The First Serological Detection and Risk Factors Analysis of Akabane Virus in Egyptian Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
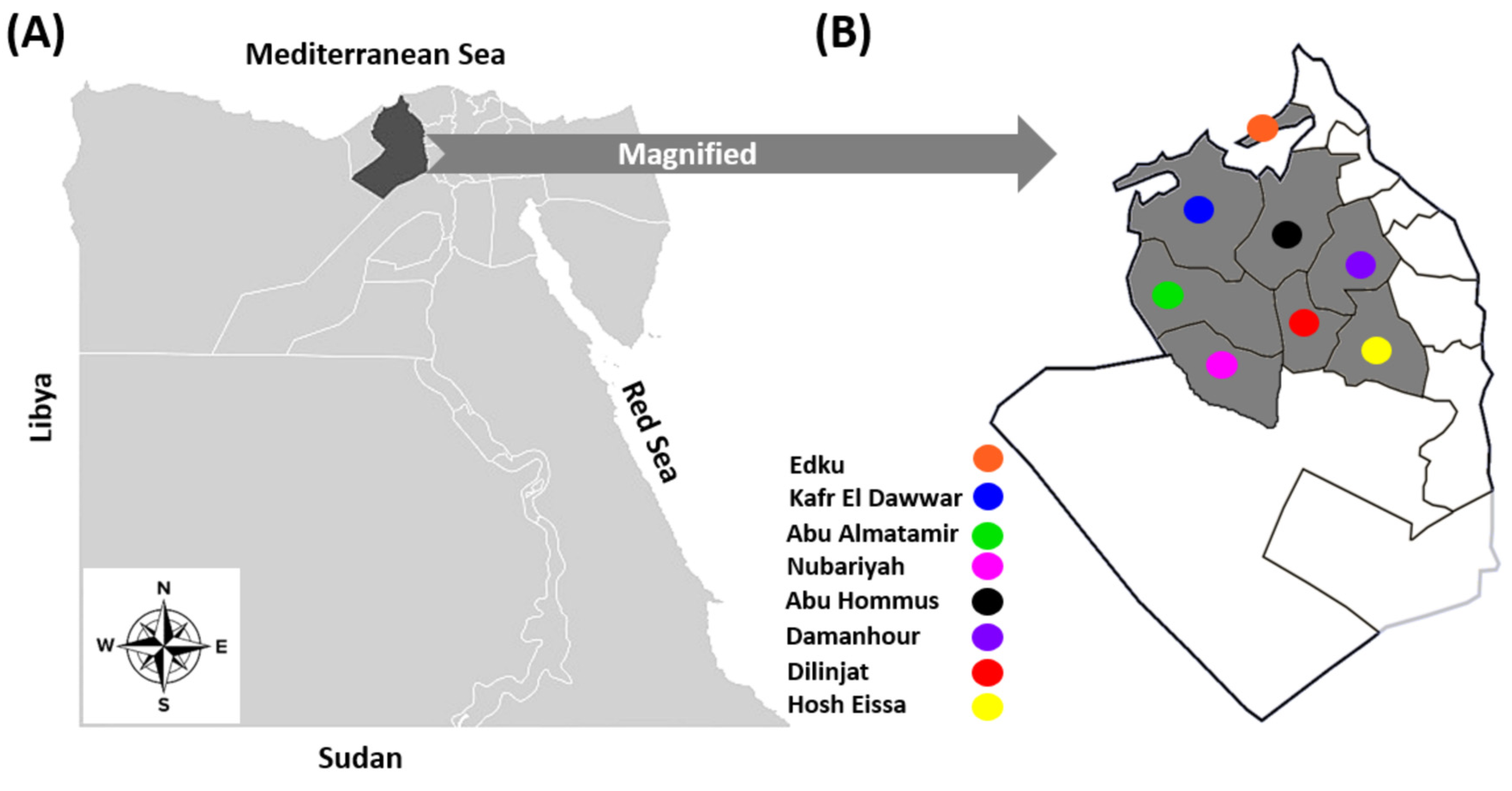
2.2. Farms and Animals
2.3. Blood Sampling and Plasma Separation
2.4. Serological Detection of Specific Anti-Akabane Antibodies by ELISA
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, R.M.; Blakqori, G. Molecular Biology of Orthobunyaviruses. Bunyaviridae: Molecular and Cellular Biology; Caister Academic Press: Norfolk, UK, 2011; pp. 1–39. [Google Scholar]
- Yanase, T.; Murota, K.; Hayama, Y. Endemic and emerging arboviruses in domestic ruminants in East Asia. Front. Vet. Sci. 2020, 7, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, A.; Okuno, T.; Ogata, T.; Kobayashi, I.; Matsuyama, T. Akabane, a new arbor virus isolated in Japan. Jpn. J. Med. Sci. Biol. 1961, 14, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akashi, H.; Kaku, Y.; Kong, X.-G.; Pang, H. Sequence determination and phylogenetic analysis of the Akabane bunyavirus S RNA genome segment. J. Gen. Virol. 1997, 78, 2847–2851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, J.E.; Crabtree, M.B.; Nam, V.S.; Yen, N.T.; Duc, H.M.; Miller, B.R. Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am. J. Trop. Med. Hyg. 2005, 73, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, P.D. Akabane virus infection. Rev. Sci. Tech. 2015, 34, 403–410. [Google Scholar] [CrossRef]
- Kurogi, H.; Inaba, Y.; Goto, Y.; Miura, Y.; Takahashi, H.; Sato, K.; Omori, T.; Matumoto, M. Serologic evidence for etiologic role of Akabane virus in epizootic abortion-arthrogryposis-hydranencephaly in cattle in Japan, 1972–1974. Arch. Virol. 1975, 47, 71–83. [Google Scholar] [CrossRef]
- Horikita, T.; Yoshinaga, S.; Okatani, A.T.; Yamane, I.; Honda, E.; Hayashidani, H. Loss of milk yield due to Akabane disease in dairy cows. J. Vet. Med. Sci. 2005, 67, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Elhassan, A.M.; Mansour, M.E.A.; Shamon, A.A.A.; El Hussein, A.M. A serological survey of Akabane virus infection in cattle in Sudan. ISRN Vet. Sci. 2014, 2014, 23904. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yanase, T.; Yamakawa, M.; Kato, T.; Yoshida, K.; Tsuda, T. Genetic diversity and reassortments among Akabane virus field isolates. Virus Res. 2007, 130, 162–171. [Google Scholar] [CrossRef]
- Ogawa, Y.; Sugiura, K.; Kato, K.; Tohya, Y.; Akashi, H. Rescue of Akabane virus (family Bunyaviridae) entirely from cloned cDNAs by using RNA polymerase I. J. Gen. Virol. 2007, 88, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Kono, R.; Hirata, M.; Kaji, M.; Goto, Y.; Ikeda, S.; Yanase, T.; Kato, T.; Tanaka, S.; Tsutsui, T.; Imada, T. Bovine epizootic encephalomyelitis caused by Akabane virus in southern Japan. BMC Vet. Res. 2008, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanase, T.; Kato, T.; Hayama, Y.; Akiyama, M.; Itoh, N.; Horiuchi, S.; Hirashima, Y.; Shirafuji, H.; Yamakawa, M.; Tanaka, S. Transition of Akabane virus genogroups and its association with changes in the nature of disease in Japan. Transbound. Emerg. Dis. 2018, 65, e434–e443. [Google Scholar] [CrossRef]
