Alien vs. Predator: Impacts of Invasive Species and Native Predators on Urban Nest Box Use by Native Birds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Area and Study Design
2.2. Nest Box Monitoring
2.3. Environmental Characteristics
2.4. Statistical Analyses
3. Results
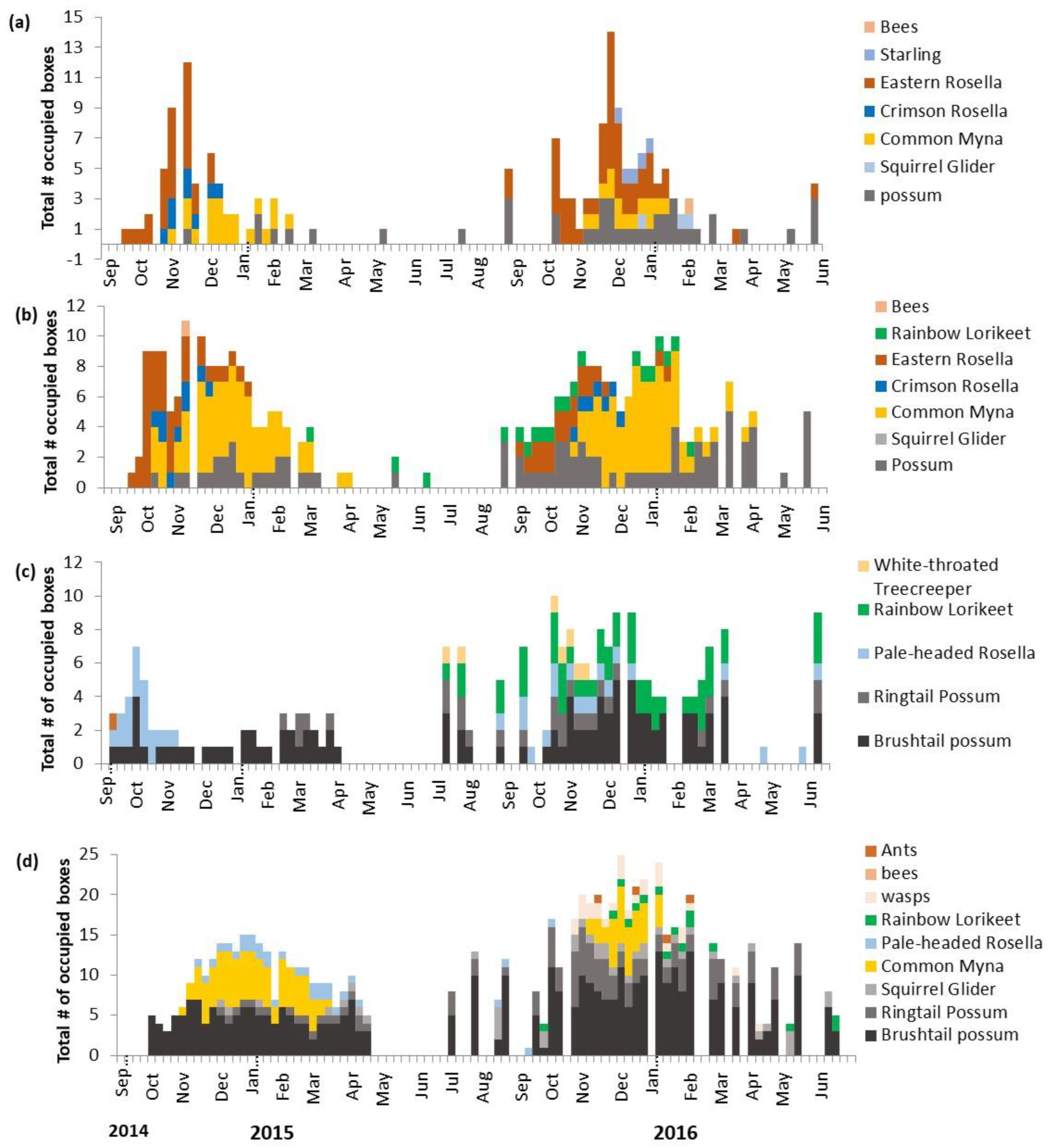
3.1. Weekly Box Occupancy
3.2. Nesting along Urban Front–Source Sites
3.3. Drivers of Box Occupancy and Nesting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LaMontagne, J.M.; Kilgour, R.J.; Anderson, E.C.; Magle, S. Tree cavity availability across forest, park, and residential habitats in a highly urban area. Urban Ecosyst. 2015, 18, 151–167. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Blanchard, W.; Manning, A.D.; Gibbons, P. Reduced availability of habitat structures in urban landscapes: Implications for policy and practice. Landsc. Urban Plan. 2014, 125, 57–64. [Google Scholar] [CrossRef]
- Anderies, J.M.; Katti, M.; Shochat, E. Living in the city: Resource availability, predation, and bird population dynamics in urban areas. J. Theor. Biol. 2007, 247, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, M.I.; Leveau, L.M.; Filloy, J. Urbanisation and Bird Communities: Spatial and Temporal Patterns Emerging from Southern South America. In Ecology and Conservation of Birds in Urban Environments; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Rohweder, D.; Taylor, B.D. Nest box contentions: Are nest boxes used by the species they target? Ecol. Manag. Restor. 2020, 21, 115–122. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Bistricer, G.; Manning, A.D.; Gibbons, P. Enriching small trees with artificial nest boxes cannot mimic the value of large trees for hollow-nesting birds. Restor. Ecol. 2016, 24, 252–258. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Crane, M.; Blanchard, W.; Okada, S.; Montague-Drake, R. Do nest boxes in restored woodlands promote the conservation of hollow-dependent fauna? Restor. Ecol. 2016, 24, 244–251. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Welsh, A.; Donnelly, C.; Crane, M.; Michael, D.; Macgregor, C.; McBurney, L.; Montague-Drake, R.; Gibbons, P. Are nest boxes a viable alternative source of cavities for hollow-dependent animals? Long-term monitoring of nest box occupancy, pest use and attrition. Biol. Conserv. 2009, 142, 33–42. [Google Scholar] [CrossRef]
- Harper, M.J.; McCarthy, M.A.; van der Ree, R. The use of nest boxes in urban natural vegetation remnants by vertebrate fauna. Wildl. Res. 2005, 32, 509–516. [Google Scholar] [CrossRef]
