The Role of Wildlife and Pests in the Transmission of Pathogenic Agents to Domestic Pigs: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
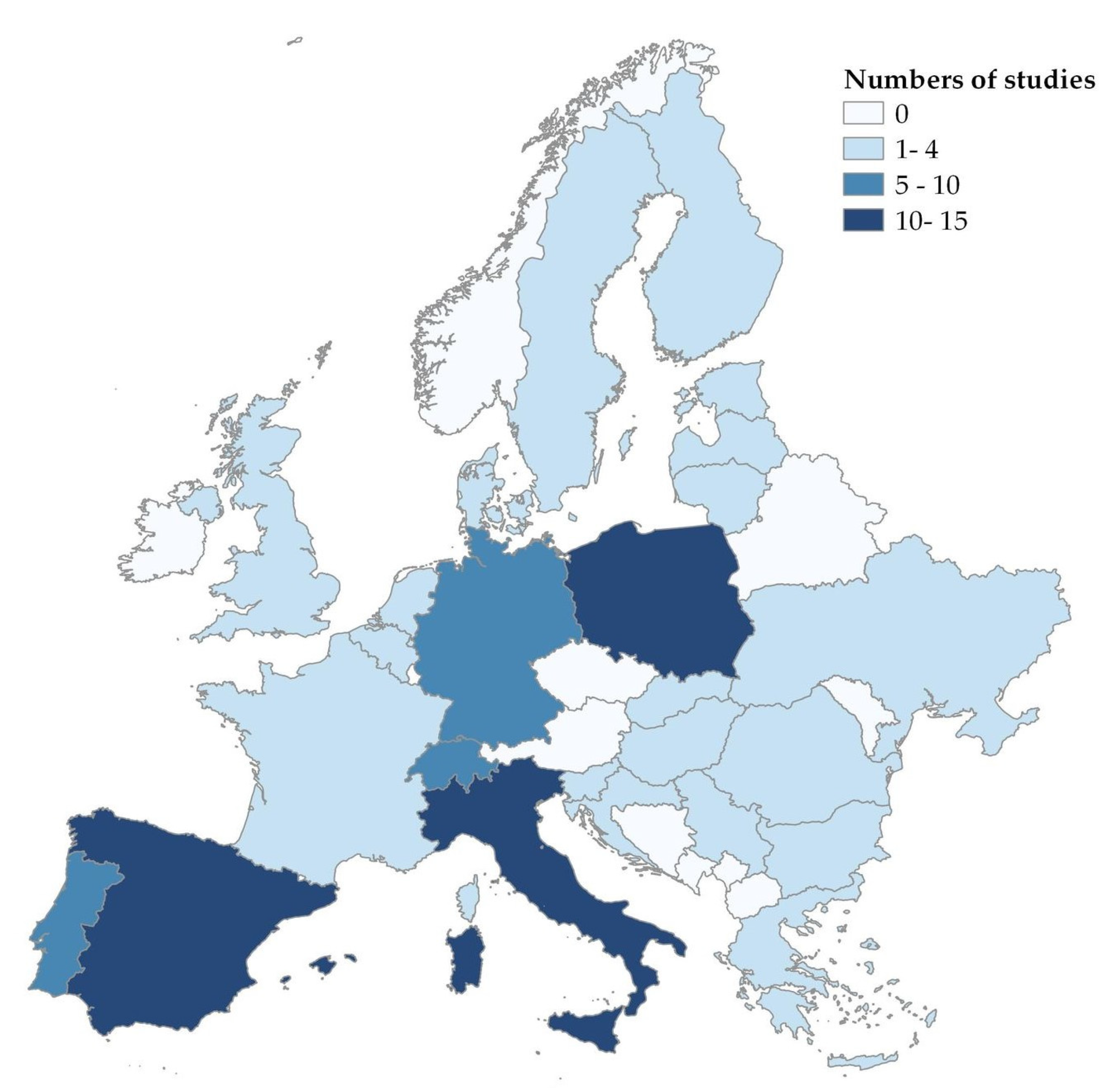
3. Results
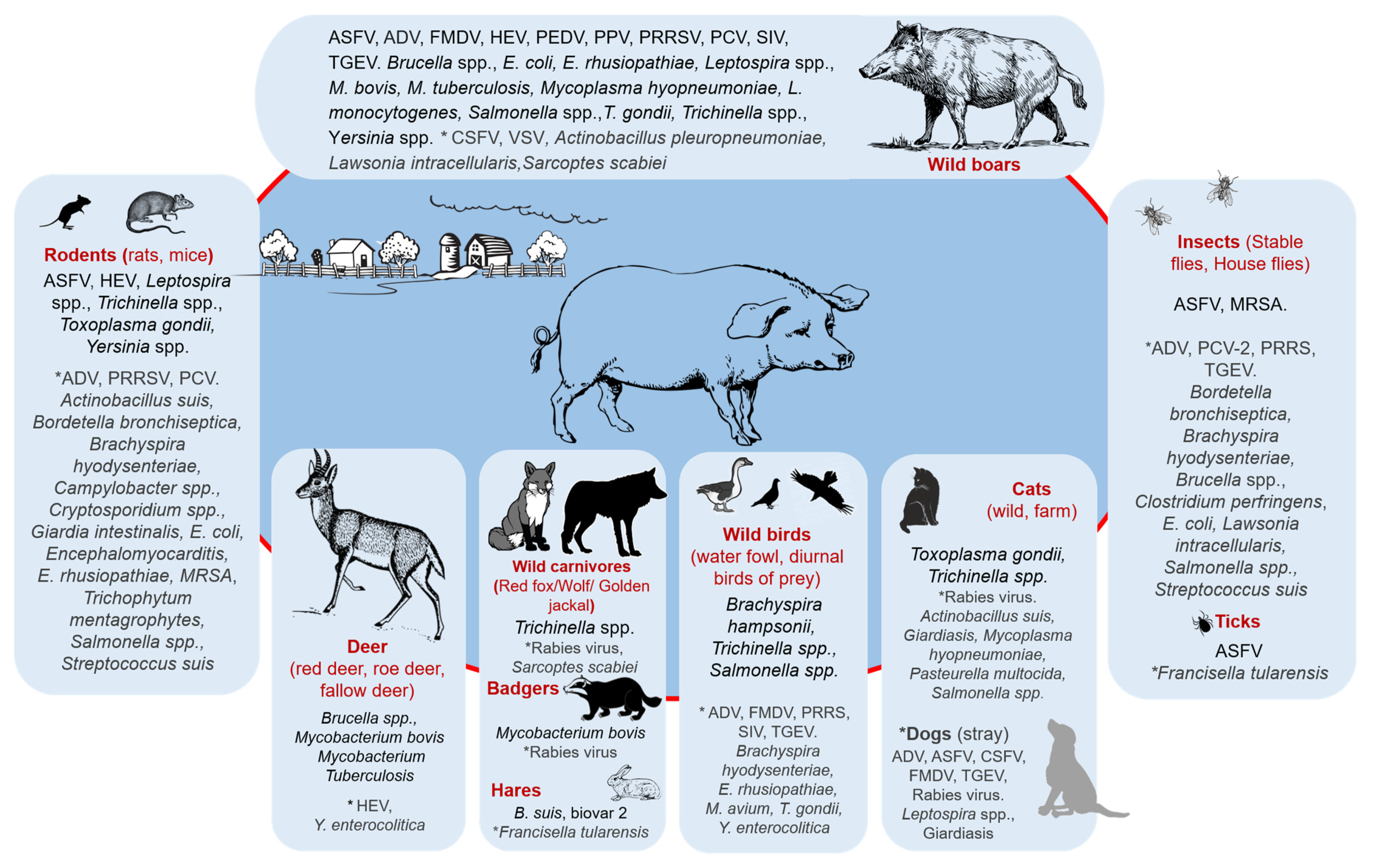
3.1. Sources of Infection
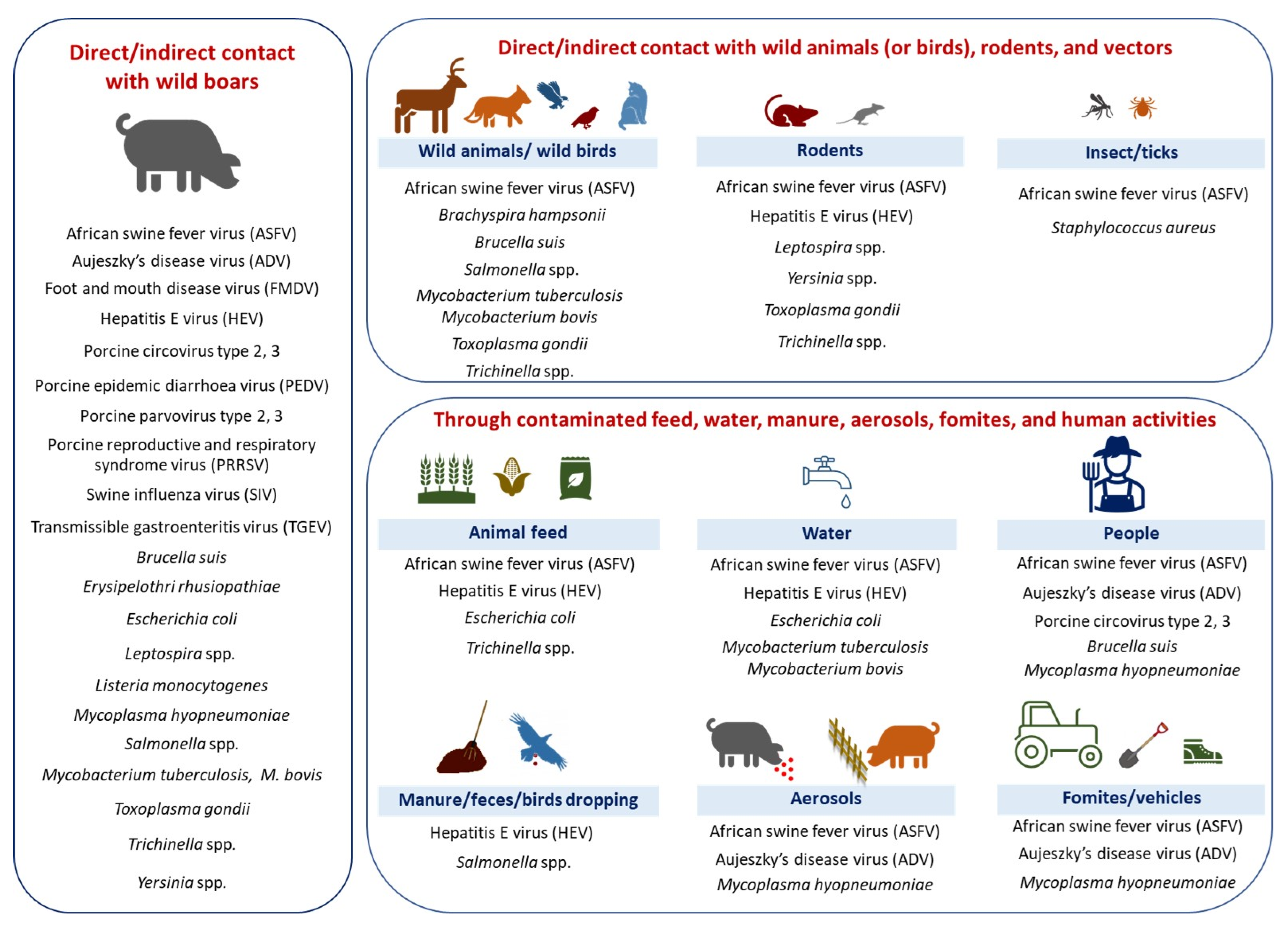
3.2. Transmission Pathways
3.2.1. Direct Transmission
3.2.2. Feed/Swill and Water
3.2.3. Airborne/Aerosol Transmission
3.2.4. Manure/Feces
3.2.5. Venereal Transmission
3.2.6. Indirect Transmission
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desrosiers, R. Transmission of Swine Pathogens: Different Means, Different Needs. Anim. Health Res. Rev. 2011, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gortázar, C.; Ferroglio, E.; Höfle, U.; Frölich, K.; Vicente, J. Diseases Shared between Wildlife and Livestock: A European Perspective. Eur. J. Wildl. Res. 2007, 53, 241–256. [Google Scholar] [CrossRef]
- Pearson, H.E.; Toribio, J.-A.L.M.L.; Lapidge, S.J.; Hernández-Jover, M. Evaluating the Risk of Pathogen Transmission from Wild Animals to Domestic Pigs in Australia. Prev. Vet. Med. 2016, 123, 39–51. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Deen, J. Global Trends in Infectious Diseases of Swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, S.A.; Dixon, L.K.; Gubbins, S.; Kucharski, A.J.; Drewe, J.A. Towards a Unified Generic Framework to Define and Observe Contacts between Livestock and Wildlife: A Systematic Review. PeerJ 2020, 8, e10221. [Google Scholar] [CrossRef] [PubMed]
- Magouras, I.; Brookes, V.J.; Jori, F.; Martin, A.; Pfeiffer, D.U.; Dürr, S. Emerging Zoonotic Diseases: Should We Rethink the Animal–Human Interface? Front. Vet. Sci. 2020, 7, 582743. [Google Scholar] [CrossRef]
- Bacigalupo, S.A.; Dixon, L.K.; Gubbins, S.; Kucharski, A.J.; Drewe, J.A. Wild Boar Visits to Commercial Pig Farms in Southwest England: Implications for Disease Transmission. Eur. J. Wildl. Res. 2022, 68, 69. [Google Scholar] [CrossRef]
- Soulsbury, C.D.; White, P.C.L.; Soulsbury, C.D.; White, P.C.L. Human–Wildlife Interactions in Urban Areas: A Review of Conflicts, Benefits and Opportunities. Wildl. Res. 2015, 42, 541–553. [Google Scholar] [CrossRef]
- Jori, F.; Hernandez-Jover, M.; Magouras, I.; Dürr, S.; Brookes, V.J. Wildlife–Livestock Interactions in Animal Production Systems: What Are the Biosecurity and Health Implications?|Animal Frontiers|Oxford Academic. Available online: https://academic.oup.com/af/article/11/5/8/6404338 (accessed on 20 January 2023).
- Miller, R.S.; Sweeney, S.J.; Slootmaker, C.; Grear, D.A.; Di Salvo, P.A.; Kiser, D.; Shwiff, S.A. Cross-Species Transmission Potential between Wild Pigs, Livestock, Poultry, Wildlife, and Humans: Implications for Disease Risk Management in North America. Sci. Rep. 2017, 7, 7821. [Google Scholar] [CrossRef]
- Alarcón, L.V.; Allepuz, A.; Mateu, E. Biosecurity in Pig Farms: A Review. Porc. Health Manag. 2021, 7, 5. [Google Scholar] [CrossRef]
- Dewulf, J.; Joosten, P.; Chantziaras, I.; Bernaerdt, E.; Vanderhaeghen, W.; Postma, M.; Maes, D. Antibiotic Use in European Pig Production: Less Is More. Antibiotics 2022, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- EFSA. African Swine Fever: Risks from Outdoor Pig Farms. Available online: https://www.efsa.europa.eu/en/news/african-swine-fever-risks-outdoor-pig-farms (accessed on 19 January 2023).
- Dixon, L.K.; Sun, H.; Roberts, H. African Swine Fever. Antiviral Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Niemi, J.K. Impacts of African Swine Fever on Pigmeat Markets in Europe. Front. Vet. Sci. 2020, 7, 634. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V. The Control of Classical Swine Fever in Wild Boar. Front. Microbiol. 2015, 6, 1211. [Google Scholar] [CrossRef]
- Backhans, A.; Fellström, C. Rodents on Pig and Chicken Farms—A Potential Threat to Human and Animal Health. Infect. Ecol. Epidemiol. 2012, 2, 17093. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Vermeer, H.M.; Kijlstra, A. Controlling Risks of Pathogen Transmission by Flies on Organic Pig Farms: A Review. Outlook Agric. 2007, 36, 193–197. [Google Scholar] [CrossRef]
- Bottoms, K.; Dewey, C.; Richardson, K.; Poljak, Z. Investigation of Biosecurity Risks Associated with the Feed Delivery: A Pilot Study. Can. Vet. J. 2015, 56, 502. [Google Scholar]
- Gordon, R.K.; Kotowski, I.K.; Coulson, K.F.; Link, D.; MacKenzie, A.; Bowling-Heyward, J. The Role of Non-Animal Origin Feed Ingredients in Transmission of Viral Pathogens of Swine: A Review of Scientific Literature. Front. Vet. Sci. 2019, 6, 273. [Google Scholar] [CrossRef]
- Filippitzi, M.E.; Brinch Kruse, A.; Postma, M.; Sarrazin, S.; Maes, D.; Alban, L.; Nielsen, L.R.; Dewulf, J. Review of Transmission Routes of 24 Infectious Diseases Preventable by Biosecurity Measures and Comparison of the Implementation of These Measures in Pig Herds in Six European Countries. Transbound. Emerg. Dis. 2018, 65, 381–398. [Google Scholar] [CrossRef]
- Chadegani, A.A.; Salehi, H.; Yunus, M.M.; Farhadi, H.; Fooladi, M.; Farhadi, M.; Ebrahim, N.A. A Comparison between Two Main Academic Literature Collections: Web of Science and Scopus Databases. Asian Soc. Sci. 2013, 9, 18. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Dei Giudici, S.; Lo Presti, A.; Bonelli, P.; Angioi, P.P.; Sanna, G.; Zinellu, S.; Balzano, F.; Salis, F.; Ciccozzi, M.; Oggiano, A. Phylogenetic Analysis of Porcine Circovirus Type 2 in Sardinia, Italy, Shows Genotype 2d Circulation among Domestic Pigs and Wild Boars. Infect. Genet. Evol. 2019, 71, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Laval, M.; Maestrini, O.; Casabianca, F.; Charrier, F.; Pavio, N. Assessment of Domestic Pigs, Wild Boars and Feral Hybrid Pigs as Reservoirs of Hepatitis E Virus in Corsica, France. Viruses 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.M.; Mick, V.; Sacchini, L.; Janowicz, A.; de Miguel, M.J.; Cherfa, M.-A.; Nevado, C.R.; Girault, G.; Andrés-Barranco, S.; Jay, M.; et al. Phylogeography and Epidemiology of Brucella Suis Biovar 2 in Wildlife and Domestic Swine. Vet. Microbiol. 2019, 233, 68–77. [Google Scholar] [CrossRef]