- Oem, J.-K.; Yoon, H.-J.; Kim, H.-R.; Roh, I.-S.; Lee, K.-H.; Lee, O.-S.; Bae, Y.-C. Genetic and pathogenic characterization of Akabane viruses isolated from cattle with encephalomyelitis in Korea. Vet. Microbiol. 2012, 158, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.; Tsai, C.; Ting, L.; Liao, K.; Tu, W. Molecular epidemiology of Akabane virus in Taiwan. Vet. Med. Sci. 2022, 8, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Alsaad, K.M.; Alautaish, H.H.N.; Alamery, M.A.Y. Congenital arthrogryposis-hydranencephaly syndrome caused by Akabane virus in newborn calves of Basrah Governorate, Iraq. Vet. World 2017, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.-F.; Wang, J.-P.; Yang, Z.-X.; Xie, J.-R.; He, Y.-W.; Hong, Q.-H.; Xin, A.-G. Genetic and pathogenic characterisation of a virulent Akabane virus isolated from goats in Yunnan, China. J. Vet. Res. 2022, 66, 35–42. [Google Scholar] [CrossRef]
- Taylor, W.P.; Mellor, P.S. The distribution of Akabane virus in the Middle East. Epidemiol. Infect. 1994, 113, 175–185. [Google Scholar] [CrossRef]
- Jagoe, S.; Kirland, P.D.; Harper, P.A.W. An outbreak of Akabane virus—Induced abnormalities in calves after agistment in an endemic region. Aust. Vet. J. 1993, 70, 56–58. [Google Scholar] [CrossRef]
- Al-Busaidy, S.; Hamblin, C.; Taylor, W.P. Neutralising antibodies to Akabane virus in free-living wild animals in Africa. Trop. Anim. Health Prod. 1987, 19, 197–202. [Google Scholar] [CrossRef]
- Mathew, C.; Klevar, S.; Elbers, A.R.W.; Van der Poel, W.H.M.; Kirkland, P.D.; Godfroid, J.; Mdegela, R.H.; Mwamengele, G.; Stokstad, M. Detection of serum neutralizing antibodies to Simbu sero-group viruses in cattle in Tanzania. BMC Vet. Res. 2015, 11, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, F.G.; Jessett, D.M. A study of the host range and distribution of antibody to Akabane virus (genus bunyavirus, family Bunyaviridae) in Kenya. Epidemiol. Infect. 1985, 95, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwayelu, D.O.; Aiki-Raji, C.O.; Umeh, E.C.; Mustapha, S.O.; Adebiyi, A.I. Serological investigation of Akabane virus infection in cattle and sheep in Nigeria. Adv. Virol. 2016, 2016, 2936082. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Shirafuji, H.; Tanaka, S.; Sato, M.; Yamakawa, M.; Tsuda, T.; Yanase, T. Bovine arboviruses in Culicoides biting midges and sentinel cattle in southern Japan from 2003 to 2013. Transbound. Emerg. Dis. 2016, 63, e160–e172. [Google Scholar] [CrossRef]
