Progesterone and Androstenedione Are Important Follicular Fluid Factors Regulating Porcine Oocyte Maturation Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of In Vivo Follicular Fluid and In Vitro Oocyte Culture Medium
2.3. Ultra-High-Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS)/MS Analysis
2.4. Preparation of In Vitro Matured Oocytes
2.5. Production of Embryos
2.6. Experimental Design
2.7. Statistical Analysis
3. Results
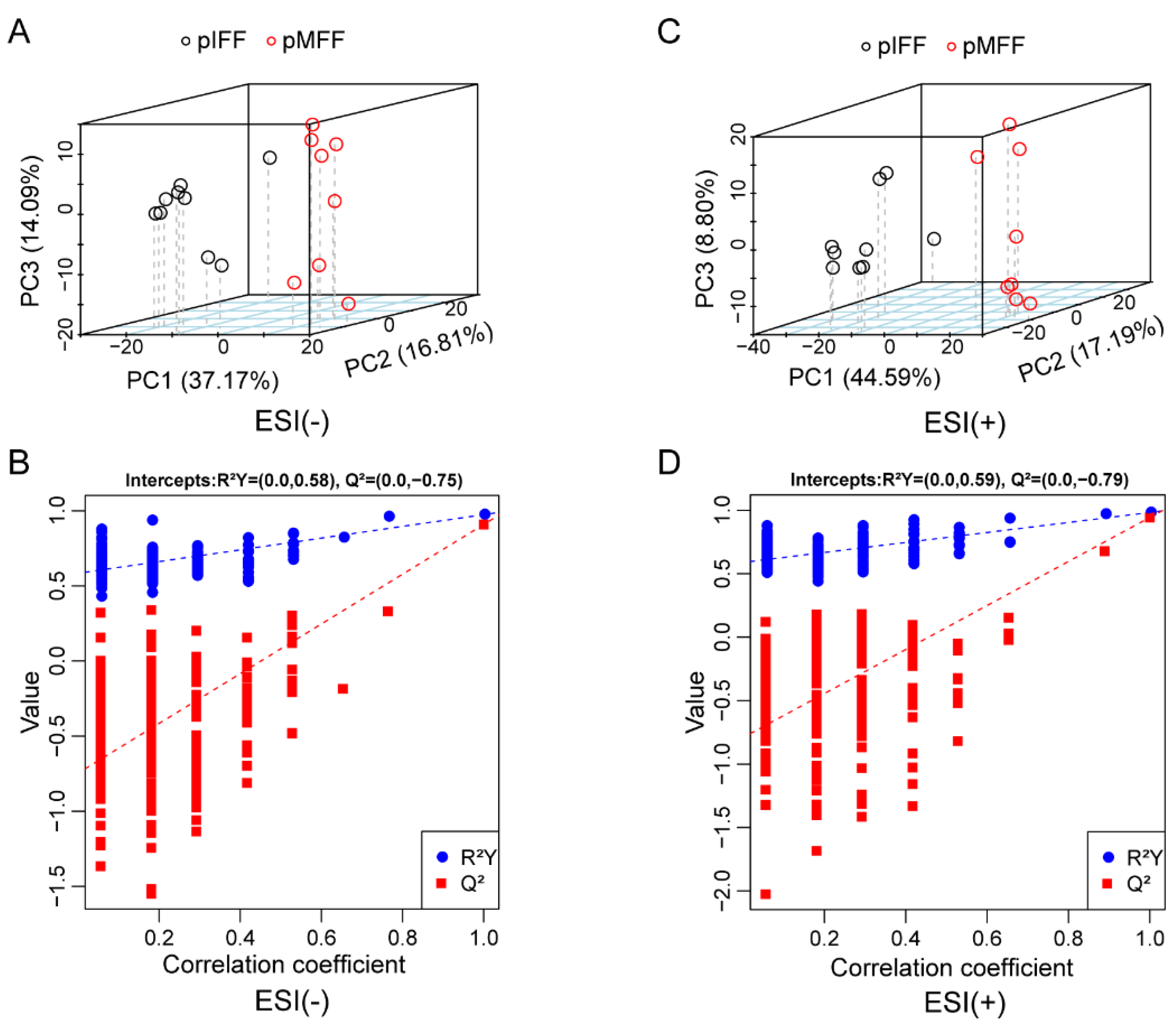
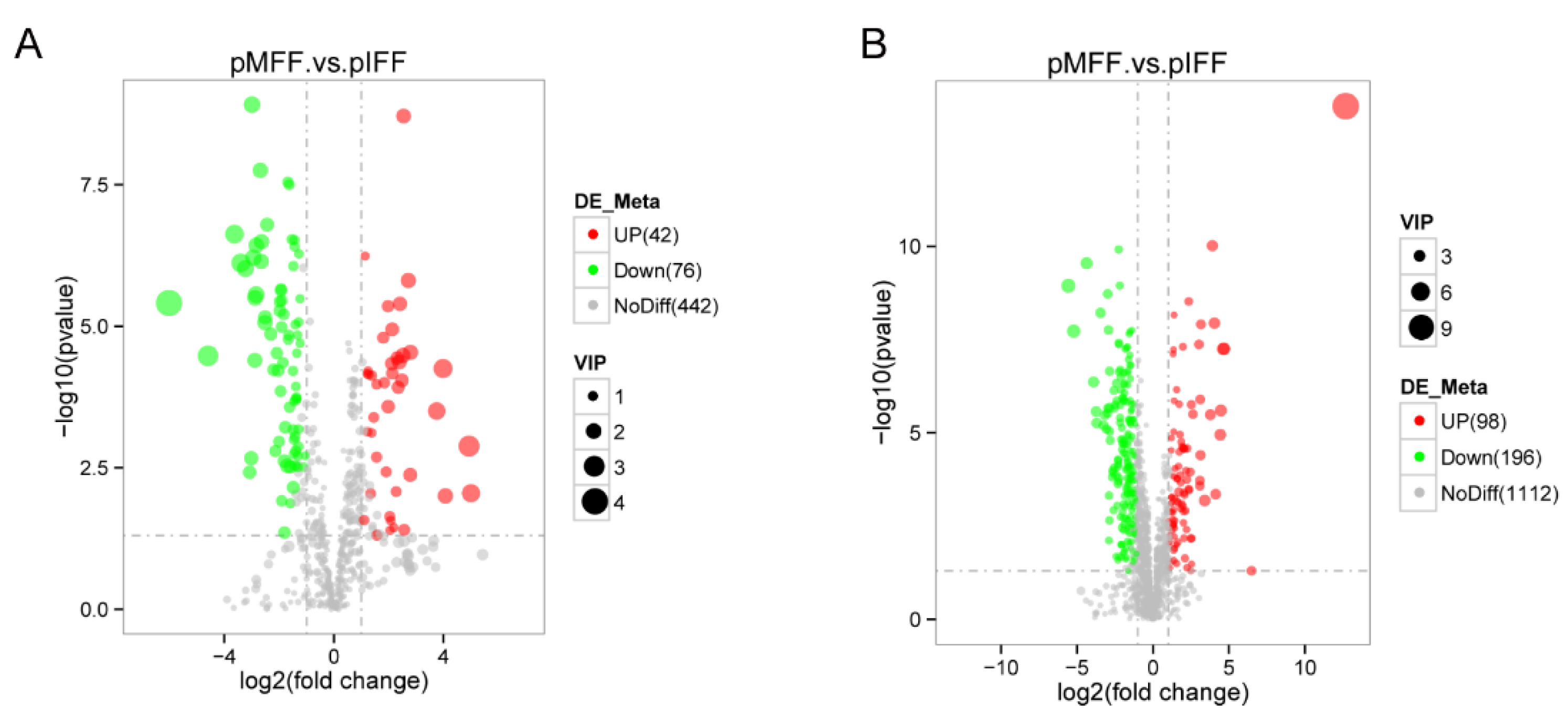
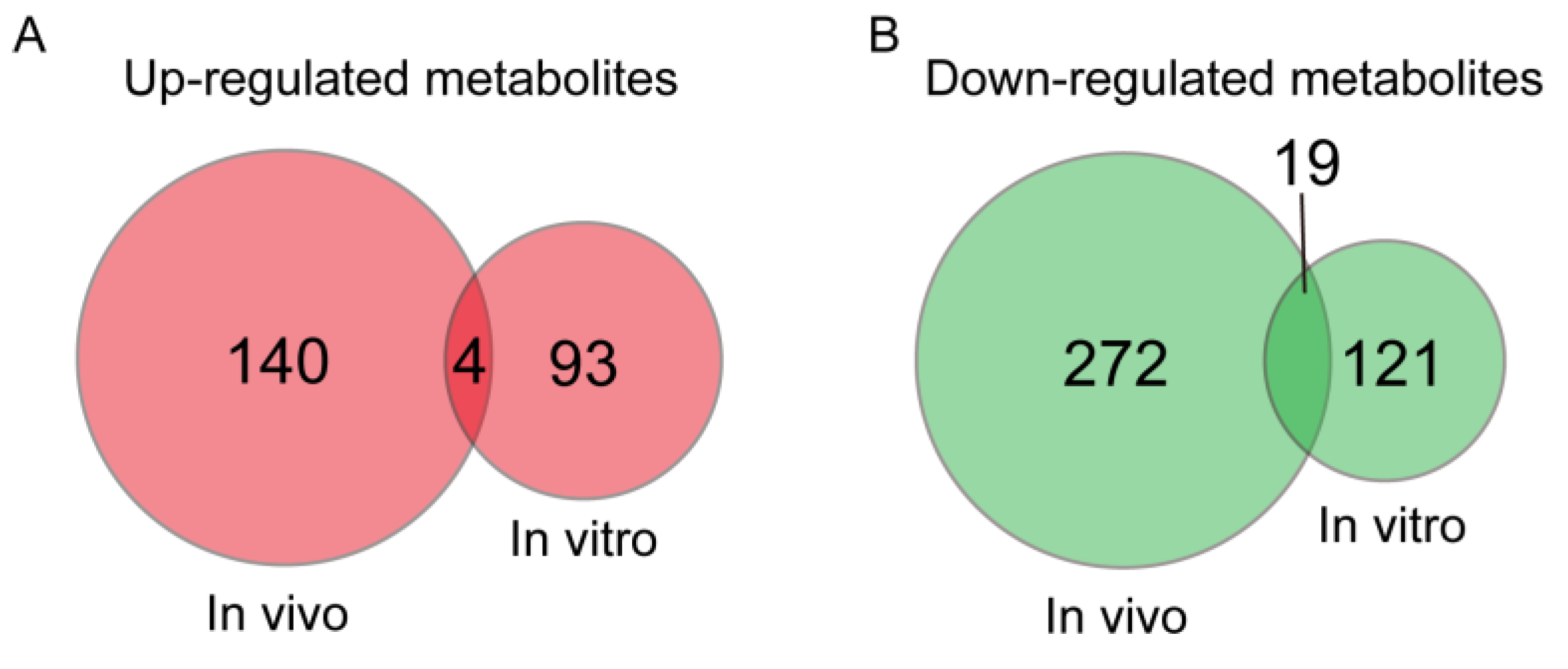
3.1. Untargeted Metabolomics Analysis of Porcine In Vivo Follicular Fluid
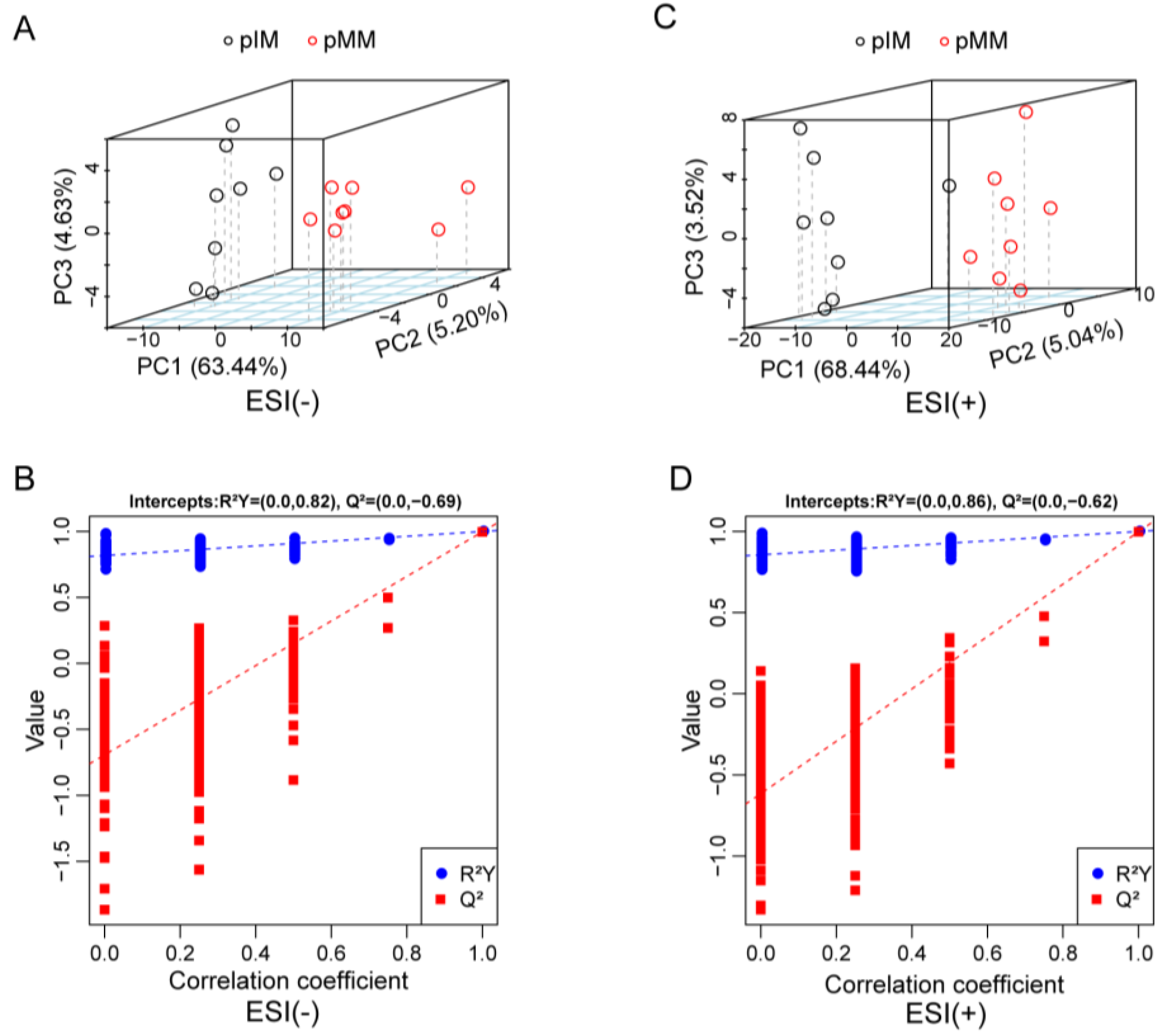
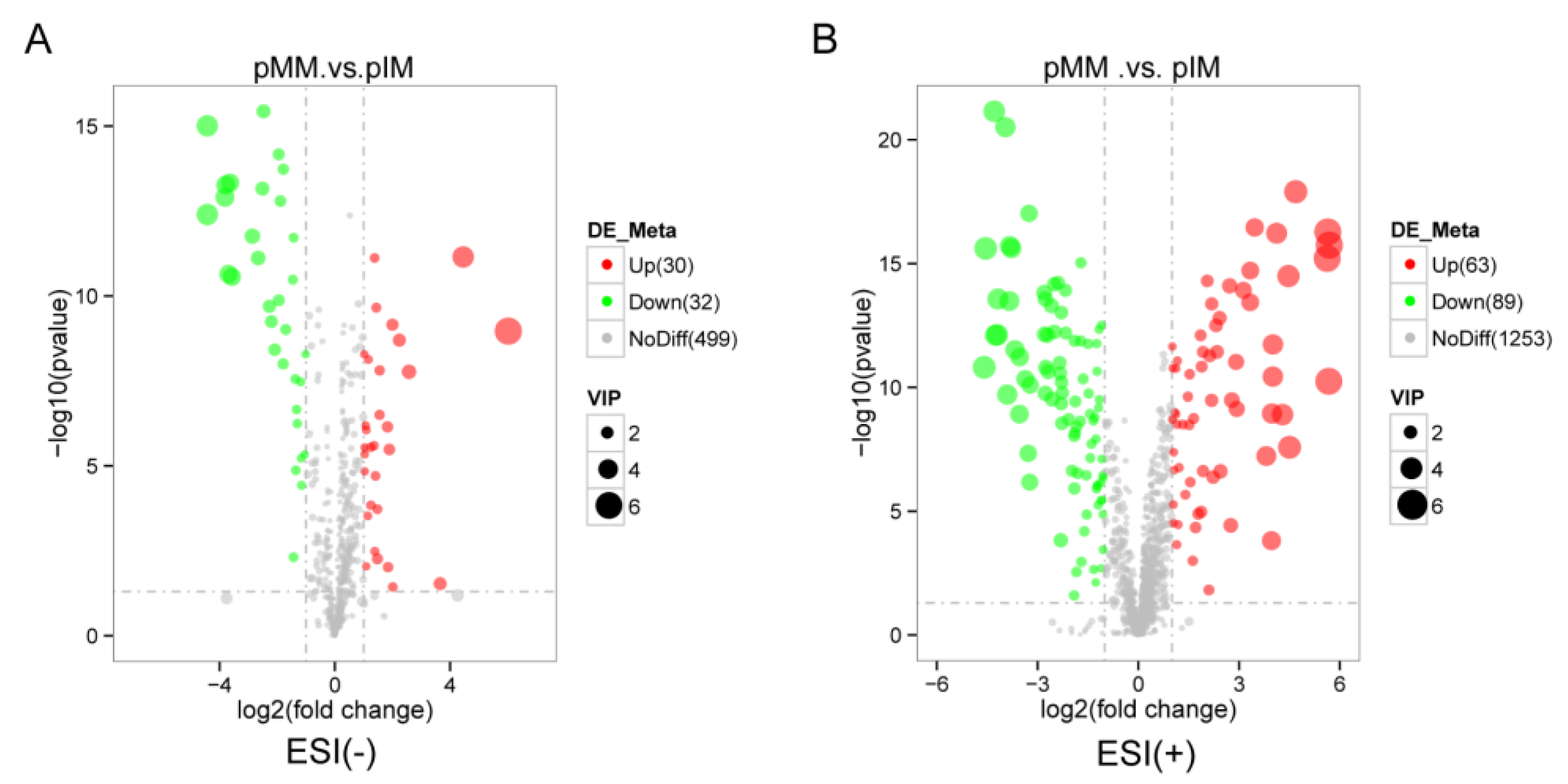
3.2. Untargeted Metabolomics Analysis of Porcine Oocyte In Vitro Culture Medium
3.3. Metabolites with a Same Change Trend during In Vivo and In Vitro Pig Oocyte Maturation
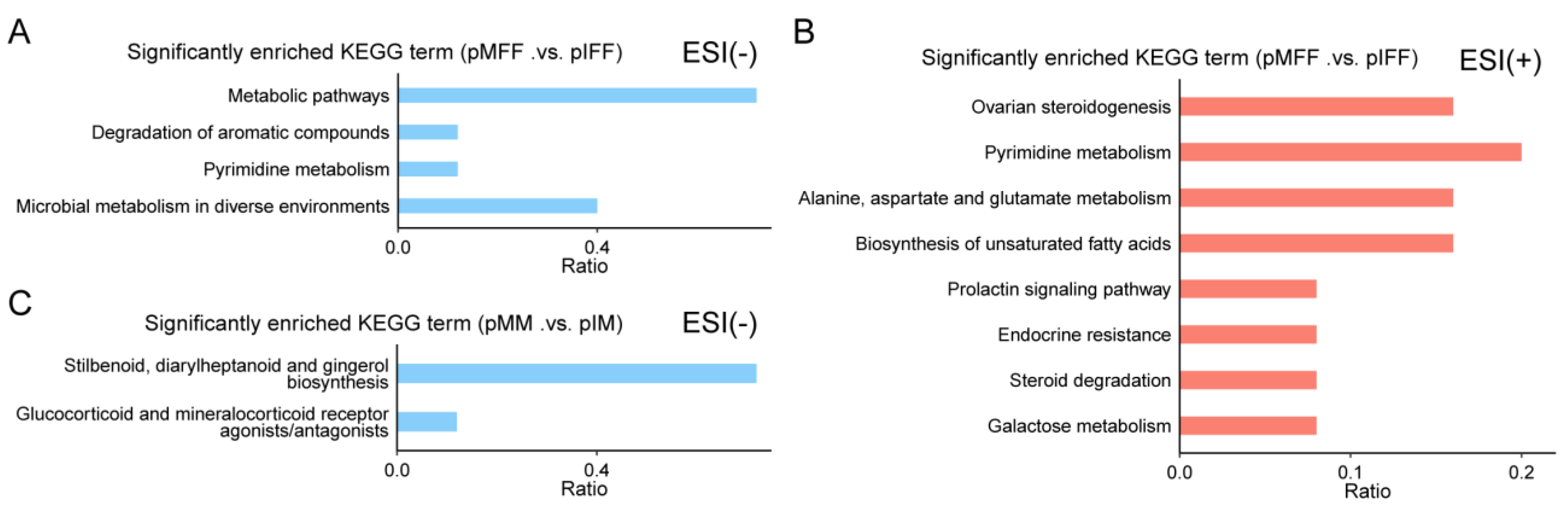
3.4. Untargeted Metabolomics Analysis of Porcine In Vivo Follicular Fluid
3.5. Untargeted Metabolomics Analysis of Porcine In Vivo Follicular Fluid
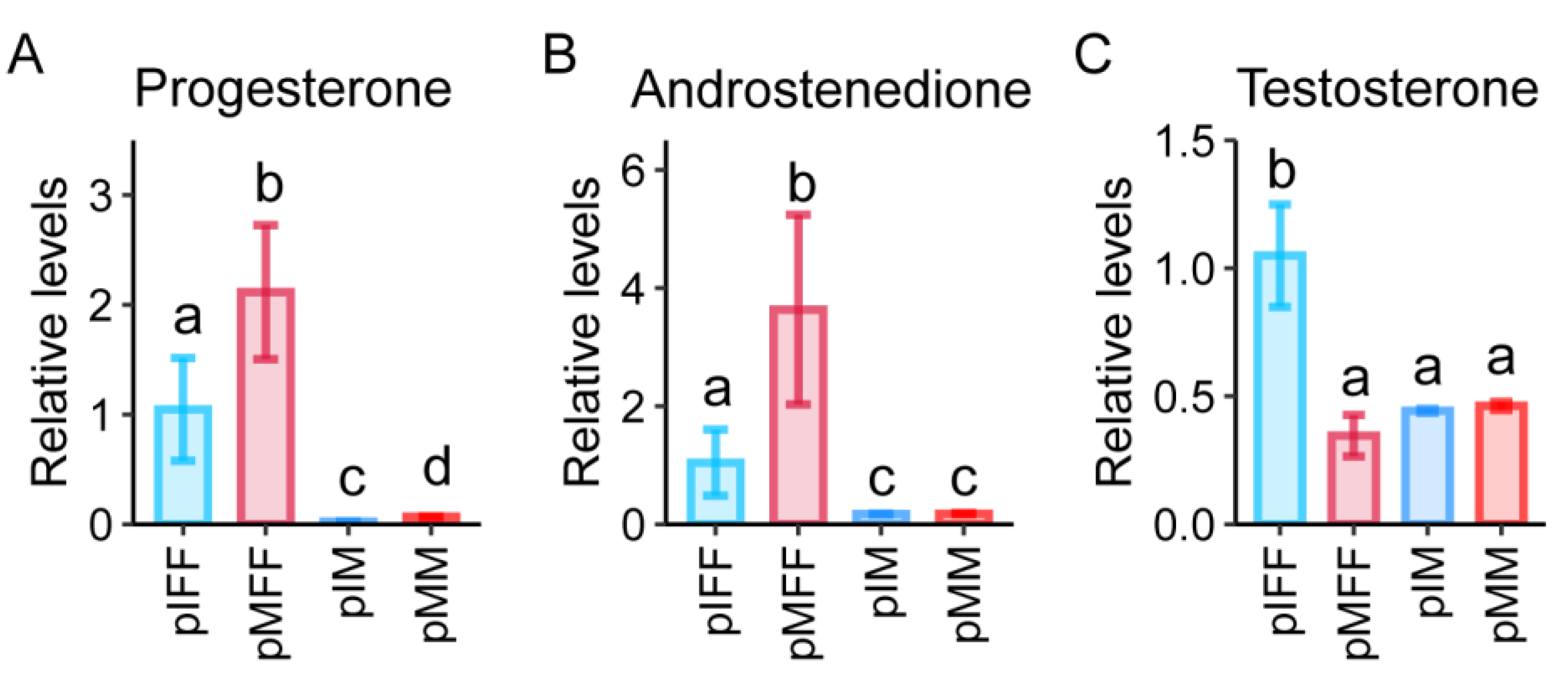
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Krisher, R.L. The effect of oocyte quality on development. J. Anim. Sci. 2004, 82, E14–E23. [Google Scholar] [PubMed]
- Shalom-Paz, E.; Almog, B.; Shehata, F.; Huang, J.; Holzer, H.; Chian, R.C.; Son, W.Y.; Tan, S.L. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod. Biomed. Online 2010, 21, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tu, Z.; Liu, Z.; Fan, N.; Yang, H.; Yang, S.; Yang, W.; Zhao, Y.; Ouyang, Z.; Lai, C.; et al. A Huntingtin Knockin Pig Model Recapitulates Features of Selective Neuro-degeneration in Huntington’s Disease. Cell 2018, 173, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Kaneyama, K.; Adachi, N.; Tsuneishi, B.; Matsukawa, K.; Watanabe, S.; Kubo, M.; Takahashi, S. Bovine nuclear transfer using fresh cumulus cell nuclei and in vivo- or in vitro-matured cytoplasts. Cloning Stem Cells 2008, 10, 173–180. