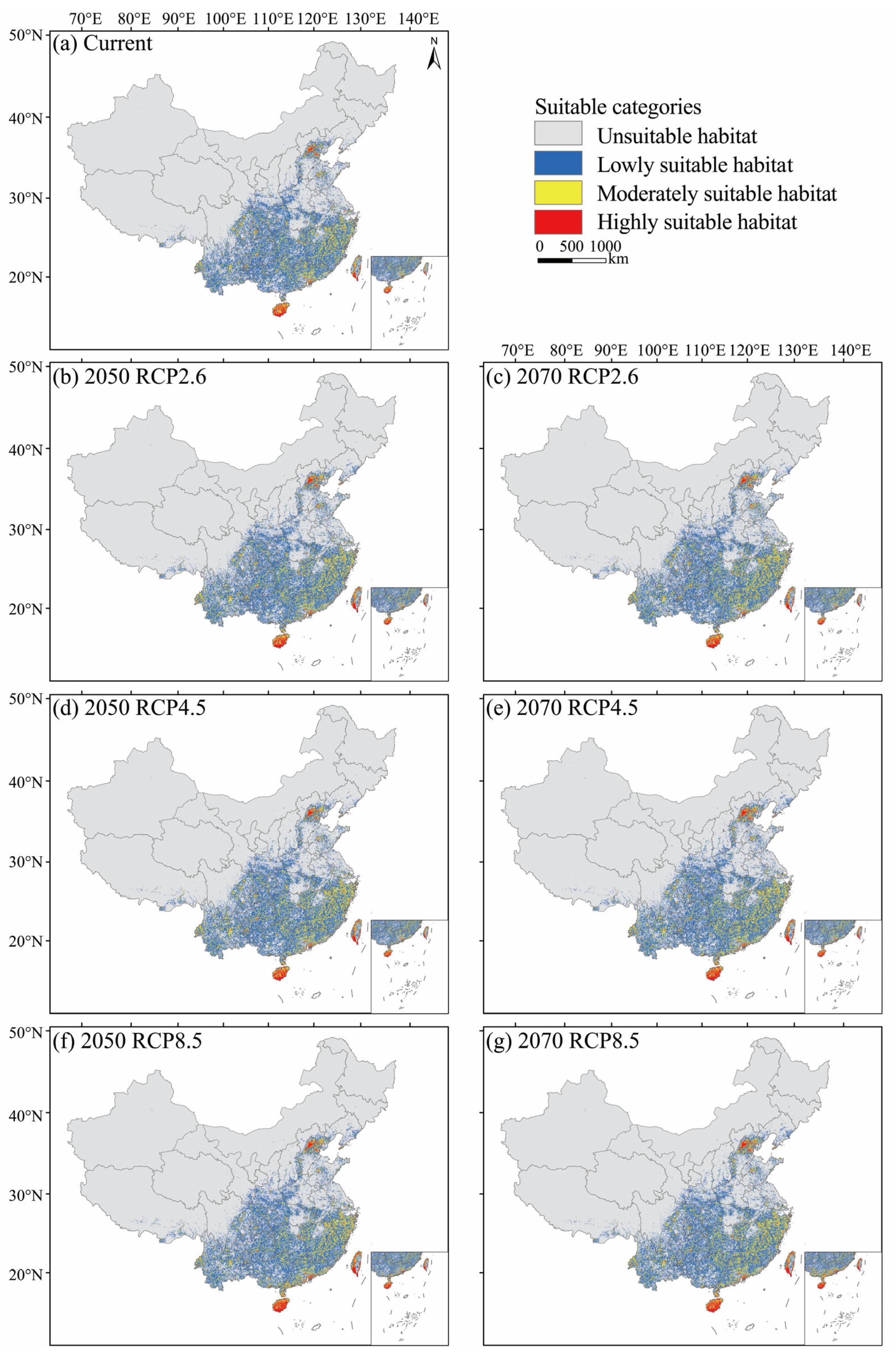
3.2. Current Potential Suitable Habitats
3.3. Future Potential Suitable Habitats
3.4. Shift in Centroid Position
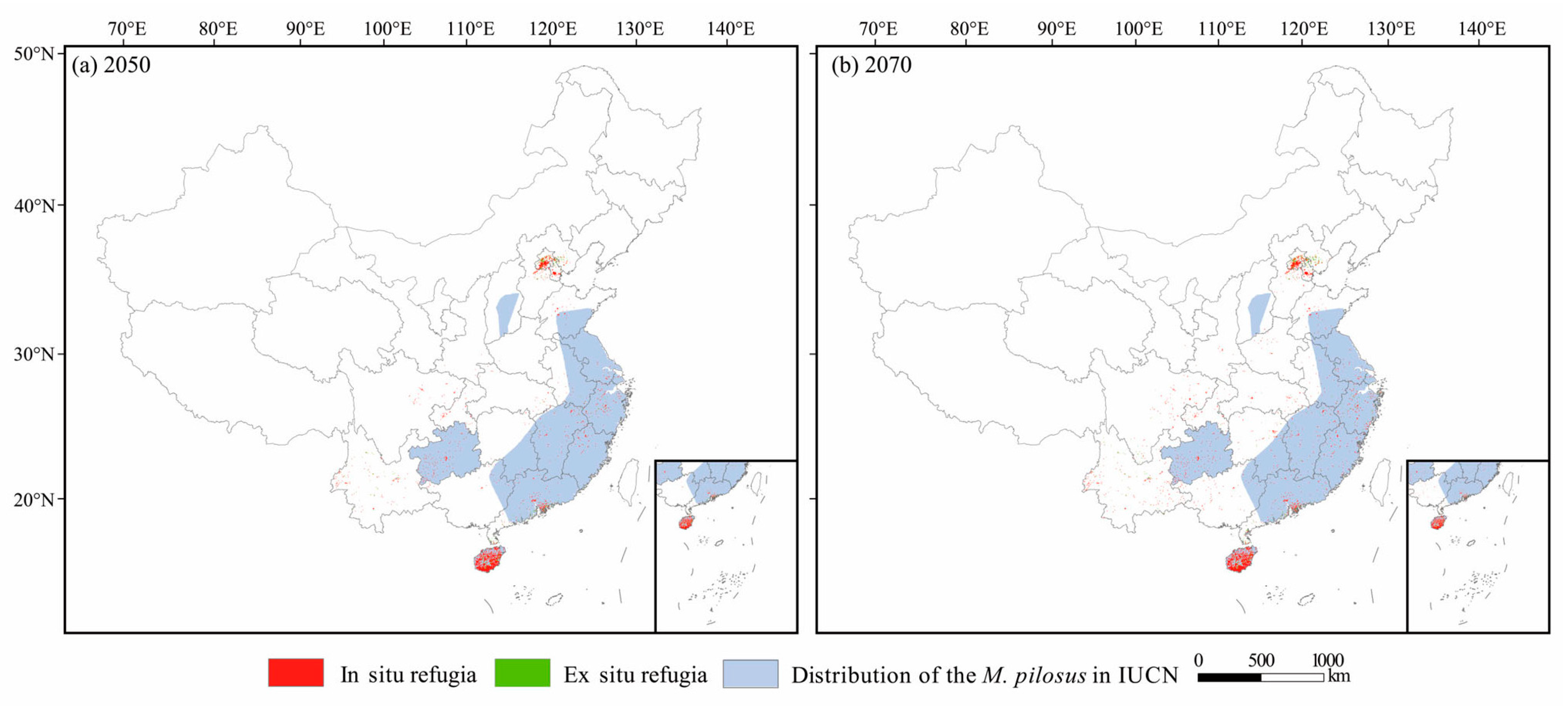
3.5. Future Climate Refugia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, Y.J.; Midgley, G.F.; Archer, E.R.; Arneth, A.; Barnes, D.K.; Chan, L.; Hashimoto, S.; Hoegh-Guldberg, O.; Insarov, G.; Leadley, P. Actions to halt biodiversity loss generally benefit the climate. Glob. Chang. Biol. 2022, 28, 2846–2874. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2022—Building a Nature Positive Society; Almond, R.E.A., Grooten, M., Juffe Bignoli, D., Petersen, T., Eds.; WWF: Gland, Switzerland, 2022. [Google Scholar]
- Jones, K.R.; Watson, J.E.; Possingham, H.P.; Klein, C.J. Incorporating climate change into spatial conservation prioritisation: A review. Biol. Conserv. 2016, 194, 121–130. [Google Scholar] [CrossRef]
- Davis, M.; Faurby, S.; Svenning, J.-C. Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proc. Natl. Acad. Sci. USA 2018, 115, 11262–11267. [Google Scholar] [CrossRef] [PubMed]