- Tsuda, T.; Yoshida, K.; Yanase, T.; Ohashi, S.; Yamakawa, M. Competitive enzyme-linked immunosorbent assay for the detection of the antibodies specific to Akabane virus. J. Vet. Diagn. Investig. 2004, 16, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jing, H.; Liu, X.; Wang, Q.; Qiu, S.; Liu, D.; Wu, S.; Lin, X. Comparative evaluation of two commercial ELISA kits for detection of antibodies against Akabane virus in cattle serum. BMC Vet. Res. 2019, 15, 408. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsuda, T. Rapid detection of antigenic diversity of Akabane virus isolates by dot immunobinding assay using neutralizing monoclonal antibodies. Clin. Diagn. Lab. Immunol. 1998, 5, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Haritani, M.; Hirogari, Y.; Kubo, M.; Sato, H.; Kobayashi, M.; Goto, Y. Effects of antigen-retrieval pretreatments for immunohistochemical detection of Akabane viral antigen. J. Vet. Diagn. Investig. 2000, 12, 361–363. [Google Scholar] [CrossRef] [Green Version]
- Akashi, H.; Onuma, S.; Nagano, H.; Ohta, M.; Fukutomi, T. Detection and differentiation of Aino and Akabane Simbu serogroup bunyaviruses by nested polymerase chain reaction. Arch. Virol. 1999, 144, 2101–2109. [Google Scholar] [CrossRef]
- Stram, Y.; Kuznetzova, L.; Guini, M.; Rogel, A.; Meirom, R.; Chai, D.; Yadin, H.; Brenner, J. Detection and quantitation of akabane and aino viruses by multiplex real-time reverse-transcriptase PCR. J. Virol. Methods 2004, 116, 147–154. [Google Scholar] [CrossRef]
- Ohashi, S.; Yoshida, K.; Yanase, T.; Kato, T.; Tsuda, T. Simultaneous detection of bovine arboviruses using single-tube multiplex reverse transcription-polymerase chain reaction. J. Virol. Methods 2004, 120, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, J.; Meng, Q.; Wang, G.; Liu, Y.; He, Z.; Yang, H.; Zhang, Z.; Cai, X.; Chen, C. Rapid detection of Akabane virus by a novel reverse transcription loop-mediated isothermal amplification assay (RT-LAMP). Virol. J. 2013, 10, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organaisation (FAO); Agency, U.S. Development, Livestock Production Systems Spotlight Cattle and Buffaloes and Poultry Sectors Livestock Production Systems Spotlight Cattle and Buffaloes, and Poultry Sectors in Egypt. 2018. Available online: http://www.fao.org/ag/againfo/programmes/en/ASL2050.html (accessed on 20 June 2022).
- Hosmer, D.; Lemeshow, S. Applied Logistic Regression; Wiley & Sons: New York, NY, USA, 1989; p. 581. [Google Scholar]
- Wilson, P.; Grigoropoulos, D. The West Delta Regional Survey, Beheira and Kafr el-Sheikh Provinces. Available online: https://en.wikipedia.org/wiki/Beheira_Governorate#cite_note-sis.gov.eg-32009 (accessed on 25 December 2022).