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, S.; Zhang, N.; Ao, Z.; Wu, X.; Yang, L.; Shi, J.; Mai, R.; Zheng, E.; Cai, G.; et al. Source and Follicular Fluid Treatment During the In Vitro Maturation of Recipient Oocytes Affects the Development of Cloned Pig Embryo. Cell. Reprogramming 2020, 22, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Egashira, J.; Ihara, Y.; Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Efficient in vitro embryo production using in vivo-matured oocytes from superstimulated Japanese Black cows. J. Reprod. Dev. 2019, 65, 183–190. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tajima, S.; Kikuchi, K. The quality after culture invitro or invivo of porcine oocytes matured and fertilized invitro and their ability to develop to term. Anim. Sci. J. 2017, 88, 1916–1924. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Zhang, Y.; Wu, X.; Zhang, X.; Liang, Y.; Li, Y.; Zeng, F.; Shi, J.; Zhou, R.; et al. Sup-plementation of SDF1 during Pig Oocyte In Vitro Maturation Improves Subsequent Embryo Development. Molecules 2022, 27, 6830. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Kim, M.; Hyun, S.H. Neurotrophic factors in the porcine ovary: Their effects on follicular growth, oocyte maturation, and developmental competence. Front. Vet. Sci. 2022, 9, 931402. [Google Scholar] [CrossRef]
- Shi, J.M.; Tian, X.Z.; Zhou, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009, 47, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.E. Ovarian follicular growth and development in mammals. Biol. Reprod. 1994, 50, 225–232. [Google Scholar] [CrossRef]