- Scheel, D.; Vincent, T.; Cameron, G.N. Global warming and the species richness of bats in Texas. Conserv. Biol. 1996, 10, 452–464. [Google Scholar] [CrossRef]
- Sachanowicz, K.; Ciechanowski, M. First winter record of the migratory bat Pipistrellus nathusii (Keyserling and Blasius 1839) (Chiroptera: Vespertilionidae) in Poland: Yet more evidence of global warming?/Premiere observation hivernale de la chauve-souris migratrice Pipistrellus nathusii (Keyserling and Blasius 1839) (Chiroptera: Vespertilionidae) en Pologne: Un autre signe du réchauffement de la planete? Mammalia 2006, 70, 168–169. [Google Scholar]
- Dool, S.E. Conservation genetic studies in bats. In Conservation Genetics in Mammals: Integrative Research Using Novel Approaches; Ortega, J., Maldonado, J.E., Eds.; Springer: Basel, Switzerland, 2020; pp. 29–62. [Google Scholar]
- Jones, G.; Rebelo, H. Responses of bats to climate change: Learning from the past and predicting the future. In Bat Evolution, Ecology, and Conservation; Adams, R.A., Pedersen, S.C., Eds.; Springer: Berlin, Germany, 2013; pp. 457–478. [Google Scholar]
- Jones, G.; Jacobs, D.S.; Kunz, T.H.; Willig, M.R.; Racey, P.A. Carpe noctem: The importance of bats as bioindicators. Endanger. Species Res. 2009, 8, 93–115. [Google Scholar] [CrossRef]
- Amorim, F.; Mata, V.A.; Beja, P.; Rebelo, H. Effects of a drought episode on the reproductive success of European free-tailed bats (Tadarida teniotis). Mamm. Biol. 2015, 80, 228–236. [Google Scholar] [CrossRef]
- Adams, R.A. Bat reproduction declines when conditions mimic climate change projections for western North America. Ecology 2010, 91, 2437–2445. [Google Scholar] [CrossRef]
- Lučan, R.; Weiser, M.; Hanák, V. Contrasting effects of climate change on the timing of reproduction and reproductive success of a temperate insectivorous bat. J. Zool. 2013, 290, 151–159. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2023. Available online: https://www.iucnredlist.org (accessed on 10 October 2022).