- Grarock, K.; Tidemann, C.R.; Wood, J.T.; Lindenmayer, D.B. Are invasive species drivers of native species decline or passengers of habitat modification? A case study of the impact of the common myna (Acridotheres tristis) on Australian bird species. Austral Ecol. 2014, 39, 106–114. [Google Scholar] [CrossRef]
- Pell, A.S.; Tidemann, C.R. The ecology of the Common Myna in urban nature reserves in the Australian iocon territory. EMU 1997, 97, 141–149. [Google Scholar] [CrossRef]
- Rogers, A.M.; Griffins, A.S.; van Rensburg, B.J.; Kark, S. Noisy neighbours and myna problems: Interaction webs and aggression around tree hollows in urban habitats. J. Appl. Ecol. 2020, 57, 1891–1901. [Google Scholar] [CrossRef]
- Global Invasive Species Database. IUCN Species Survival Commission. 2015. Available online: http://www.issg.org/database (accessed on 17 January 2019).
- McKinney, M.; Kark, S. Factors shaping avian alien species richness in Australia vs. Europe. Divers. Distrib. 2017, 23, 1334–1342. [Google Scholar] [CrossRef]
- Martin, W. The Current and Potential Distribution of the Common Myna Acridotheres tristis in Australia. EMU 1996, 96, 166–173. [Google Scholar] [CrossRef]
- Grarock, K.; Lindenmayer, D.B.; Wood, J.T.; Tidemann, C.R. Does Human-Induced Habitat Modification Influence the Impact of Introduced Species? A Case Study on Cavity-Nesting by the Introduced Common Myna (Acridotheres tristis) and Two Australian Native Parrots. Environ. Manag. 2013, 52, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Pell, A.; Tidemann, C. The impact of two exotic hollow-nesting birds on two native parrots in savannah and woodland in eastern Australia. Biol. Conserv. 1997, 79, 145–153. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Crane, M.; Evans, M.C.; Maron, M.; Gibbons, P.; Bekessy, S.; Blanchard, W. The anatomy of a failed offset. Biol. Conserv. 2017, 210, 286–292. [Google Scholar] [CrossRef]
- Garnett, S.T.; Pedler, L.P.; Crowley, G.M. The Breeding Biology of the Glossy Black-Cockatoo Calyptorhynchus lathami on Kangaroo Island, South Australia. EMU 1999, 99, 262–279. [Google Scholar] [CrossRef]
- Stojanovic, D.; Webb, M.H.; Alderman, R.; Porfirio, L.L.; Heinsohn, R. Discovery of a novel predator reveals extreme but highly variable mortality for an endangered migratory bird. Divers. Distrib. 2014, 20, 1200–1207. [Google Scholar] [CrossRef]