- Mohamed, M.E.H.; Mellor, P.S.; Taylor, W.P. Akabane virus: Serological survey of antibodies in livestock in the Sudan. Rev. D Elev. Med. Vet. Pays Trop. 1996, 49, 285–288. [Google Scholar] [CrossRef]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013, 100, 102–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oem, J.K.; Lee, K.H.; Kim, H.R.; Bae, Y.C.; Chung, J.Y.; Lee, O.S.; Roh, I.S. Bovine epizootic encephalomyelitis caused by Akabane virus infection in Korea. J. Comp. Pathol. 2012, 147, 101–105. [Google Scholar] [CrossRef]
- Fereig, R.M.; Rezk, M.; Mohamed, S.G.A.; Mahmoud, H.Y.A.H.; Ali, A.O.; Ali, A.F.; Hilali, M.; Zaid, A.; Elsayed, A.; Mohamed, A.; et al. Acta Tropica Serological detection and epidemiology of Neospora caninum and Cryptosporidium parvum antibodies in cattle in southern Egypt. Acta Trop. 2016, 162, 206–211. [Google Scholar] [CrossRef]
- Christensen, C.M. External parasites of dairy cattle. J. Dairy Sci. 1982, 65, 2189–2193. [Google Scholar] [CrossRef]
- Abdi, A.; Roushdy, S.; Beillard, M.J. Egypt Livestock and Products Annual 2019 Egyptian Beef Prices Stable, U.S. Beef Imports Challenged; USDA Foreign Agricultural Service: Washington, DC, USA, 2020; pp. 1–12.
- Hamada, R.; Metwally, S.; Polat, M.; Borjigin, L.; Ali, A.O.; Abdel-Hady, A.A.A.; Mohamed, A.E.A.; Wada, S.; Aida, Y. Detection and molecular characterization of bovine leukemia virus in Egyptian dairy cattle. Front. Vet. Sci. 2020, 7, 608. [Google Scholar] [CrossRef]
| Location | Damanhour | Edku | Abu Hommus | Nubariyah | Abu Almatamer | Dilinjat | Kafr El-Dawar | Housh Eissa | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Population | ||||||||||
| No. of tested farms/abattoirs | 0/2 | 1/0 | 1/2 | 1/0 | 1/0 | 2/1 | 1/2 | 0/2 | 7/9 | |
| No. of samples from farms/abattoirs | 0/20 | 22/0 | 40/43 | 57/0 | 27/0 | 23/37 | 50/26 | 0/23 | 368 | |
| Farm size (Cattle per farm) * | - | 195 b | 750 a | 2000 a | 100 b | 630 a | 700 a | - | 4375 | |
| Location | No. of Tested | No. of Negative (%) | No. of Doubtful (%) | No. of Positive (%) | 95% CI * |
|---|---|---|---|---|---|
| Damanhour | 20 | 15 (75.0) | 0 (0.0) | 5 (25.0) | 9.6–49.4 |
| Edku | 22 | 2 (9.1) | 1 (4.5) | 19 (86.4) | 68.2–98.3 |
| Abu Hommus | 83 | 41 (49.4) | 4 (4.8) | 38 (45.8) | 36.8–59.5 |
| Nubariyah | 57 | 17 (29.8) | 0 (0.0) | 40 (70.2) | 56.4–81.2 |
| Abu Almatamer | 27 | 3 (11.1) | 0 (0.0) | 24 (88.9) | 69.7–97.1 |
| Dilinjat | 60 | 26 (43.3) | 3 (5.0) | 31 (51.7) | 40.8–67.4 |
| Kafr El-Dawar | 76 | 31 (40.8) | 4 (5.3) | 41 (53.9) | 44.8–68.4 |
| Housh Eissa | 23 | 21 (91.3) | 0 (0.0) | 2 (8.7) | 15.2–29.5 |
| Total | 368 | 156 (42.4) | 12 (3.3) | 200 (54.3) | 50.8–61.4 |