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2020, 1469, 5–25. [Google Scholar] [CrossRef]
- Aizpurua, O.; Alberdi, A. Ecology and evolutionary biology of fishing bats. Mammal Rev. 2018, 48, 284–297. [Google Scholar] [CrossRef]
- Ma, J.; Jones, G.; Zhang, S.; Shen, J.; Metzner, W.; Zhang, L.; Liang, B. Dietary analysis confirms that Rickett’s big-footed bat (Myotis ricketti) is a piscivore. J. Zool. 2003, 261, 245–248. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Liang, B.; Zhang, L.; Zhang, S.; Metzner, W. Dietary characteristics of Myotis ricketti in Beijing, north China. J. Mammal. 2006, 87, 339–344. [Google Scholar] [CrossRef]
- Chang, Y.; Song, S.; Li, A.; Zhang, Y.; Li, Z.; Xiao, Y.; Jiang, T.; Feng, J.; Lin, A. The roles of morphological traits, resource variation and resource partitioning associated with the dietary niche expansion in the fish-eating bat Myotis pilosus. Mol. Ecol. 2019, 28, 2944–2954. [Google Scholar] [CrossRef]
- Jiang, T.L.; Feng, J.; Csorba, G.; Bates, P. Myotis pilosus. In The IUCN Red List of Threatened Species 2019; International Union for Conservation of Nature: Cambridge, UK, 2019; p. E.T14193A22062554. [Google Scholar] [CrossRef]
- Jie, M.; Qiang, D.; Shuyi, Z.; Junxian, S.; Bing, L. Distribution of ricketti’s big footed bat (Myotis ricketti). Sichuan Dong Wu Sichuan Dongwu Sichuan J. Zool. 2003, 22, 155–156. [Google Scholar]
- Jie, M.; Gareth, J.; Bing, L.; Junxian, S.; Shuyi, Z. Biology of the Bid footed Bat, Myotis ricketti. Chin. J. Zool. 2003, 38, 93–95. [Google Scholar]
- Jiang, F.; Zhenxin, L.; Min, C.; Ying, L.; Xichen, Z.; Jiang, Z.; Shuyi, Z. Morphological features, echolocation calls and foraging strategy in the trawling piscivorous bat: Rickett’s big-footed bat Myotis ricketti. Acta Ecol. Sin. 2003, 23, 1712–1718. [Google Scholar]
- Thong, V.D.; Limbert, H.; Limbert, D. First Records of Bats (Mammalia: Chiroptera) from the World’s Largest Cave in Vietnam. Diversity 2022, 14, 534. [Google Scholar] [CrossRef]
- Dong, D.; Lei, M.; Liu, Y.; Zhang, S. Comparative inner ear transcriptome analysis between the Rickett’s big-footed bats (Myotis ricketti) and the greater short-nosed fruit bats (Cynopterus sphinx). BMC Genom. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Lu, G.; Lin, A.; Luo, J.; Blondel, D.V.; Meiklejohn, K.A.; Sun, K.; Feng, J. Phylogeography of the Rickett’s big-footed bat, Myotis pilosus (Chiroptera: Vespertilionidae): A novel pattern of genetic structure of bats in China. BMC Evol. Biol. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Jiao, H.; Xie, H.-W.; Zhang, L.; Zhuoma, N.; Jiang, P.; Zhao, H. Loss of sweet taste despite the conservation of sweet receptor genes in insectivorous bats. Proc. Natl. Acad. Sci. USA 2021, 118, e2021516118. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Deng, X.; Xu, D.; Liao, W.; Wang, R.; Zhuo, Z. Predicting the distributions of Scleroderma guani (Hymenoptera: Bethylidae) under climate change in China. Ecol. Evol. 2022, 12, e9410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90. [Google Scholar]
- Graham, C.H.; Ferrier, S.; Huettman, F.; Moritz, C.; Peterson, A.T. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol. Evol. 2004, 19, 497–503. [Google Scholar] [CrossRef]
- Anderson, R.P. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Glob. Chang. Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef]
- Ramirez-Villegas, J.; Cuesta, F.; Devenish, C.; Peralvo, M.; Jarvis, A.; Arnillas, C.A. Using species distributions models for designing conservation strategies of Tropical Andean biodiversity under climate change. J. Nat. Conserv. 2014, 22, 391–404. [Google Scholar] [CrossRef]
- Guisan, A.; Rahbek, C. SESAM–a new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J. Biogeogr. 2011, 38, 1433–1444. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McCune, J. Species distribution models predict rare species occurrences despite significant effects of landscape context. J. Appl. Ecol. 2016, 53, 1871–1879. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Merow, C.; Silander Jr, J.A. A comparison of Maxlike and Maxent for modelling species distributions. Methods Ecol. Evol. 2014, 5, 215–225. [Google Scholar] [CrossRef]
- Harte, J.; Newman, E.A. Maximum information entropy: A foundation for ecological theory. Trends Ecol. Evol. 2014, 29, 384–389. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Otto-Bliesner, B.L.; Marshall, S.J.; Overpeck, J.T.; Miller, G.H.; Hu, A.; CAPE Last Interglacial Project members. Simulating Arctic climate warmth and icefield retreat in the last interglaciation. Science 2006, 311, 1751–1753. [Google Scholar] [CrossRef]
- Jubb, I.; Canadell, P.; Dix, M. Representative Concentration Pathways (RCPs); Australian Government, Department of the Environment: Parkes, Australia, 2013.