- Gosden, R.G.; Hunter, R.H.; Telfer, E.; Torrance, C.; Brown, N. Physiological factors underlying the formation of ovarian follicular fluid. J. Reprod. Fertil. 1988, 82, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Ishak, G.M.; Feugang, J.M.; Pechanova, O.; Pechan, T.; Peterson, D.G.; Willard, S.T.; Ryan, P.L.; Gastal, E.L. Follicular-fluid proteomics during equine follicle development. Mol. Reprod. Dev. 2022, 89, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Song, R.; Bao, P.; Yin, M.; Li, J.; Zhang, G.; Wu, F.; Luo, Z.; Wu, X.; Song, W.; et al. Differential proteomic analysis demonstrates follicle fluid participate immune reaction and protein translation in yak. BMC Vet. Res. 2022, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Shi, B.; Wang, T.; Fang, Y.; Cao, T.; Zhou, Y.; Wang, X.; Li, D. Characterization of long non-coding RNA and messenger RNA profiles in follicular fluid from mature and immature ovarian follicles of healthy women and women with polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1735–1748. [Google Scholar] [CrossRef]
- Wang, C.; Fei, X.; Zhang, H.; Zhou, W.; Cheng, Z.; Feng, Y. Proteomic Analysis of the Alterations in Follicular Fluid Proteins During Oocyte Maturation in Humans. Front. Endocrinol. 2021, 12, 830691. [Google Scholar] [CrossRef]
- Sinchak, K.; Wagner, E.J. Estradiol signaling in the regulation of reproduction and energy balance. Front. Neuroendocrinol. 2012, 33, 342–363. [Google Scholar] [CrossRef]