- Mauroy, A.; Depoorter, P.; Saegerman, C.; Cay, B.; De Regge, N.; Filippitzi, M.-E.; Fischer, C.; Laitat, M.; Maes, D.; Morelle, K.; et al. Semi-Quantitative Risk Assessment by Expert Elicitation of Potential Introduction Routes of African Swine Fever from Wild Reservoir to Domestic Pig Industry and Subsequent Spread during the Belgian Outbreak (2018–2019). Transbound. Emer. Dis. 2021, 68, 2761–2773. [Google Scholar] [CrossRef]
- Valdazo-González, B.; Polihronova, L.; Alexandrov, T.; Normann, P.; Knowles, N.J.; Hammond, J.M.; Georgiev, G.K.; Özyörük, F.; Sumption, K.J.; Belsham, G.J.; et al. Reconstruction of the Transmission History of RNA Virus Outbreaks Using Full Genome Sequences: Foot-and-Mouth Disease Virus in Bulgaria in 2011. PLoS ONE 2012, 7, e49650. [Google Scholar] [CrossRef] [PubMed]
- Takova, K.; Koynarski, T.; Minkov, I.; Ivanova, Z.; Toneva, V.; Zahmanova, G. Increasing Hepatitis E Virus Seroprevalence in Domestic Pigs and Wild Boar in Bulgaria. Animals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Prpić, J.; Černi, S.; Škorić, D.; Keros, T.; Brnić, D.; Cvetnić, Ž.; Jemeršić, L. Distribution and Molecular Characterization of Hepatitis E Virus in Domestic Animals and Wildlife in Croatia. Food Environ. Virol. 2015, 7, 195–205. [Google Scholar] [CrossRef]
- Prpić, J.; Keros, T.; Vucelja, M.; Bjedov, L.; Rode, O.Đ.; Margaletić, J.; Habrun, B.; Jemeršić, L. First Evidence of Hepatitis E Virus Infection in a Small Mammal (Yellow-Necked Mouse) from Croatia. PLoS ONE 2019, 14, e225583. [Google Scholar] [CrossRef]
- Jemeršić, L.; Prpić, J.; Brnić, D.; Keros, T.; Pandak, N.; Đaković Rode, O. Genetic Diversity of Hepatitis E Virus (HEV) Strains Derived from Humans, Swine and Wild Boars in Croatia from 2010 to 2017. BMC Infect. Dis. 2019, 19, 269. [Google Scholar] [CrossRef]
- Olesen, A.S.; Hansen, M.F.; Rasmussen, T.B.; Belsham, G.J.; Bødker, R.; Bøtner, A. Survival and Localization of African Swine Fever Virus in Stable Flies (Stomoxys Calcitrans) after Feeding on Viremic Blood Using a Membrane Feeder. Vet. Microbiol. 2018, 222, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Olesen, A.S.; Lohse, L.; Hansen, M.F.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A.; Bødker, R. Infection of Pigs with African Swine Fever Virus via Ingestion of Stable Flies (Stomoxys Calcitrans). Transbound. Emer. Dis. 2018, 65, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Stelder, J.J.; Kjær, L.J.; Jensen, L.B.; Boklund, A.E.; Denwood, M.; Carlsen, M.; Bødker, R. Livestock-Associated MRSA Survival on House Flies (Musca Domestica) and Stable Flies (Stomoxys Calcitrans) after Removal from a Danish Pig Farm. Sci. Rep. 2021, 11, 3527. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.T.; Westergaard, I.L.; Guldbech, G.K.; Nielsen, H.V.; Johansen, M.V. The Prevalence of Toxoplasma Gondii in Mice Living in Danish Indoor Sow Herds. Acta Vet. Scand. 2019, 61, 48. [Google Scholar] [CrossRef] [PubMed]
- Viltrop, A.; Reimus, K.; Niine, T.; Mõtus, K. Biosecurity Levels and Farm Characteristics of African Swine Fever Outbreak and Unaffected Farms in Estonia—What Can Be Learned from Them? Animals 2022, 12, 68. [Google Scholar] [CrossRef]
- Nurmoja, I.; Mõtus, K.; Kristian, M.; Niine, T.; Schulz, K.; Depner, K.; Viltrop, A. Epidemiological Analysis of the 2015–2017 African Swine Fever Outbreaks in Estonia. Prev. Vet. Med. 2020, 181, 104556. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; London, L.; Skrzypczak, T.; Kantala, T.; Laamanen, I.; Biström, M.; Maunula, L.; Gadd, T. Foodborne Zoonoses Common in Hunted Wild Boars. EcoHealth 2020, 17, 512–522. [Google Scholar] [CrossRef]
- Barth, S.A.; Blome, S.; Cornelis, D.; Pietschmann, J.; Laval, M.; Maestrini, O.; Geue, L.; Charrier, F.; Etter, E.; Menge, C.; et al. Faecal Escherichia Coli as Biological Indicator of Spatial Interaction between Domestic Pigs and Wild Boar (Sus scrofa) in Corsica. Transbound. Emer. Dis. 2018, 65, 746–757. [Google Scholar] [CrossRef]
- Jori, F.; Petit, G.; Civil, N.; Decors, A.; Charrier, F.; Casabianca, F.; Grosbois, V. A Questionnaire Survey for the Assessment of Wild–Domestic Pig Interactions in a Context Oedema Disease Outbreaks among Wild Boars (Sus scrofa) in South-Eastern France. Transbound. Emer. Dis. 2022, 69, 4009–4015. [Google Scholar] [CrossRef]
- Pannwitz, G.; Freuling, C.; Denzin, N.; Schaarschmidt, U.; Nieper, H.; Hlinak, A.; Burkhardt, S.; Klopries, M.; Dedek, J.; Hoffmann, L.; et al. A Long-Term Serological Survey on Aujeszky’s Disease Virus Infections in Wild Boar in East Germany. Epidemiol. Infect. 2012, 140, 348–353. [Google Scholar] [CrossRef]
- Schlosser, J.; Vina-Rodriguez, A.; Fast, C.; Groschup, M.H.; Eiden, M. Chronically Infected Wild Boar Can Transmit Genotype 3 Hepatitis E Virus to Domestic Pigs. Vet. Microbiol. 2015, 180, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Eiden, M.; Vina-Rodriguez, A.; Fast, C.; Dremsek, P.; Lange, E.; Ulrich, R.G.; Groschup, M.H. Natural and Experimental Hepatitis E Virus Genotype 3-Infection in European Wild Boar Is Transmissible to Domestic Pigs. Vet. Res. 2014, 45, 121. [Google Scholar] [CrossRef] [PubMed]
- Methner, U.; Merbach, S.; Peters, M. Salmonella enterica Subspecies Enterica Serovar Choleraesuis in a German Wild Boar Population: Occurrence and Characterisation. Acta Vet. Scand. 2018, 60, 65. [Google Scholar] [CrossRef]
- Marinou, K.A.; Papatsiros, V.G.; Gkotsopoulos, E.K.; Odatzoglou, P.K.; Athanasiou, L.V. Exposure of Extensively Farmed Wild Boars (Sus Scrofa Scrofa) to Selected Pig Pathogens in Greece. Vet. Q. 2015, 35, 97–101. [Google Scholar] [CrossRef]
- Di Sabatino, D.; Garofolo, G.; Di Provvido, A.; Zilli, K.; Foschi, G.; Di Giannatale, E.; Ciuffetelli, M.; De Massis, F. Brucella Suis Biovar 2 Multi Locus Sequence Type ST16 in Wild Boars (Sus scrofa) from Abruzzi Region, Italy. Introduction from Central-Eastern Europe? Infect. Genet. Evol. 2017, 55, 63–67. [Google Scholar] [CrossRef] [PubMed]
- De Massis, F.; Zilli, K.; Donato, G.D.; Nuvoloni, R.; Pelini, S.; Sacchini, L.; D’Alterio, N.; Giannatale, E.D. Distribution of Brucella Field Strains Isolated from Livestock, Wildlife Populations, and Humans in Italy from 2007 to 2015. PLoS ONE 2019, 14, e213689. [Google Scholar] [CrossRef]