- Araujo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Engler, R.; Hordijk, W.; Guisan, A. The MIGCLIM R package–seamless integration of dispersal constraints into projections of species distribution models. Ecography 2012, 35, 872–878. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 3 May 2023).
- Dupont-Doaré, C.; Alagador, D. Overlooked effects of temporal resolution choice on climate-proof spatial conservation plans for biodiversity. Biol. Conserv. 2021, 263, 109330. [Google Scholar] [CrossRef]
- Brambilla, M.; Rubolini, D.; Appukuttan, O.; Calvi, G.; Karger, D.N.; Kmecl, P.; Mihelič, T.; Sattler, T.; Seaman, B.; Teufelbauer, N. Identifying climate refugia for high-elevation Alpine birds under current climate warming predictions. Glob. Chang. Biol. 2022, 28, 4276–4291. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, M.; Caprio, E.; Assandri, G.; Scridel, D.; Bassi, E.; Bionda, R.; Celada, C.; Falco, R.; Bogliani, G.; Pedrini, P. A spatially explicit definition of conservation priorities according to population resistance and resilience, species importance and level of threat in a changing climate. Divers. Distrib. 2017, 23, 727–738. [Google Scholar] [CrossRef]
- Morelli, T.L.; Barrows, C.W.; Ramirez, A.R.; Cartwright, J.M.; Ackerly, D.D.; Eaves, T.D.; Ebersole, J.L.; Krawchuk, M.A.; Letcher, B.H.; Mahalovich, M.F. Climate-change refugia: Biodiversity in the slow lane. Front. Ecol. Environ. 2020, 18, 228–234. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Tong, J.; Yang, Y. Habitat suitability of neotenic net-winged beetles (Coleoptera: Lycidae) in China using combined ecological models, with implications for biological conservation. Divers. Distrib. 2022, 28, 2806–2823. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Gaston, K.J.; Fuller, R.A. The sizes of species’ geographic ranges. J. Appl. Ecol. 2009, 46, 1–9. [Google Scholar] [CrossRef]
- Jiang, D.; Goodale, E.; Yang, G.; Yu, L.; Jiang, A.; Luo, X. Modeling the distribution of Nonggang Babbler Stachyris nonggangensis, a threatened bird of limestone karst forests of the Sino-Vietnam border, and implications for its conservation. Bird Conserv. Int. 2020, 30, 75–86. [Google Scholar] [CrossRef]