- Tong, J.; Sheng, S.; Sun, Y.; Li, H.; Li, W.P.; Zhang, C.; Chen, Z.J. Melatonin levels in follicular fluid as markers for IVF out-comes and predicting ovarian reserve. Reproduction 2017, 153, 443–451. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.Y. Evaluation of oocyte quality: Morphological, cellular and molecular predictors. Reprod. Fertil. Dev. 2007, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Da, B.M.G.; Giorgi, V.S.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar]
- Zhang, X.; Li, W.; Sun, X.; Li, J.; Wu, W.; Liu, H. Vitamin C protects against defects induced by juglone during porcine oocyte maturation. J. Cell. Physiol. 2019, 234, 19574–19581. [Google Scholar] [PubMed]
- Yu, X.X.; Liu, Y.H.; Liu, X.M.; Wang, P.C.; Liu, S.; Miao, J.K.; Du, Z.Q.; Yang, C.X. Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes. Sci. Rep. 2018, 8, 6132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liang, S.; Jin, Y.X.; Kwon, J.W.; Zhang, J.B.; Kim, N.H. Progesterone influences cytoplasmic maturation in porcine oocytes developing in vitro. PeerJ 2016, 4, e2454. [Google Scholar] [CrossRef] [PubMed]
- Fair, T.; Lonergan, P. The role of progesterone in oocyte acquisition of developmental competence. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 142–147. [Google Scholar] [CrossRef]
- Varnagy, A.; Koszegi, T.; Gyorgyi, E.; Szegedi, S.; Sulyok, E.; Premusz, V.; Bodis, J. Levels of total antioxidant capacity and 8-hydroxy-2′-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: Focusing on endometriosis. Hum. Fertil. 2020, 23, 200–208. [Google Scholar] [CrossRef]
- Da, B.M.G.; de Albuquerque, F.O.; de Andrade, A.Z.; Cardoso, R.L.; Jordao, J.A.A.; Navarro, P.A. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016, 366, 231–242. [Google Scholar]
- Shi, C.; Yan, Z.; Zhang, Y.; Qin, L.; Wu, W.; Gao, C.; Gao, L.; Liu, J.; Cui, Y. Effects of putrescine on the quality and epigenetic modification of mouse oocytes during in vitro maturation. Reprod. Fertil. Dev. 2022, 34, 957–970. [Google Scholar]