- De Sabato, L.; Ianiro, G.; Monini, M.; De Lucia, A.; Ostanello, F.; Di Bartolo, I. Detection of Hepatitis E Virus RNA in Rats Caught in Pig Farms from Northern Italy. Zoonoses Public Health 2020, 67, 62–69. [Google Scholar] [CrossRef]
- Aprea, G.; Amoroso, M.G.; Di Bartolo, I.; D’Alessio, N.; Di Sabatino, D.; Boni, A.; Cioffi, B.; D’Angelantonio, D.; Scattolini, S.; De Sabato, L.; et al. Molecular Detection and Phylogenetic Analysis of Hepatitis E Virus Strains Circulating in Wild Boars in South-Central Italy. Transbound. Emer. Dis. 2018, 65, e25–e31. [Google Scholar] [CrossRef]
- Lo Presti, A.; Bruni, R.; Villano, U.; Marcantonio, C.; Equestre, M.; Ciuffetelli, M.; Grimaldi, A.; Suffredini, E.; Di Pasquale, S.; De Medici, D.; et al. Phylogenetic Analysis and Epidemiological History of Hepatitis E Virus 3f and 3c in Swine and Wild Boar, Italy. Heliyon 2020, 6, e05110. [Google Scholar] [CrossRef]
- Cilia, G.; Bertelloni, F.; Piredda, I.; Ponti, M.N.; Turchi, B.; Cantile, C.; Parisi, F.; Pinzauti, P.; Armani, A.; Palmas, B.; et al. Presence of Pathogenic Leptospira Spp. In the Reproductive System and Fetuses of Wild Boars (Sus scrofa) in Italy. PLoS. Negl. Trop. Dis. 2020, 14, e8982. [Google Scholar] [CrossRef]
- Amato, B.; Di Marco Lo Presti, V.; Gerace, E.; Capucchio, M.T.; Vitale, M.; Zanghì, P.; Pacciarini, M.L.; Marianelli, C.; Boniotti, M.B. Molecular Epidemiology of Mycobacterium Tuberculosis Complex Strains Isolated from Livestock and Wild Animals in Italy Suggests the Need for a Different Eradication Strategy for Bovine Tuberculosis. Transbound. Emer. Dis. 2018, 65, e416–e424. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.G.; Serra, F.; Esposito, C.; D’Alessio, N.; Ferrara, G.; Cioffi, B.; Anzalone, A.; Pagnini, U.; De Carlo, E.; Fusco, G.; et al. Prevalence of Infection with Porcine Circovirus Types 2 and 3 in the Wild Boar Population in the Campania Region (Southern Italy). Animals 2021, 11, 3215. [Google Scholar] [CrossRef]
- Ferrara, G.; Nocera, F.P.; Longobardi, C.; Ciarcia, R.; Fioretti, A.; Damiano, S.; Iovane, G.; Pagnini, U.; Montagnaro, S. Retrospective Serosurvey of Three Porcine Coronaviruses among the Wild Boar (Sus scrofa) Population in the Campania Region of Italy. J. Wildl. Dis. 2022, 58, 887–891. [Google Scholar] [CrossRef]
- Lamberga, K.; Olševskis, E.; Seržants, M.; Berzinš, A.; Viltrop, A.; Depner, K. African Swine Fever in Two Large Commercial Pig Farms in LATVIA-Estimation of the High Risk Period and Virus Spread within the Farm. Vet. Sci. 2020, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Lamberga, K.; Seržants, M.; Oļševskis, E. African Swine Fever Outbreak Investigations in a Large Commercial Pig Farm in Latvia: A Case Report. Berl. Munch. Tierarztl. Wochenschr. 2019, 132, 151–155. [Google Scholar] [CrossRef]
- Turčinavičienė, J.; Petrašiūnas, A.; Bernotienė, R.; Masiulis, M.; Jonušaitis, V. The Contribution of Insects to African Swine Fever Virus Dispersal: Data from Domestic Pig Farms in Lithuania. Med. Vet. Entomol. 2021, 35, 484–489. [Google Scholar] [CrossRef]
- Pautienius, A.; Schulz, K.; Staubach, C.; Grigas, J.; Zagrabskaite, R.; Buitkuviene, J.; Stankevicius, R.; Streimikyte, Z.; Oberauskas, V.; Zienius, D.; et al. African Swine Fever in the Lithuanian Wild Boar Population in 2018: A Snapshot. Virol. J. 2020, 17, 148. [Google Scholar] [CrossRef]
- Malakauskas, A.; Schulz, K.; Kukanauskaite, I.; Masiulis, M.; Conraths, F.; Sauter-Louis, C. African Swine Fever Outbreaks in Lithuanian Domestic Pigs in 2019. Animals 2022, 12, 115. [Google Scholar] [CrossRef]
- Stankevicius, A.; Buitkuviene, J.; Sutkiene, V.; Spancerniene, U.; Pampariene, I.; Pautienius, A.; Oberauskas, V.; Zilinskas, H.; Zymantiene, J. Detection and Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus in Lithuanian Wild Boar Populations. Acta Vet. Scand. 2016, 58, 51. [Google Scholar] [CrossRef]
- van Tulden, P.; Gonzales, J.L.; Kroese, M.; Engelsma, M.; de Zwart, F.; Szot, D.; Bisselink, Y.; van Setten, M.; Koene, M.; Willemsen, P.; et al. Monitoring Results of Wild Boar (Sus scrofa) in The Netherlands: Analyses of Serological Results and the First Identification of Brucella Suis Biovar 2. Infect. Ecol. Epidemiol. 2020, 10, 1794668. [Google Scholar] [CrossRef] [PubMed]
- Krijger, I.M.; Ahmed, A.A.A.; Goris, M.G.A.; Cornelissen, J.B.W.J.; Groot Koerkamp, P.W.G.; Meerburg, B.G. Wild Rodents and Insectivores as Carriers of Pathogenic Leptospira and Toxoplasma Gondii in The Netherlands. Vet. Med. Sci. 2020, 6, 623–630. [Google Scholar] [CrossRef]
- Frant, M.P.; Gal-Cisoń, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Szczotka-Bochniarz, A. African Swine Fever (ASF) Trend Analysis in Wild Boar in Poland (2014–2020). Animals 2022, 12, 1170. [Google Scholar] [CrossRef]
- Śmietanka, K.; Woźniakowski, G.; Kozak, E.; Niemczuk, K.; Fraçzyk, M.; Bocian, L.; Kowalczyk, A.; Pejsak, Z. African Swine Fever Epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.J.; Dziwulaki, M. Development of African Swine Fever in Poland. Agriculture 2022, 12, 119. [Google Scholar] [CrossRef]
- Weiner, M.; Tokarska-Rodak, M.; Plewik, D.; Pańczuk, A.; Szepeluk, A.; Krajewska, M. Preliminary Study on the Detection of Hepatitis E Virus (HEV) Antibodies in Pigs and Wild Boars in Poland. J. Vet. Res. 2016, 60, 385–389. [Google Scholar] [CrossRef]
- Antas, M.; Olech, M.; Szczotka-Bochniarz, A. Porcine Enteric Coronavirus Infections in Wild Boar in Poland—A Pilot Study. J. Vet. Res. 2021, 65, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kornacka, A.; Moskwa, B.; Werner, A.; Nowosad, P.; Jankowska, W.; Cybulska, A.; Majewska, A.C. The Seroprevalence of Toxoplasma Gondii in Wild Boars from Three Voivodeships in Poland, MAT Analyses. Acta Parasitol. 2020, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Bilska-Zając, E.; Różycki, M.; Grądziel-Krukowska, K.; Bełcik, A.; Mizak, I.; Karamon, J.; Sroka, J.; Zdybel, J.; Cencek, T. Diversity of Trichinella Species in Relation to the Host Species and Geographical Location. Vet. Parasitol. 2020, 279, 109052. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; Pereira-Vaz, J.; Duque, V.; Bandeira, V.; Fonseca, C.; Donato, A.; Luxo, C.; Matos, A.M. First Serological Evidence on Endemicity of HEV Infection in Wild Boar (Sus scrofa) Populations from Portugal. Virol. Sin. 2018, 33, 197–200. [Google Scholar] [CrossRef]