- e Silva, V.d.N.; Pressey, R.L.; Machado, R.B.; VanDerWal, J.; Wiederhecker, H.C.; Werneck, F.P.; Colli, G.R. Formulating conservation targets for a gap analysis of endemic lizards in a biodiversity hotspot. Biol. Conserv. 2014, 180, 1–10. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Ancillotto, L.; Santini, L.; Ranc, N.; Maiorano, L.; Russo, D. Extraordinary range expansion in a common bat: The potential roles of climate change and urbanisation. Sci. Nat. 2016, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Detection and attribution of the effects of climate change on bat distributions over the last 50 years. Clim. Chang. 2016, 134, 681–696. [Google Scholar] [CrossRef]
- Smeraldo, S.; Bosso, L.; Salinas-Ramos, V.B.; Ancillotto, L.; Sánchez-Cordero, V.; Gazaryan, S.; Russo, D. Generalists yet different: Distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mammal Rev. 2021, 51, 571–584. [Google Scholar] [CrossRef]
- Sherwin, H.A.; Montgomery, W.I.; Lundy, M.G. The impact and implications of climate change for bats. Mammal Rev. 2013, 43, 171–182. [Google Scholar] [CrossRef]
- Burles, D.; Brigham, R.; Ring, R.; Reimchen, T. Influence of weather on two insectivorous bats in a temperate Pacific Northwest rainforest. Can. J. Zool. 2009, 87, 132–138. [Google Scholar] [CrossRef]
- Anthony, E.; Stack, M.; Kunz, T. Night roosting and the nocturnal time budget of the little brown bat, Myotis lucifugus: Effects of reproductive status, prey density, and environmental conditions. Oecologia 1981, 51, 151–156. [Google Scholar] [CrossRef]
- Park, K.J.; Jones, G.; Ransome, R.D. Torpor, arousal and activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum). Funct. Ecol. 2000, 14, 580–588. [Google Scholar] [CrossRef]
- Kurta, A. Torpor patterns in food-deprived Myotis lucifugus (Chiroptera: Vespertilionidae) under simulated roost conditions. Can. J. Zool. 1991, 69, 255–257. [Google Scholar] [CrossRef]
- McNab, B.K. Evolutionary alternatives in the physiological ecology of bats. In Ecology of Bats; Springer: Boston, MA, USA, 1982; pp. 151–200. [Google Scholar]
- Lundy, M.; Montgomery, I.; Russ, J. Climate change-linked range expansion of Nathusius’ pipistrelle bat, Pipistrellus nathusii (Keyserling & Blasius, 1839). J. Biogeogr. 2010, 37, 2232–2242. [Google Scholar]