- Hu, X.; Cheng, L.; Wang, X.; Luo, G.; Zhao, T.; Tian, J.; An, L. N-acetyl-l-cysteine protects porcine oocytes undergoing meiotic resumption from heat stress. Reprod. Toxicol. 2020, 91, 27–34. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Felix, M.R.; Turner, R.M.; Dobbie, T.; Hinrichs, K. Successful in vitro fertilization in the horse: Production of blastocysts and birth of foals after prolonged sperm incubation for capacitationdagger. Biol. Reprod. 2022, 107, 1551–1564. [Google Scholar] [CrossRef]
- Revelli, A.; Delle, P.L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Hernándezbello, R.; Moralesmontor, J. Regulation of Steroidogenesis in Reproductive, Adrenal and Neural Tissues by Cytokines. Bello 2010, 94, 132–135. [Google Scholar]
- Chen, M.; Zhang, B.; Cai, S.; Zeng, X.; Ye, Q.; Mao, X.; Zhang, S.; Zeng, X.; Ye, C.; Qiao, S. Metabolic disorder of amino acids, fatty acids and purines reflects the decreases in oocyte quality and potential in sows. J. Proteom. 2019, 200, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Mehta, J.; Gordon, L.I. Rust and corrosion in hematopoietic stem cell transplantation: The problem of iron and oxidative stress. Bone Marrow Transpl. 2004, 34, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Feng, T.; Li, S.; Zhang, X.; Qian, Y. Human follicular fluid shows diverse metabolic profiles at different follicle developmental stages. Reprod. Biol. Endocrinol. 2020, 18, 74. [Google Scholar] [CrossRef]
- Huang, Y.; Tu, M.; Qian, Y.; Ma, J.; Chen, L.; Liu, Y.; Wu, Y.; Chen, K.; Liu, J.; Ying, Y.; et al. Age-Dependent Metabolomic Profile of the Follicular Fluids From Women Undergoing Assisted Reproductive Technology Treatment. Front. Endocrinol. 2022, 13, 818888. [Google Scholar] [CrossRef]