- Muñoz, P.M.; Boadella, M.; Arnal, M.; de Miguel, M.J.; Revilla, M.; Martínez, D.; Vicente, J.; Acevedo, P.; Oleaga, T.; Ruiz-Fons, F.; et al. Spatial Distribution and Risk Factors of Brucellosis in Iberian Wild Ungulates. BMC Infect. Dis. 2010, 10, 46. [Google Scholar] [CrossRef]
- Boinas, F.; Ribeiro, R.; Madeira, S.; Palma, M.; de Carvalho, I.L.; Núncio, S.; Wilson, A.J. The Medical and Veterinary Role of Ornithodoros Erraticus Complex Ticks (Acari: Ixodida) on the Iberian Peninsula. J Vector Ecol 2014, 39, 238–248. [Google Scholar] [CrossRef]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The Persistence of African Swine Fever Virus in Field-Infected Ornithodoros Erraticus during the ASF Endemic Period in Portugal. PLoS ONE 2011, 6, e20383. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Bastos, A.D.; Penrith, M.L. New Insights into the Role of Ticks in African Swine Fever Epidemiology. Rev. Sci. Tech. 2015, 34, 503–511. [Google Scholar] [CrossRef]
- Barbuceanu, F.; Tamba, P.; Nitescu, C.; Lazar, N.; Donescu, D.; Predoi, G.; Baraitareanu, S. African swine fever in Romania: Retrospective analysis 2017–2020. Rev. Romana Med. Vet. 2020, 31, 59–63. [Google Scholar]
- Boklund, A.; Dhollander, S.; Chesnoiu Vasile, T.; Abrahantes, J.C.; Bøtner, A.; Gogin, A.; Gonzalez Villeta, L.C.; Gortázar, C.; More, S.J.; Papanikolaou, A.; et al. Risk Factors for African Swine Fever Incursion in Romanian Domestic Farms during 2019. Sci. Rep. 2020, 10, 10215. [Google Scholar] [CrossRef] [PubMed]
- Nešković, M.; Ristić, B.; Došenović, R.; Grubač, S.; Petrović, T.; Prodanov-Radulović, J.; Polaček, V. African Swine Fever Outbreak Investigation on Large Commercial Pig Farm in Serbia. Acta Vet. 2021, 71, 219–229. [Google Scholar] [CrossRef]
- Zurovac Sapundzic, Z.; Zutic, J.; Stevic, N.; Milicevic, V.; Radojicic, M.; Stanojevic, S.; Radojicic, S. First Report of Brucella Seroprevalence in Wild Boar Population in Serbia. Vet. Sci. 2022, 9, 575. [Google Scholar] [CrossRef]
- Cvetkovic, J.; Teodorovic, V.; Marucci, G.; Vasilev, D.; Vasilev, S.; Cirovic, D.; Sofronic-Milosavljevic, L. First Report of Trichinella britovi in Serbia. Acta Parasitol. 2011, 56, 232–235. [Google Scholar] [CrossRef]
- Zivojinovic, M.; Sofronic-Milosavljevic, L.; Cvetkovic, J.; Pozio, E.; Interisano, M.; Plavsic, B.; Radojicic, S.; Kulisic, Z. Trichinella Infections in Different Host Species of an Endemic District of Serbia. Vet. Parasitol. 2013, 194, 136–138. [Google Scholar] [CrossRef]
- Sliz, I.; Vlasakova, M.; Jackova, A.; Vilcek, S. Characterization of Porcine Parvovirus Type 3 and Porcine Circovirus Type 2 in Wild Boars (Sus scrofa) in Slovakia. J. Wildl. Dis. 2015, 51, 703–711. [Google Scholar] [CrossRef]
- Hurníková, Z.; Miterpáková, M.; Zaleśny, G.; Komorová, P.; Chovancová, G. Fifteen Years since the First Record of Trichinella Pseudospiralis in Slovakia: What’s New? Vet. Parasitol. 2021, 297, 109129. [Google Scholar] [CrossRef]
- Papić, B.; Kušar, D.; Mićunović, J.; Vidrih, Š.; Pirš, M.; Ocepek, M.; Avberšek, J. Genomic Insights into Salmonella Choleraesuis Var. Kunzendorf Outbreak Reveal Possible Interspecies Transmission. Vet. Microbiol. 2021, 263, 109282. [Google Scholar] [CrossRef]
- Gil Molino, M.; García Sánchez, A.; Risco Pérez, D.; Gonçalves Blanco, P.; Quesada Molina, A.; Rey Pérez, J.; Martín Cano, F.; Cerrato Horrillo, R.; Hermoso-de-Mendoza Salcedo, J.; Fernández Llario, P. Prevalence of Salmonella Spp. in Tonsils, Mandibular Lymph Nodes and Faeces of Wild Boar from Spain and Genetic Relationship between Isolates. Transbound. Emer. Dis. 2019, 66, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ruiz, S.; Laguna, E.; Vicente, J.; García-Bocanegra, I.; Martínez-Guijosa, J.; Cano-Terriza, D.; Risalde, M.A.; Acevedo, P. Characterization and Management of Interaction Risks between Livestock and Wild Ungulates on Outdoor Pig Farms in Spain. Porc. Health Manag. 2022, 8, 2. [Google Scholar] [CrossRef]
- Boadella, M.; Gortázar, C.; Vicente, J.; Ruiz-Fons, F. Wild Boar: An Increasing Concern for Aujeszky’s Disease Control in Pigs? BMC Vet. Res. 2012, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Casades-Martí, L.; González-Barrio, D.; Royo-Hernández, L.; Díez-Delgado, I.; Ruiz-Fons, F. Dynamics of Aujeszky’s Disease Virus Infection in Wild Boar in Enzootic Scenarios. Transboundary Emer. Dis. 2020, 67, 388–405. [Google Scholar] [CrossRef]
- Vicente-Rubiano, M.; Martínez-López, B.; Sánchez-Vizcaíno, F.; Sánchez-Vizcaíno, J.M. A New Approach for Rapidly Assessing the Risk of Aujeszky’s Disease Reintroduction into a Disease-Free Spanish Territory by Analysing the Movement of Live Pigs and Potential Contacts with Wild Boar. Transbound. Emer. Dis. 2014, 61, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Risco, D.; García, A.; Serrano, E.; Fernandez-Llario, P.; Benítez, J.M.; Martínez, R.; García, W.L.; de Mendoza, J.H. High-Density Dependence but Low Impact on Selected Reproduction Parameters of Brucella Suis Biovar 2 in Wild Boar Hunting Estates from South-Western Spain. Transbound. Emer. Dis. 2014, 61, 555–562. [Google Scholar] [CrossRef]
- Aller-Morán, L.M.; Martínez-Lobo, F.J.; Rubio, P.; Carvajal, A. Experimental Infection of Conventional Pigs with a ‘Brachyspira Hampsonii’ Isolate Recovered from a Migrating Waterfowl in Spain. Vet. J. 2016, 214, 10–13. [Google Scholar] [CrossRef]
- Cowie, C.E.; Hutchings, M.R.; Barasona, J.A.; Gortázar, C.; Vicente, J.; White, P.C.L. Interactions between Four Species in a Complex Wildlife: Livestock Disease Community: Implications for Mycobacterium Bovis Maintenance and Transmission. Eur. J. Wildl. Res. 2016, 62, 51–64. [Google Scholar] [CrossRef]