- Festa, F.; Ancillotto, L.; Santini, L.; Pacifici, M.; Rocha, R.; Toshkova, N.; Amorim, F.; Benítez-López, A.; Domer, A.; Hamidović, D. Bat responses to climate change: A systematic review. Biol. Rev. 2023, 98, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Perry, R.W. Migration and recent range expansion of Seminole bats (Lasiurus seminolus) in the United States. J. Mammal. 2018, 99, 1478–1485. [Google Scholar] [CrossRef]
- Blomberg, A.S.; Vasko, V.; Salonen, S.; Pētersons, G.; Lilley, T.M. First record of a Nathusius’ pipistrelle (Pipistrellus nathusii) overwintering at a latitude above 60° N. Mammalia 2021, 85, 74–78. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Agnelli, P.; Bosso, L.; Ancillotto, L.; Russo, D. Body size of Italian greater horseshoe bats (Rhinolophus ferrumequinum) increased over one century and a half: A response to climate change? Mamm. Biol. 2021, 101, 1127–1131. [Google Scholar] [CrossRef]
- Stepanian, P.M.; Wainwright, C.E. Ongoing changes in migration phenology and winter residency at Bracken Bat Cave. Glob. Chang. Biol. 2018, 24, 3266–3275. [Google Scholar] [CrossRef]
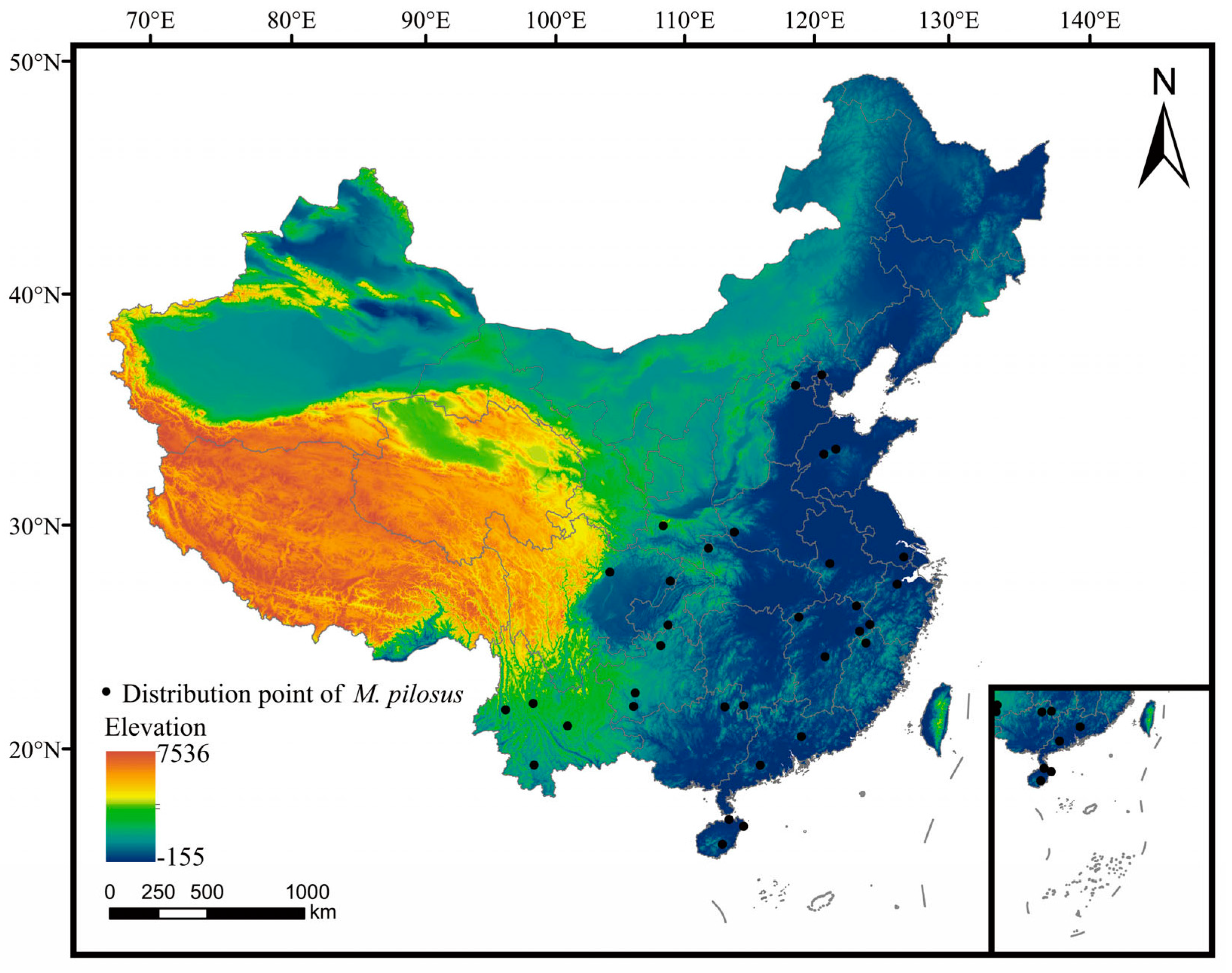


| Category of Environmental Variables | Environmental Variables | Abbreviation |
|---|---|---|
| Climate | Annual Mean Temperature | Bio1 |
| Mean Diurnal Range | Bio2 | |
| Isothermality | Bio3 | |
| Temperature Seasonality | Bio4 | |
| Maximum Temperature of Warmest Month | Bio5 | |
| Minimum Temperature of Coldest Month | Bio6 | |
| Temperature Annual Range | Bio7 | |
| Mean Temperature of Wettest Quarter | Bio8 | |
| Mean Temperature of Driest Quarter | Bio9 | |
| Mean Temperature of Warmest Quarter | Bio10 | |
| Mean Temperature of Coldest Quarter | Bio11 | |
| Annual Precipitation | Bio12 | |
| Precipitation of Wettest Month | Bio13 | |
| Precipitation of Driest Month | Bio14 | |
| Precipitation Seasonality | Bio15 | |
| Precipitation of Wettest Quarter | Bio16 | |
| Precipitation of Driest Quarter | Bio17 | |
| Precipitation of Warmest Quarter | Bio18 | |
| Precipitation of Coldest Quarter | Bio19 | |
| Land cover and vegetation | Land Use Type | Landuse |
| Normalized Vegetation Index | NDVI | |
| Percent Tree Cover | TREE | |