- Moorhouse, R.; Greene, T.; Dilks, P.; Powlesland, R.; Moran, L.; Taylor, G.; Jones, A.; Knegtmans, J.; Wills, D.; Pryde, M.; et al. Control of introduced mammalian predators improves kaka Nestor meridionalis breeding success: Reversing the decline of a threatened New Zealand parrot. Biol. Conserv. 2003, 110, 33–44. [Google Scholar] [CrossRef]
- Tompkins, D.M.; Veltman, C.J. Unexpected consequences of vertebrate pest control: Predictions from a four-species community model. Ecol. Appl. 2006, 16, 1050–1061. [Google Scholar] [CrossRef]
- Durant, R.; Luck, G.W.; Matthews, A. Nest-box use by arboreal mammals in a peri-urban landscape. Wildl. Res. 2009, 36, 565–573. [Google Scholar] [CrossRef]
- Ewart, K.M.; Griffin, A.S.; Johnson, R.N.; Kark, S.; Cohen, T.M.; Lo, N.; Major, R.E. Two speed invasion: Assisted and intrinsic dispersal of common mynas over 150 years of colonization. J. Biogeogr. 2019, 46, 45–57. [Google Scholar] [CrossRef]
- Hyett, J. Review—A Field Guide to Nests and Eggs of Australian Birds. Aust. Field Ornithol. 1980, 259–260. [Google Scholar]
- Larson, E.R.; Eastwood, J.R.; Buchanan, K.L.; Bennett, A.T.; Berg, M.L. How does nest box temperature affect nestling growth rate and breeding success in a parrot? Emu 2015, 115, 247–255. [Google Scholar] [CrossRef]
- Davis, A.; Major, R.E.; Taylor, C.E. Distribution of tree-hollows and hollow preferences by parrots in an urban landscape. EMU 2014, 114, 295–303. [Google Scholar] [CrossRef]
- Fournier, D.A.; Skaug, H.J.; Ancheta, J.; Ianelli, J.; Magnusson, A.; Maunder, M.N.; Nielsen, A.; Sibert, J. AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 2012, 27, 233–249. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org (accessed on 23 June 2019).
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 209–259. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Stevens, J.R. Use of artificial tree hollows by Australian birds and bats. Wildl. Res. 2009, 36, 81–97. [Google Scholar] [CrossRef]
- Stojanovic, D.; Owens, G.; Young, C.M.; Alves, F.; Heinsohn, R. Do nest boxes breed the target species or its competitors? A case study of a critically endangered bird. Restor. Ecol. 2021, 29, e13319. [Google Scholar] [CrossRef]
- Grarock, K.; Tidemann, C.R.; Wood, J.; Lindenmayer, D.B. Is It Benign or Is It a Pariah? Empirical Evidence for the Impact of the Common Myna (Acridotheres tristis) on Australian Birds. PLoS ONE 2012, 7, e40622. [Google Scholar] [CrossRef]