- Triguero-Ocaña, R.; Laguna, E.; Jiménez-Ruiz, S.; Fernández-López, J.; García-Bocanegra, I.; Barasona, J.Á.; Risalde, M.Á.; Montoro, V.; Vicente, J.; Acevedo, P. The Wildlife-Livestock Interface on Extensive Free-Ranging Pig Farms in Central Spain during the “Montanera” Period. Transbound. Emer. Dis. 2021, 68, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Barasona, J.A.; Vicente, J.; Díez-Delgado, I.; Aznar, J.; Gortázar, C.; Torres, M.J. Environmental Presence of Mycobacterium Tuberculosis Complex in Aggregation Points at the Wildlife/Livestock Interface. Transboundary Emer. Dis. 2017, 64, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Prieto, V.; Kukielka, D.; Martínez-López, B.; de las Heras, A.I.; Barasona, J.Á.; Gortázar, C.; Sánchez-Vizcaíno, J.M.; Vicente, J. Porcine Reproductive and Respiratory Syndrome (PRRS) Virus in Wild Boar and Iberian Pigs in South-Central Spain. Eur. J. Wildl. Res. 2013, 59, 859–867. [Google Scholar] [CrossRef]
- Castillo-Cuenca, J.C.; Díaz-Cao, J.M.; Martínez-Moreno, Á.; Cano-Terriza, D.; Jiménez-Ruiz, S.; Almería, S.; García-Bocanegra, I. Seroepidemiology of Toxoplasma Gondii in Extensively Raised Iberian Pigs in Spain. Prev. Vet. Med. 2020, 175, 104854. [Google Scholar] [CrossRef]
- Malmsten, A.; Magnusson, U.; Ruiz-Fons, F.; González-Barrio, D.; Dalin, A.-M. A Serologic Survey of Pathogens in Wild Boar (Sus scrofa) in Sweden. J. Wildl. Dis. 2018, 54, 229–237. [Google Scholar] [CrossRef]
- Backhans, A.; Fellström, C.; Lambertz, S.T. Occurrence of Pathogenic Yersinia enterocolitica and Yersinia pseudotuberculosis in Small Wild Rodents. Epidemiol. Infect. 2011, 139, 1230–1238. [Google Scholar] [CrossRef]
- Wacheck, S.; Fredriksson-Ahomaa, M.; König, M.; Stolle, A.; Stephan, R. Wild Boars as an Important Reservoir for Foodborne Pathogens. Foodborne Pathog. Dis. 2010, 7, 307–312. [Google Scholar] [CrossRef]
- Vargas-Amado, M.E.; Carmo, L.P.; Berezowski, J.; Fischer, C.; Santos, M.J.; Grütter, R. Towards Risk-Based Surveillance of African Swine Fever in Switzerland. Prev. Vet. Med. 2022, 204, 105661. [Google Scholar] [CrossRef]
- Wu, N.; Abril, C.; Hinić, V.; Brodard, I.; Thür, B.; Fattebert, J.; Hüssy, D. Ryser-Degiorgis MP Free-Ranging Wild Boar: A Disease Threat to Domestic Pigs in Switzerland? J. Wildl. Dis. 2011, 47, 868–879. [Google Scholar] [CrossRef]
- Wu, N.; Abril, C.; Thomann, A.; Grosclaude, E.; Doherr, M.G.; Boujon, P.; Ryser-Degiorgis, M.-P. Risk Factors for Contacts between Wild Boar and Outdoor Pigs in Switzerland and Investigations on Potential Brucella Suis Spill-Over. BMC Vet. Res. 2012, 8, 116. [Google Scholar] [CrossRef]
- Linhares, M.B.; Belloy, L.; Origgi, F.C.; Lechner, I.; Segner, H.; Ryser-Degiorgis, M.-P. Investigating the Role of Free-Ranging Wild Boar (Sus scrofa) in the Re-Emergence of Enzootic Pneumonia in Domestic Pig Herds: A Pathological, Prevalence and Risk-Factor Study. PLoS ONE 2015, 10, e119060. [Google Scholar] [CrossRef]
- Overesch, G.; Kuhnert, P. Persistence of Mycoplasma Hyopneumoniae Sequence Types in Spite of a Control Program for Enzootic Pneumonia in Pigs. Prev. Vet. Med. 2017, 145, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bassi, A.M.G.; Steiner, J.C.; Stephan, R.; Nüesch-Inderbinen, M. Seroprevalence of Toxoplasma Gondii and Salmonella in Hunted Wild Boars from Two Different Regions in Switzerland. Animals 2021, 11, 2227. [Google Scholar] [CrossRef]
- De Lucia, A.; Rabie, A.; Smith, R.P.; Davies, R.; Ostanello, F.; Ajayi, D.; Petrovska, L.; Martelli, F. Role of Wild Birds and Environmental Contamination in the Epidemiology of Salmonella Infection in an Outdoor Pig Farm. Vet. Microbiol. 2018, 227, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.S.; Crawshaw, T.R.; Smith, N.H.; Palgrave, C.J. Mycobacterium Bovis Infection in Domestic Pigs in Great Britain. Vet. J. 2013, 198, 391–397. [Google Scholar] [CrossRef]
- Dudar, L.V.; Budzanivska, I.G.; Polishchuk, V.P. Genetic Characterization of Porcine Circovirus Type 2 (PCV2) from Wild Boars Detected in Different Regions of Ukraine. Bioplym. Cell 2018, 34, 41–48. [Google Scholar] [CrossRef]
- Ferri, G.; Piccinini, A.; Olivastri, A.; Vergara, A. Hepatitis E Virus Detection in Hunted Wild Boar (Sus scrofa) Livers in Central Italy. Ital. J. Food Saf. 2022, 11, 9979. [Google Scholar] [CrossRef]
- Wiethoelter, A.K.; Beltrán-Alcrudo, D.; Kock, R.; Mor, S.M. Global Trends in Infectious Diseases at the Wildlife–Livestock Interface. Proc. Natl. Acad. Sci. USA 2015, 112, 9662–9667. [Google Scholar] [CrossRef]
- Thiry, D.; Mauroy, A.; Saegerman, C.; Licoppe, A.; Fett, T.; Thomas, I.; Brochier, B.; Thiry, E.; Linden, A. Belgian Wildlife as Potential Zoonotic Reservoir of Hepatitis E Virus. Transbound Emerg Dis 2017, 64, 764–773. [Google Scholar] [CrossRef]
- De Nardi, M.; Léger, A.; Adkin, A.; Ru, G.; Stärk, K.D.C. Description of Surveillance Components Related to Classical Swine Fever, Blue Tongue and Rabies in Selected European Countries: An Experts’ Knowledge Elicitation. Microbial Risk Analysis 2019, 13, 100081. [Google Scholar] [CrossRef]
- Cameron, A.R.; Meyer, A.; Faverjon, C.; Mackenzie, C. Quantification of the Sensitivity of Early Detection Surveillance. Transbound. Emerg. Dis. 2020, 67, 2532–2543. [Google Scholar] [CrossRef]
- Kukielka, E.; Barasona, J.A.; Cowie, C.E.; Drewe, J.A.; Gortazar, C.; Cotarelo, I.; Vicente, J. Spatial and Temporal Interactions between Livestock and Wildlife in South Central Spain Assessed by Camera Traps. Prev. Vet. Med. 2013, 112, 213–221. [Google Scholar] [CrossRef]
- Gilbert, M.; Nicolas, G.; Cinardi, G.; Van Boeckel, T.P.; Vanwambeke, S.O.; Wint, G.R.W.; Robinson, T.P. Global Distribution Data for Cattle, Buffaloes, Horses, Sheep, Goats, Pigs, Chickens and Ducks in 2010. Sci. Data 2018, 5, 180227. [Google Scholar] [CrossRef] [PubMed]
- Mollalo, A.; Mao, L.; Rashidi, P.; Glass, G.E. A GIS-Based Artificial Neural Network Model for Spatial Distribution of Tuberculosis across the Continental United States. Int. J. Environ. Publica Health 2019, 16, 157. [Google Scholar] [CrossRef]