- Grupen, C.G.; Mcilfatrick, S.M.; Ashman, R.J.; Boquest, A.C.; Armstrong, D.T.; Nottle, M.B. Relationship between donor animal age, follicular fluid steroid content and oocyte developmental competence in the pig. Reprod. Fertil. Dev. 2003, 15, 81–87. [Google Scholar] [CrossRef]
- Eroglu, A. Experimental studies on in vitro maturation of porcine oocytes. II. Effects of estradiol-17 beta and progesterone. Berl. Und Münchener Tierrztliche Wochenschr. 1993, 106, 157–159. [Google Scholar]
- Tasaki, H.; Iwata, H.; Sato, D.; Monji, Y.; Kuwayama, T. Estradiol has a major role in antrum formation of porcine preantral follicles cultured in vitro. Theriogenology 2013, 79, 809–814. [Google Scholar] [CrossRef]
- Makita, M.; Miyano, T. Steroid hormones promote bovine oocyte growth and connection with granulosa cells. Theriogenology 2014, 82, 605–612. [Google Scholar] [PubMed]
| Number | Metabolites | log2FC (pMFF vs. pIFF) | log2FC (pMM vs. pIM) | Up/ Down | Ion Modern |
|---|---|---|---|---|---|
| 1 | (2R)-2-Hydroxy-3-(phosphonooxy) propyl (8Z,11Z,14Z)-8,11,14-Icosatrienoate | 1.84 | 1.16 | Up | Negative |
| 2 | Prostaglandin A1 ethyl ester | 1.91 | 1.03 | Up | Negative |
| 3 | Progesterone | 1.36 | 1.51 | Up | Positive |
| 4 | Oxprenolol | 2.23 | 1.14 | Up | Positive |
| 5 | Maleic acid | −1.80 | −1.16 | Down | Negative |
| 6 | Vitamin C | −1.91 | −1.37 | Down | Negative |
| 7 | 5-(4-acetoxybut-1-ynyl)-2,2′-Bithiophene | −6.02 | −1.18 | Down | Negative |
| 8 | Glycerol 3-phosphate | −2.69 | −1.30 | Down | Negative |
| 9 | Sinapyl alcohol | −2.12 | −2.50 | Down | Positive |
| 10 | 8-hydroxy-deoxyguanosine | −2.931 | −1.05 | Down | Positive |