| Terrain | Altitude | |
| Slope | ||
| Aspect | ||
| Light index | Night Light Brightness | Light |
| Human disturbance | Human Influence Index | Hii |
| Distance to Settlements | Dis_set | |
| Distance to Roads | Dis_roa | |
| River | Distance to Freshwater | Dis_riv |
| Environmental Variable | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|
| Bio6 | 25.9 | 52 |
| Bio16 | 16.1 | 0.6 |
| TREE | 15.8 | 14.4 |
| Bio14 | 14.6 | 0.9 |
| Slope | 7.1 | 10.9 |
| Light | 6.5 | 3.8 |
| Landuse | 6.2 | 1.3 |
| Dis_riv | 2.5 | 6.1 |
| Bio15 | 2 | 4.2 |
| Aspect | 1.7 | 1.5 |
| Dis_set | 1.6 | 4.4 |
| Years | Climate Scenario | Altitude (m) | Area (×104 km2) | ||||
|---|---|---|---|---|---|---|---|
| Average Altitude | Highly Suitable Habitats | Moderately Suitable Habitats | Lowly Suitable Habitats | Total Area | Suitable Habitats with Limited Dispersal | ||
| Current | - | 648.19 | 5.74 | 34.82 | 119.98 | 160.54 | - |
| 2050 | RCP2.6 | 681.94 | 6.87 | 39.15 | 123.55 | 169.58 | 94.00 |
| RCP4.5 | 698.58 | 6.92 | 40.71 | 123.86 | 171.48 | 95.00 | |
| RCP8.5 | 692.79 | 7.49 | 38.00 | 124.25 | 169.74 | 94.14 | |
| 2070 | RCP2.6 | 678.94 | 6.97 | 41.15 | 123.35 | 171.47 | 154.16 |
| RCP4.5 | 687.75 | 7.67 | 41.14 | 124.86 | 173.67 | 155.16 | |
| RCP8.5 | 708.56 | 9.70 | 41.43 | 127.87 | 179.00 | 156.99 | |
| Climate Scenario | In Situ Refugia Area/(×104 km2) | Ex Situ Refugia Area/(×104 km2) | ||
|---|---|---|---|---|
| 2050 | 2070 | 2050 | 2070 | |
| RCP2.6 | 3.87 | 4.77 | 0.86 | 1.22 |
| RCP4.5 | 3.80 | 4.76 | 0.92 | 1.74 |
| RCP8.5 | 3.77 | 4.51 | 1.36 | 3.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Li, Z.; Liu, T.; Feng, J. Effects of Climate Change on the Distribution of Threatened Fishing Bat Myotis pilosus in China. Animals 2023, 13, 1784. https://doi.org/10.3390/ani13111784
Guo W, Li Z, Liu T, Feng J. Effects of Climate Change on the Distribution of Threatened Fishing Bat Myotis pilosus in China. Animals. 2023; 13(11):1784. https://doi.org/10.3390/ani13111784
Chicago/Turabian StyleGuo, Wei, Zixuan Li, Tong Liu, and Jiang Feng. 2023. "Effects of Climate Change on the Distribution of Threatened Fishing Bat Myotis pilosus in China" Animals 13, no. 11: 1784. https://doi.org/10.3390/ani13111784