- Mänd, R.; Tilgar, V.; Lõhmus, A.; Leivits, A. Providing nest boxes for hole-nesting birds—Does habitat matter? Biodivers. Conserv. 2005, 14, 1823–1840. [Google Scholar] [CrossRef]
- Tomasevic, J.A.; Marzluff, J.M. Cavity nesting birds along an urban-wildland gradient: Is human facilitation structuring the bird community? Urban Ecosyst. 2016, 20, 435–448. [Google Scholar] [CrossRef]
- Finch, D.M. Effects of Predation and Competitor Interference on Nesting Success of House Wrens and Tree Swallows. Condor 1990, 92, 674. [Google Scholar] [CrossRef]
- Ingold, D.J. The influence of starlings on flicker reproduction when both naturally excavated cavities and artificial nest boxes are available. Wilson Bull. 1998, 110, 218–225. [Google Scholar]
- Orchan, Y.; Chiron, F.; Shwartz, A.; Kark, S. The complex interaction network among multiple invasive bird species in a cavity-nesting community. Biol. Invasions 2013, 15, 429–445. [Google Scholar] [CrossRef]
- Charter, M.; Izhaki, I.; Ben Mocha, Y.; Kark, S. Nest-site competition between invasive and native cavity nesting birds and its implication for conservation. J. Environ. Manag. 2016, 181, 129–134. [Google Scholar] [CrossRef]
- Diamond, J.M.; Ross, M.S. Overlap in reproductive phenology increases the likelihood of cavity nest usurpation by invasive species in a tropical city. Condor 2020, 122, duaa013. [Google Scholar] [CrossRef]
- Lowe, K.A.; Taylor, C.E.; Major, R.E. Do Common Mynas significantly compete with native birds in urban environments? J. Ornithol. 2011, 152, 909–921. [Google Scholar] [CrossRef]
- Smith, D.H.V.; Wilson, D.J.; Moller, H.; Murphy, E.C. Using artificial nests to explore predation by introduced predators inhabiting alpine areas in New Zealand. New Zealand J. Zool. 2008, 35, 119–128. [Google Scholar] [CrossRef]
- Davis, A.; Major, R.E.; Taylor, C.E. Housing Shortages in Urban Regions: Aggressive Interactions at Tree Hollows in Forest Remnants. PLoS ONE 2013, 8, e59332. [Google Scholar] [CrossRef]
- Rogers, A.M.; Kark, S. Australia’s urban cavity nesters and introduced parrots: Patterns, processes, and impacts. In Naturalised Parrots of the World; Pruett-Jones, S., Ed.; Princeton University Press: Princeton, NJ, USA, 2021; pp. 277–292. [Google Scholar] [CrossRef]
- Tidemann, S.C.; Boyden, J.; Elvish, R.; Elvish, J.; O’Gorman, B. Comparison of the breeding sites and habitat of two hole-nesting estrildid finches, one endangered, in northern Australia. J. Trop. Ecol. 1992, 8, 373–388. [Google Scholar] [CrossRef]