| 11 | Putrescine | −1.18 | −1.72 | Down | Positive |
| 12 | S-3-oxodecanoyl cysteamine | −1.57 | −1.48 | Down | Positive |
| 13 | N-Acetyl-L-cysteine | −2.69 | −1.16 | Down | Positive |
| 14 | N-Acetylcadaverine | −1.25 | −2.29 | Down | Positive |
| 15 | (2S)-2-Amino-5-({(2R)-3-{[(2R)-2-amino-2-Carboxyethyl]disulfanyl}-1-[(carboxymethyl)amino]-1-oxo-2-propanyl}amino)-5-Oxopentanoic acid | −1.65 | −2.16 | Down | Positive |
| 16 | 2E-Crotamiton | −2.86 | −4.54 | Down | Positive |
| 17 | Hypotaurine | −1.95 | −1.30 | Down | Positive |
| 18 | Tetraacetylethylenediamine | −1.50 | −1.05 | Down | Positive |
| 19 | 3,4-dehydrothiomorpholine-3-Carboxylic acid | −1.87 | −1.05 | Down | Positive |
| 20 | Guanine | −3.26 | −4.24 | Down | Positive |
| 21 | 3-Methylhistamine | −2.02 | −1.73 | Down | Positive |
| 22 | 3,3-Dimethyl-1,2-dithiolane | −3.45 | −4.59 | Down | Positive |
| 23 | Phosphonoacetaldehyde | −2.31 | −1.11 | Down | Positive |
| Groups | No. of Cultured Oocytes | No. of Matured Oocytes | Maturation Rate (%) |
|---|---|---|---|
| Control | 644 | 378 | 58.70 a |
| Progesterone | 678 | 414 | 71.63 b |
| Groups | No. of Cultured Embryos | No. of Cleaved Embryos (%) | No. of Blastocytes (%) |
|---|---|---|---|
| Control | 100 | 77 (77.00) | 33 (33.00) |
| Progesterone | 100 | 74 (74.00) | 33 (33.00) |
| Groups | No. of Cultured Embryos | No. of Cleaved Embryos (%) | No. of Blastocytes (%) |
|---|---|---|---|
| Control | 231 | 171 (74.03) | 33 (14.29) |
| Progesterone | 214 | 160 (74.77) | 35 (16.36) |
| Concentration (ng/mL) | No. of Cultured Oocytes | No. of Matured Oocytes | Maturation Rate (%) |
|---|---|---|---|
| 0 | 627 | 440 | 71.08 |
| 5 | 640 | 463 | 72.69 |
| 75 | 576 | 412 | 72.71 |
| 125 | 635 | 455 | 71.72 |
| 250 | 622 | 340 | 67.83 |