- Haas, C.; Origgi, F.C.; Rossi, S.; López-Olvera, J.R.; Rossi, L.; Castillo-Contreras, R.; Malmsten, A.; Dalin, A.-M.; Orusa, R.; Robetto, S.; et al. Serological Survey in Wild Boar (Sus scrofa) in Switzerland and Other European Countries: Sarcoptes scabiei May Be More Widely Distributed than Previously Thought. BMC Vet. Res. 2018, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A Review of Environmental Risk Factors for African Swine Fever in European Wild Boar. Animals 2021, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Viani, A.; Orusa, T.; Borgogno-Mondino, E.; Orusa, R. Snow Metrics as Proxy to Assess Sarcoptic Mange in Wild Boar: Preliminary Results in Aosta Valley (Italy). Life 2023, 13, 987. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, K.C.; Beltrán-Alcrudo, D.; Hovari, M.; Tabakovski, B.; Martínez-López, B. Descriptive and Multivariate Analysis of the Pig Sector in North Macedonia and Its Implications for African Swine Fever Transmission. Front. Vet. Sci. 2021, 8, 733157. [Google Scholar] [CrossRef]
- Ortega, N.; Fanelli, A.; Serrano, A.; Martínez-Carrasco, C.; Escribano, F.; Tizzani, P.; Candela, M.G. Salmonella Seroprevalence in Wild Boar from Southeast Spain Depends on Host Population Density. Res. Vet. Sci. 2020, 132, 400–403. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. A Systematic Review of Influenza A Virus Prevalence and Transmission Dynamics in Backyard Swine Populations Globally. Porc. Health Manag. 2022, 8, 10. [Google Scholar] [CrossRef]
- Brookes, V.J.; Barrett, T.E.; Ward, M.P.; Roby, J.A.; Hernandez-Jover, M.; Cross, E.M.; Donnelly, C.M.; Barnes, T.S.; Wilson, C.S.; Khalfan, S. A Scoping Review of African Swine Fever Virus Spread between Domestic and Free-Living Pigs. Transbound. Emerg. Dis. 2021, 68, 2643–2656. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, J.; Immerseel, F.V. Biosecurity in Animal Production and Veterinary Medicine; ACCO: Leuven, Belgium, 2018. [Google Scholar]
- Herm, R.; Tummeleht, L.; Jürison, M.; Vilem, A.; Viltrop, A. Trace Amounts of African Swine Fever Virus DNA Detected in Insects Collected from an Infected Pig Farm in Estonia. Vet. Med. Sci. 2020, 6, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S.; Filipello, V.; Pavoni, E.; Carta, V.; Bolzoni, L.; Corradi, M.; Gilioli, S.; Losio, M.N. Geographical Restriction of Hepatitis E Virus Circulation in Wild Boars (Sus scrofa) in Emilia-Romagna Region, Northern Italy. Ital. J. Food Saf. 2020, 9, 8463. [Google Scholar] [CrossRef]
- Papatsiros, V.; Athanasiou, L.V.; Stougiou, D.; Christodoulopoulos, G.; Boutsini, S. Trichinella britovi as a Risk Factor for Alternative Pig Production Systems in Greece and Europe. Vet. Res. Forum 2020, 11, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, P.; Chantziaras, I.; Vijay, D.; Bedi, J.S.; Makovska, I.; Biebaut, E.; Dewulf, J. Can Improved Farm Biosecurity Reduce the Need for Antimicrobials in Food Animals? A Scoping Review. Antibiotics 2023, 12, 893. [Google Scholar] [CrossRef]
- Rossi, S.; Artois, M.; Pontier, D.; Cruciere, C.; Hars, J.; Barrat, J.; Pacholek, X.; Fromont, E. Long-Term Monitoring of Classical Swine Fever in Wild Boar (Sus scrofa Sp.) Using Serological Data. Vet. Res. 2005, 36, 27–42. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
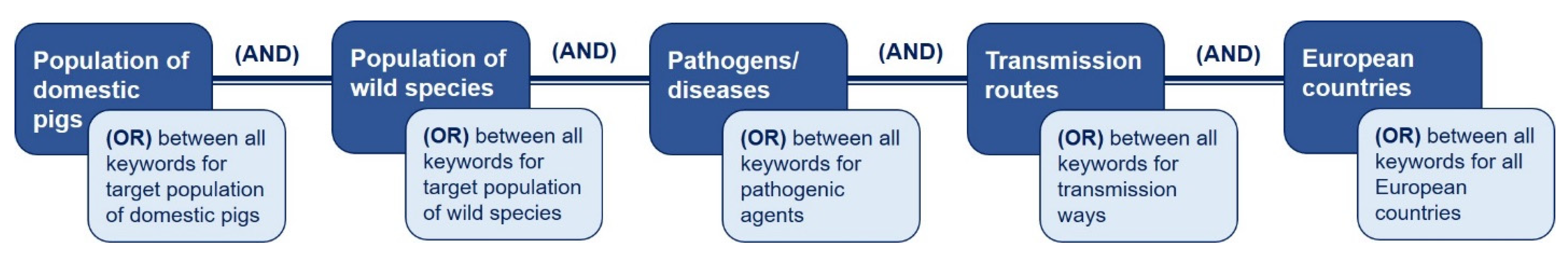


| Sl. No. | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| 1. | Peer-reviewed, original research articles from the studies carried out in the European region | A manuscript that was not peer-reviewed and outside the European region |
| 2. | Written in the English language | A manuscript not written in the English language |
| 3. | The study involved domestic pigs and wildlife species, pests | Studies carried out only among domestic pigs |
| 4. | Study collected or analyzed data ‘to investigate the interface/interaction between wild animals and domestic pigs’ OR ‘the prevalence of common domestic pig pathogen(s) in wildlife and mention possible transmission risk factors’ | Conference abstracts, review articles, commentary, and gray literature |
| 5. | The study included information about the role of wild animals in spreading pathogenic agents on pig farms | The study did not involve pathogenic agents of domestic pigs |
| 6. | The study described possible transmission routes of pathogenic agents from wildlife species to domestic pigs | The study focused exclusively on either wild pork products or domestic pork products |
| 7. | The study described information relating to the risks of transmission of pathogenic agents from wildlife species to domestic pigs | The study on the livestock–wildlife interface was based only on the results from simulation models |
| Country | Pathogen/ Disease | Prevalence in Domestic Pig | Source | Epidemiological Role | Prevalence in Wildlife | Evidence of Transmission Based on | Reference |
|---|---|---|---|---|---|---|---|
| Belgium | African swine fever virus (ASFV) | NA | Wild boars | Reservoir | NA | Expert opinion | [27] |
| Bulgaria | Foot-and-mouth disease virus (FMDV) | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [28] |
| Hepatitis E virus (HEV) | 60% (260/433) | Wild boars | Reservoir | 12.5% (4/32) | Observation | [29] | |
| Croatia | HEV | 24.5% | Wild boars | Reservoir | 12.3% | Observation | [30] |
| NA | Rodents (Yellow-necked mice) | Potential reservoirs or/and transmitters | 0.21% (1/483) | Observation | [31] | ||
| 15.2% (216/1419) | Wild boars | Reservoir | 11.5% (83/720) | Authors’ hypothesis | [32] | ||
| Denmark | ASFV | NA | Insects (stable flies) | Mechanical vectors | NA | Authors’ hypothesis | [33] |
| NA | Insects (stable flies) | Mechanical vectors | NA | Authors’ hypothesis | [34] | ||
| Methicillin-resistant Staphylococcus aureus (MRSA) | NA | Insects (stable flies) | Mechanical vectors | 5.4% | Observation | [35] | |
| NA | Insects (house flies) | Mechanical vectors | 7.8% | Observation | |||
| Toxoplasma gondii | NA | Rodents (mice (Mus musculus)) | Reservoir | 8% (11/137) | Observation | [36] | |
| Estonia | ASFV | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [37] |
| 29.7% | Wild boars | Reservoir | NA | Authors’ hypothesis | [38] | ||
| Finland | HEV | NA | Wild boars | Reservoir | 18% (32/181) | Observation | [39] |