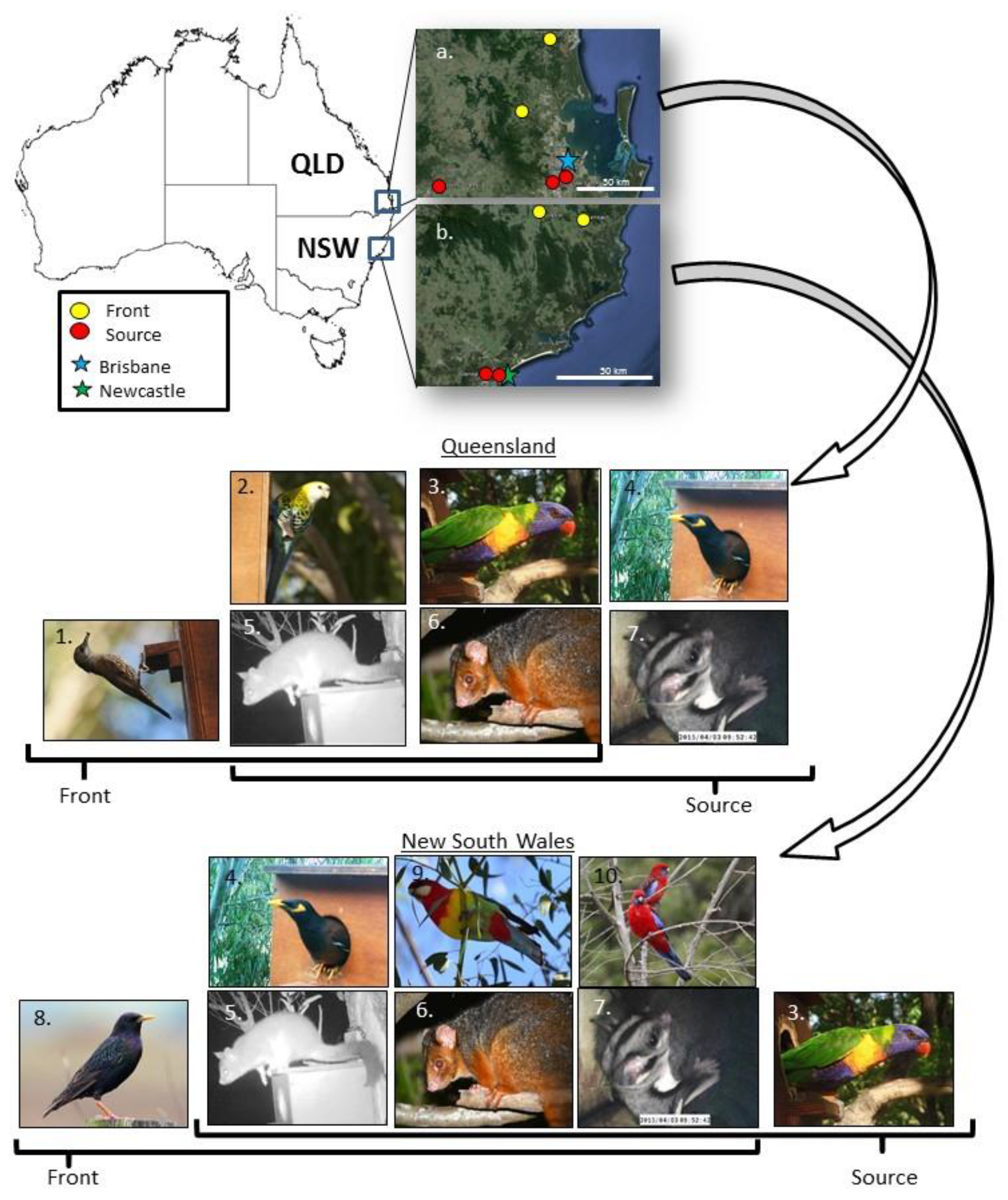


| Region | Site | Human Population (Year of the Census) | Front/Source | Sub-Environment | Average NDVI | Total Hollows | Percent Grass | Percent Sealed Surface | Percent Bush | Total Trees |
|---|---|---|---|---|---|---|---|---|---|---|
| N.S.W. | Gloucester | 2336 (2011) | Front | Bush | 0.804 | 0 | 20.6 | 0 | 79.3 | 334 |
| Park | 0.698 | 0 | 56.1 | 20 | 23.8 | 83 | ||||
| Urban | 0.463 | 0 | 40 | 54.375 | 5.6 | 33 | ||||
| Krambach | 392 (2011) | Bush | 0.762 | 0 | 21.8 | 13.75 | 64.3 | 199 | ||
| Park | 0.726 | 0 | 87.5 | 12.5 | 0 | 91 | ||||
| Urban | 0.589 | 0 | 4.6 | 94.75 | 0.6 | 70 | ||||
| Blackbutt | 436,200 (2016) | Source | Bush | 0.814 | 0 | 37.5 | 0 | 62.5 | 480 | |
| Park | 0.566 | 0 | 36.6 | 42.875 | 20.5 | 128 | ||||
| Urban | 0.311 | 0 | 27.7 | 70 | 2.25 | 59 | ||||
| Glendale | Bush | 0.591 | 0 | 62.5 | 0 | 37.5 | 191 | |||
| Park | 0.603 | 0 | 74.7 | 12.625 | 12.6 | 84 | ||||
| Urban | 0.447 | 0 | 35.6 | 58.125 | 6.2 | 70 | ||||
| QLD | Dayboro | 1692 (2011) | Front | Bush | 0.611 | 1 | 25 | 0 | 75 | 126 |
| Park | 0.559 | 1 | 78.7 | 18.125 | 3.1 | 40 | ||||
| Urban | 0.495 | 3 | 46.8 | 50 | 2.5 | 181 | ||||
| Landsborough | 3706 (2011) | Bush | 0.719 | 7 | 38.1 | 9.375 | 52.5 | 136 | ||
| Park | 0.5235 | 0 | 52.5 | 38.125 | 9.375 | 68 | ||||
| Urban | 0.496 | 5 | 46.2 | 31.875 | 20 | 102 | ||||
| Norman Park | 6003 (2011) | Source | Bush | 0.518 | 0 | 43.7 | 20 | 36.25 | 76 | |
| Park | 0.554 | 0 | 90 | 6.875 | 4.375 | 68 | ||||
| Urban | 0.571 | 4 | 72.5 | 26.875 | 0.625 | 109 | ||||
| Oxley Common | 7291 (2011) | Bush | 0.621 | 0 | 21.6 | 0.0 | 78.3333 | 168 | ||
| Park | 0.616 | 6 | 55.0 | 2.85714 | 42.1429 | 103 | ||||
| Urban | 0.518 | 0 | 40.0 | 12.14 | 47.8571 | 302 | ||||
| University of Queensland Gatton campus | 304 (2011) | Bush | 0.587 | 0 | 80.0 | 1.87 | 18.125 | 84 | ||
| Park | 0.636 | 2 | 68.7 | 28.75 | 3.75 | 69 | ||||
| Urban | 0.532 | 7 | 75.0 | 22.5 | 2.5 | 35 |
| Species Sharing Boxes | |||||
|---|---|---|---|---|---|
| Region | Front/Source | Site | Bird + Mammal | Myna + Native Bird | Native Birds + Native Bird |
| N.S.W. | front | Gloucester | 3 | 0 | 0 |
| Krambach | 0 | 0 | 0 | ||
| source | Blackbutt | 5 | 2 | 1 | |
| Glendale | 3 | 6 | 2 | ||
| QLD | front | Dayboro | 6 | 0 | 4 |
| Landsborough | 1 | 0 | 0 | ||
| source | Gatton | 3 | 3 | 0 | |
| Norman Park | 4 | 0 | 0 | ||
| Oxley Creek Common | 1 | 2 | 0 | ||