| Concentration (ng/mL) | No. of Cultured Embryos | No. of Cleaved Embryos (%) | No. of Blastocytes (%) |
|---|---|---|---|
| 0 | 110 | 103 (93.46) | 38 (34.72 a) |
| 5 | 104 | 100 (95.98) | 48 (46.40 ab) |
| 75 | 105 | 100 (94.79) | 49 (46.67 ab) |
| 125 | 117 | 103 (88.39) | 58 (50.11 b) |
| 250 | 108 | 100 (93.50) | 40 (36.70 a) |
| Concentration (ng/mL) | No. of Cultured Embryos | No. of Cleaved Embryos (%) | No. of Blastocytes (%) |
|---|---|---|---|
| 0 | 112 | 48 (42.84 a) | 11 (9.86) |
| 125 | 139 | 80 (57.63 b) | 12 (8.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; He, X.; Zhang, X.; Shi, J.; Zhou, R.; Mai, R.; Su, Q.; Cai, G.; Huang, S.; Xu, Z.; et al. Progesterone and Androstenedione Are Important Follicular Fluid Factors Regulating Porcine Oocyte Maturation Quality. Animals 2023, 13, 1811. https://doi.org/10.3390/ani13111811
Zhao H, He X, Zhang X, Shi J, Zhou R, Mai R, Su Q, Cai G, Huang S, Xu Z, et al. Progesterone and Androstenedione Are Important Follicular Fluid Factors Regulating Porcine Oocyte Maturation Quality. Animals. 2023; 13(11):1811. https://doi.org/10.3390/ani13111811
Chicago/Turabian StyleZhao, Huaxing, Xiaohua He, Xianjun Zhang, Junsong Shi, Rong Zhou, Ranbiao Mai, Qiaoyun Su, Gengyuan Cai, Sixiu Huang, Zheng Xu, and et al. 2023. "Progesterone and Androstenedione Are Important Follicular Fluid Factors Regulating Porcine Oocyte Maturation Quality" Animals 13, no. 11: 1811. https://doi.org/10.3390/ani13111811
APA StyleZhao, H., He, X., Zhang, X., Shi, J., Zhou, R., Mai, R., Su, Q., Cai, G., Huang, S., Xu, Z., Wu, Z., & Li, Z. (2023). Progesterone and Androstenedione Are Important Follicular Fluid Factors Regulating Porcine Oocyte Maturation Quality. Animals, 13(11), 1811. https://doi.org/10.3390/ani13111811