| Salmonella spp. | NA | Reservoir | 38% (69/181) | Observation | |||
| T. gondii | NA | Reservoir | 9% (17/181) | Observation | |||
| Trichinella spp. | NA | Reservoir | 1% (2/181) | Observation | |||
| Brucella spp. | NA | Reservoir | 9% (8/87) | Observation | |||
| Yersinia spp. | NA | Reservoir | 56% (102/181) | Observation | |||
| France (Corsica) Corsica | Escherichia coli (E. coli) | NA | Wild boars | Reservoir | 87.5% (7/8) | Observation | [40] |
| NA | Wild boars | Reservoir | NA | Expert opinion | [41] | ||
| HEV | 88% | Wild boars | Reservoir | 29.2% (101/346) | Genetic typing | [25] | |
| Brucella suis, biovar 2 | 104 isolates | Wild boars | Reservoir | 45 isolates | Genetic typing | [26] | |
| 104 isolates | European hares | Reservoir | 54 isolates | Genetic typing | |||
| Germany | Aujeszky’s disease virus (ADV) | NA | Wild boars | Potential host | 6.64% (6795/ 102,387) | Observation | [42] |
| B. suis, biovar 2 | 3 isolates | Wild boars | Reservoir | 22 isolates | Genetic typing | [26] | |
| 3 isolates | European hares | Reservoir | 1 isolate | Genetic typing | |||
| HEV 3 | NA | Wild boars | Reservoir and transmission host | 16.8% (39/232) | Observation | [43] | |
| 100% (4/4) | Wild boars | Reservoir and transmission host | 100% (4/4) | Observation | [44] | ||
| Salmonella choleraesuis | NA | Wild boars | Reservoir | 84.7% (39/46) | Observation | [45] | |
| Greece | ADV | NA | Wild boars | Reservoir | 32.0% (115/359) | Observation | [46] |
| Erysipelothrix rhusiopathiae | NA | Reservoir | 2.4% (4/167) | Observation | |||
| PRRSV | NA | Reservoir | 5.6% (18/321) | Observation | |||
| Mycoplasma hyopneumoniae | NA | Reservoir | 72.55% (229/316) | Observation | |||
| Hungary | B. suis, biovar 2 | 8 isolates | Wild boars | Reservoir | 55 isolates | Genetic typing | [26] |
| 8 isolates | European hares | Reservoir | 5 isolates | Genetic typing | |||
| Italy | B. suis, biovar 2 | NA | Wild boars | Reservoir | 1.3% (5/389) | Observation | [47] |
| 9 isolates | Wild boars | Reservoir | 114 isolates | Genetic typing | [26] | ||
| 11 strains | Wild boars | Reservoir | 98.3% (170/173 strains) | Observation | [48] | ||
| B. melitensis, biovar 3 | NA | Wild boars | Reservoir | 1.7% (3/173) | Observation | ||
| HEV | NA | Rodents [black rats (Rattus rattus)] | Reservoir | 2% (1/47) | Observation | [49] | |
| NA | Wild boars | Reservoir | 13.7% (40/291) | Authors’ hypothesis | [50] | ||
| HEV-3 | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [51] | |
| Leptospira spp. | NA | Wild boars | Potential sources | NA | Authors’ hypothesis | [52] | |
| Mycobacterium bovis | 4.9% (330/6714) | Deer (fallow deer) | Reservoir | 25.5% (12/47) | Observation | [53] | |
| 4.9% (330/6714) | Wild boars | Reservoir | 6.8% (19/280) | Observation | |||
| Porcine circovirus type 2 (PCV-2) | NA | Wild boars | Reservoir | 47.30% (70/148) | Observation | [54] | |
| Porcine circovirus type 3 (PCV-3) | NA | Wild boars | Reservoir | 49.32% (73/148) | Observation | ||
| PCV-2 | 60% (12/120) | Wild boars | Reservoir | 22% (28/127) | Genetic typing | [24] | |
| Porcine epidemic diarrhea virus (PEDV) | NA | Wild boars | Reservoir | 3.83% (17/444) | Observation | [55] | |
| Transmissible gastroenteritis virus (TGEV) | NA | Wild boars | Reservoir | 0.67% (3/444) | Observation | ||
| Latvia | ASFV | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [56] |
| NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [57] | ||
| Lithuania | ASFV | NA | Insects (Stomoxys) | Mechanical vector | 10.7% (217/2035) | Observation | [58] |
| NA | Wild boars | Reservoir | 75% (2513/3352) | Observation | [59] | ||
| NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [60] | ||
| Porcine reproductive and respiratory syndrome virus (PRRSV) | NA | Wild boars | Reservoir | 8.66% (298/1597) | Observation | [61] | |
| The Netherlands | Brucella spp. | NA | Wild boars | Reservoir | 6.4% (131/2057) | Observation | [62] |
| Leptospira spp. | NA | Rodents | Reservoir | 5.3% (20/379) | Observation | [63] | |
| T. gondii | NA | Rodents | Reservoir | 1.6% (5/312) | Observation | ||
| Poland | ASFV | NA | Wild boars | Reservoir | 4.6% (15,639/ 340,775) | Observation | [64] |
| NA | Wild boars | Reservoir | 14.2% (57/402) | Observation | [65] | ||
| 362 outbreaks (124,382 pigs) | Wild boars | Reservoir | 5824 cases | Observation | [66] | ||
| HEV | 44.1% (63/143) | Wild boars | Reservoir | 31% (90/290) | Observation | [67] | |
| B. suis, biovar 2 | 6 isolates | Wild boars | Reservoir | 5 isolates | Genetic typing | [26] | |
| 6 isolates | European hares | Reservoir | 3 isolates | Genetic typing | |||
| PEDV | NA | Wild boars | Reservoir | 3.2% (5/157) | Observation | [68] | |
| T. gondii | NA | Wild boars | Reservoir | 37.7% (50/398) | Observation | [69] | |
| NA | Cats | Reservoir | NA | Observation | |||
| Trichinella spiralis, T. britovi, T. nativa | 0.0002% (150/86,989,313) | Rodents (rats) | Reservoir | 23.3% (21/90) | Observation | [70] | |
| Red foxes (Vulpes vulpes) | Reservoir | 0.04% (71/1740) | Observation | ||||
| Wild boars | Reservoir | 0.34% (4690/ 1,389,865) | Observation | ||||
| Portugal | HEV | NA | Wild boars | Reservoir | 14% (4/29) | Observation | [71] |
| B. suis, biovar 2 | NA | Wild boars | Reservoir | 33–59.3% | Observation | [72] | |
| 32 isolates | Wild boars | Reservoir | 56 isolates | Genetic typing | [26] | ||
| ASFV | NA | Ticks (Ornithodoros erraticus) | Vectors | 28% (13/47) farms with ticks detected | Observation | [73] | |
| NA | Ticks (O. erraticus) | Reservoir | 8.8% (3/34) farms with ticks detected | Observation | [74] | ||
| NA | Ticks (O. erraticus) | Vectors | NA | Authors’ hypothesis | [75] | ||
| Romania | ASFV | 69.40% (3942/5680) | Wild boars | Reservoir | 30.6% (1738/5680) | Observation | [76] |
| 200 outbreaks farms | Wild boars | Reservoir | NA | Risk factors | [77] | ||
| B. suis, biovar 2 | 27 isolates | Wild boars | Reservoir | NA | Genetic typing | [26] | |
| Serbia | ASFV | 9000 pigs | Wild boars | Reservoir | NA | Authors’ hypothesis | [78] |