| Total | 26 | 13 | 7 | ||
| Response Variable | Explanatory Variables | Estimate | Std. Error | z-Value | Pr(>|z|) |
|---|---|---|---|---|---|
| Total box occupancy | (Intercept) | −1.997 | 0.397 | −5.03 | 4.90−07 |
| Avg diameter of all trees | 0.005 | 0.006 | 0.76 | 0.455 | |
| Avg distance to trees | −0.043 | 0.037 | −1.16 | 0.256 | |
| Per cent shrub cover | 0.000 | 0.006 | 0.04 | 0.971 | |
| Total number of natural hollows | 0.027 | 0.038 | 0.72 | 0.471 | |
| Total nesting attempts | (Intercept) | −1.924 | 0.367 | −5.24 | 1.60−07 |
| Avg diameter of all trees | 0.003 | 0.005 | 0.57 | 0.570 | |
| Avg distance to the nearest tree | 0.039 | 0.021 | 1.821 | 0.072 | |
| Per cent shrub cover | −0.012 | 0.007 | −1.741 | 0.082 | |
| Total number of natural hollows | −0.106 | 0.062 | −1.733 | 0.084 | |
| Total number of boxes occupied by mammals | −0.061 | 0.059 | −1.052 | 0.293 | |
| Total number of successful nesting attempts | (Intercept) | −2.191 | 0.437 | −5.012 | 5.30−07 |
| Avg diameter of all trees | 0.004 | 0.007 | 0.641 | 0.525 | |
| Avg distance to trees | 0.013 | 0.024 | 0.551 | 0.579 | |
| Per cent shrub cover | −0.008 | 0.008 | −1.07 | 0.284 | |
| Total number of natural hollows | 0.015 | 0.050 | 0.292 | 0.769 | |
| Total number of boxes occupied by mammals | −0.659 | 0.285 | −2.31 | 0.021 * | |
| Total mammal occupancy | (Intercept) | −2.431 | 0.959 | −2.54 | 0.011 |
| Total number of natural hollows | 0.0163 | 0.039 | 0.423 | 0.675 | |
| Avg diameter of all trees | 0.004 | 0.006 | 0.574 | 0.570 | |
| Avg distance to the nearest tree | −0.07 | 0.041 | −1.723 | 0.090 | |
| Per cent shrub cover | −0.004 | 0.006 | −0.681 | 0.500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, A.M.; Lermite, F.; Griffin, A.S.; van Rensburg, B.J.; Kark, S. Alien vs. Predator: Impacts of Invasive Species and Native Predators on Urban Nest Box Use by Native Birds. Animals 2023, 13, 1807. https://doi.org/10.3390/ani13111807
Rogers AM, Lermite F, Griffin AS, van Rensburg BJ, Kark S. Alien vs. Predator: Impacts of Invasive Species and Native Predators on Urban Nest Box Use by Native Birds. Animals. 2023; 13(11):1807. https://doi.org/10.3390/ani13111807
Chicago/Turabian StyleRogers, Andrew M., Françoise Lermite, Andrea S. Griffin, Berndt J. van Rensburg, and Salit Kark. 2023. "Alien vs. Predator: Impacts of Invasive Species and Native Predators on Urban Nest Box Use by Native Birds" Animals 13, no. 11: 1807. https://doi.org/10.3390/ani13111807