| Brucella spp. | NA | Wild boars | Natural hosts (and/or vectors) are likely reservoir | 9.4%, (45/480) | Observation | [79] | |
| T. spiralis | NA | Wild cat | Reservoir | 100% (1/1) | Observation | [80] | |
| T. britovi | NA | Red foxes | Reservoir | 50% (2/4) | Observation | ||
| T. spiralis, T. britovi | NA | Golden jackals (Canis aureus) | Reservoir | 100% (3/3) | Observation | ||
| T. britovi | NA | Wolves (Canis lupus) | Reservoir | 100% (4/4) | Observation | ||
| Trichinella spp. | 0.12% (344/282,960) | Wild boars | Reservoir | 11.7% (11/94) | Observation | [81] | |
| Golden jackals | Reservoir | 53.8% (7/13) | Observation | ||||
| Red foxes | Reservoir | 12.3% (7/57) | Observation | ||||
| Wolves | Reservoir | 100% (3/3) | Observation | ||||
| Slovakia | PCV-2 | NA | Wild boars | Reservoir | 43.8% (85/194) | Observation | [82] |
| Porcine parvovirus type 3 (PPV-3) | NA | Wild boars | Reservoir | 19.1% (37/194) | Observation | ||
| T. pseudospiralis | All negative (n = 1,843,464) | Wild boars | Reservoir | 0.04% | Observation | [83] | |
| Red foxes | Reservoir | 9.58% | Observation | ||||
| Wild birds of prey | Reservoir | 1.11% | Observation | ||||
| Slovenia | Salmonella spp. | 50% (9/18) isolates | Wild boars | Reservoir | 11.1% (2/18) isolates | Authors’ hypothesis | [84] |
| Spain | Salmonella spp. | NA | Wild boars | Reservoir | 7.7% (81/1041) | Observation | [85] |
| ASFV | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [86] | |
| NA | Ticks (O. erraticus) | Vectors | 28% (13/47) farms with ticks detected | Observation | [73] | ||
| ADV | 1.7% | Wild boars | Reservoir | 49.6 ± 2.4% | Authors’ hypothesis | [87] | |
| NA | Wild boars | Reservoir | 80–100% | Authors’ hypothesis | [88] | ||
| NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [89] | ||
| B. suis, biovar 2 | NA | Wild boars | Reservoir | 33–59.3% | Observation | [72] | |
| 96 isolates | Wild boars | Reservoir | 48 isolates | Genetic typing | [26] | ||
| 96 isolates | European hares | Reservoir | 2 isolates | Genetic typing | |||
| NA | Wild boars | Reservoir | 59.3% (121/204) | Observation | [90] | ||
| Brachyspira hampsonii | NA | Wild birds (waterfowl) | Reservoir | NA | Authors’ hypothesis | [91] | |
| M. bovis | NA | Red deer | Reservoir | NA | Authors’ hypothesis | [92] | |
| 0.7% | Wild boars | Reservoir | 22.4% | Observation | [93] | ||
| NA | Red deer | Reservoir | 6.2% | Observation | |||
| Mycobacterium tuberculosis complex (MTC) | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [94] | |
| NA | Deer | Reservoir | NA | Authors’ hypothesis | |||
| PRRSV | 1.25% | Wild boars | Reservoir | 2.0% (7/294) | Observation | [95] | |
| T. gondii | 24.3% | Cats | Reservoir | NA | Authors’ hypothesis | [96] | |
| Sweden | E. rhusiopathiae | NA | Wild boars | Reservoir | 17.5% | Observation | [97] |
| M. hyopneumoniae | NA | Wild boars | Reservoir | 24.8% | Observation | ||
| PCV-2 | NA | Wild boars | Reservoir | 99.0% | Observation | ||
| PPV | NA | Wild boars | Reservoir | 78.0% (233/286) | Observation | ||
| Swine influenza virus (SIV) | NA | Wild boars | Reservoir | 3.8% (11/286) | Observation | ||
| T. gondii | NA | Wild boars | Reservoir | 28.6% (82/286) | Observation | ||
| Y. enterocolitica | 18% (11/60) | Rodents | Reservoir | 5% (9/190) | Observation | [98] | |
| Y. pseudotuberculosis | 0% (0/60) | Rodents | Reservoir | 0.5% (1/190) | Observation | ||
| Switzerland | E. coli | NA | Wild boars | Reservoir | 9% (14/153) | Observation | [99] |
| Salmonella spp. | NA | Wild boars | Reservoir | 12% (19/153) | Observation | ||
| Y. enterocolitica | NA | Wild boars | Reservoir | 35% (53/153) | Observation | ||
| L. monocytogenes | NA | Wild boars | Reservoir | 17% (26/153) | Observation | ||
| Y. pseudotuberculosis | NA | Wild boars | Reservoir | 20% (30/153) | Observation | ||
| ASFV | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [100] | |
| B. suis, biovar 2 | 7 isolates | Wild boars | Reservoir | 30 isolates | Genetic typing | [26] | |
| 7 isolates | European hares | Reservoir | 4 isolates | Genetic typing | |||
| B. suis, biovar 2 | NA | Wild boars | Reservoir | 35.8% (86/240) | Observation | [101] | |
| PRRSV | NA | Wild boars | Reservoir | 0.43% (1/233) | Observation | ||
| B. suis | 5.3% (17/322) contact cases | Wild boars | Reservoir | 22.4% | Observation | [102] | |
| M. hyopneumoniae | NA | Wild boars | Reservoir | 26.2% (256/978) | Expert opinion | [103] | |
| NA | Wild boars | More likely recipient rather than transmitter | 22 cases diagnosed by real-time PCR | Observation | [104] | ||
| Salmonella spp. | NA | Wild boars | Reservoir | 17% (21/126) | Observation | [105] | |
| T. gondii | NA | Wild boars | Reservoir | 35% (44/126) | Observation | ||
| United Kingdom (UK) | Salmonella spp. | 80.8% (97/120) | Wild birds | Reservoir | 84% (28/33) | Observation | [106] |
| M. bovis | 12.8% (112/874) | Wild boars | Host | NA | Authors’ hypothesis | [107] | |
| 12.8% (112/874) | Badgers | Host | NA | Authors’ hypothesis | |||
| Ukraine | PCV-2 | NA | Wild boars | Reservoir | NA | Authors’ hypothesis | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makovska, I.; Dhaka, P.; Chantziaras, I.; Pessoa, J.; Dewulf, J. The Role of Wildlife and Pests in the Transmission of Pathogenic Agents to Domestic Pigs: A Systematic Review. Animals 2023, 13, 1830. https://doi.org/10.3390/ani13111830
Makovska I, Dhaka P, Chantziaras I, Pessoa J, Dewulf J. The Role of Wildlife and Pests in the Transmission of Pathogenic Agents to Domestic Pigs: A Systematic Review. Animals. 2023; 13(11):1830. https://doi.org/10.3390/ani13111830
Chicago/Turabian StyleMakovska, Iryna, Pankaj Dhaka, Ilias Chantziaras, Joana Pessoa, and Jeroen Dewulf. 2023. "The Role of Wildlife and Pests in the Transmission of Pathogenic Agents to Domestic Pigs: A Systematic Review" Animals 13, no. 11: 1830. https://doi.org/10.3390/ani13111830
APA StyleMakovska, I., Dhaka, P., Chantziaras, I., Pessoa, J., & Dewulf, J. (2023). The Role of Wildlife and Pests in the Transmission of Pathogenic Agents to Domestic Pigs: A Systematic Review. Animals, 13(11), 1830. https://doi.org/10.3390/ani13111830