| Sample Origin | Region | Sex | Breed | Type of Production | No. of Tested | No. of Negative (%) | No. of Doubtful (%) | No. of Positive (%) | Seropositive Status | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| Abattoir #1 | Damanhour | Male | Mixed | Beef | 15 | 11 (73.3) | 0 (0.0) | 4 (26.7) | Yes | 8.9–55.2 |
| Abattoir #2 | Damanhour | Male | Colombian Zebu | Beef | 5 | 4 (80) | 0 (0.0) | 1 (20) | Yes | 1.1–70.1 |
| Farm #1 | Edku | Female | Mixed | Dairy | 22 | 2 (9.1) | 1 (4.5) | 19 (86.4) | Yes | 68.2–98.3 |
| Farm #2 | Abu Hommus | Female | Holstein | Dairy | 40 | 10 (25.0) | 3 (7.5) | 27 (67.5) | Yes | 55.6–85.6 |
| Abattoir #3 | Abu Hommus | Male | Mixed and Colombian Zebu | Beef | 23 | 19 (82.6) | 0 (0.0) | 4 (17.4) | Yes | 5.7–39.5 |
| Abattoir #4 | Abu Hommus | Male | Mixed and Colombian Zebu | Beef | 20 | 12 (60.0) | 1 (5.0) | 7 (35.0) | Yes | 17.2–61.3 |
| Farm #3 | Nubariyah | Female | Holstein | Dairy | 57 | 17 (29.8) | 0 (0.0) | 40 (70.2) | Yes | 56.4–81.2 |
| Farm #4 | Abu Almatamer | Female | Mixed | Dairy | 27 | 3 (11.1) | 0 (0.0) | 24 (88.9) | Yes | 69.7–97.1 |
| Farm #5 | Dilinjat | Female | Mixed | Dairy | 23 | 7 (30.4) | 1 (4.3) | 15 (65.2) | Yes | 45.1–85.3 |
| Farm #6 | Dilinjat | Male | Holstein and Mixed | Beef | 27 | 11 (40.7) | 2 (7.4) | 14 (51.9) | Yes | 35.3–75.0 |
| Abattoir #5 | Dilinjat | Male | Colombian Zebu | Beef | 10 | 8 (80.0) | 0 (0.0) | 2 (20.0) | Yes | 35.4–55.8 |
| Farm #7 | Kafr El-Dawar | Female | Mixed | Dairy | 50 | 10 (20.0) | 3 (6.0) | 37 (74.0) | Yes | 63.9–88.8 |
| Abattoir #6 | Kafr El-Dawar | Male | Mixed | Beef | 16 | 12 (75.0) | 1 (6.3) | 3 (18.7) | Yes | 5.3–48.6 |
| Abattoir #7 | Kafr El-Dawar | Male | Colombian Zebu | Beef | 10 | 9 (90.0) | 0 (0.0) | 1 (10.0) | Yes | 0–45.9 |
| Abattoir #8 | Housh Eissa | Male | Mixed | Beef | 14 | 12 (85.7) | 0 (0.0) | 2 (14.3) | Yes | 2.5–43.8 |
| Abattoir #9 | Housh Eissa | Male | Colombian Zebu | Beef | 9 | 9 (100.0) | 0 (0.0) | 0 (0.0) | No | 0–37.1 |
| Total | 8 | 2 | 3 | 2 | 368 | 156 (42.4) | 12 (3.3) | 200 (54.3) | 15/16 positive | 50.8–61.4 |
| Analyzed Factor | No. of Tested | No. of Negative (%) | No. of Positive (%) | OR (95% CI) # | p-Value * |
|---|---|---|---|---|---|
| Age | |||||
| <3 years | 149 | 111 (74.5) | 38 (25.5) | Ref. | Ref. |
| 3–5 years | 143 | 39 (27.3) | 104 (72.7) | 7.8 (4.6–13.1) | <0.0001 |
| >5 years | 76 | 18 (23.7) | 58 (76.3) | 9.4 (4.9–18.1) | <0.0001 |
| Sex | |||||
| Male | 149 | 111 (74.5) | 38 (25.5) | Ref. | Ref. |
| Female | 219 | 57 (26) | 162 (74) | 8.3 (5.1–13.2) | <0.0001 |
| Breed | |||||
| Mixed | 210 | 88 (41.9) | 122 (58.1) | 15.3 (5.5–40.6) | <0.0001 |
| Holstein | 110 | 36 (32.7) | 74 (67.3) | 22.6 (7.9–61.3) | <0.0001 |
| Colombian zebu | 48 | 44 (91.7) | 4 (8.3) | Ref. | Ref. |
| Location | |||||
| Damanhour | 20 | 15 (75) | 5 (25) | Ref. | Ref. |
| Edku | 22 | 3 (13.6) | 19 (86.4) | 19 (3.9–73.8) | 0.0001 |
| Abu Hommus | 83 | 45 (54.2) | 38 (45.8) | 2.5 (0.9–6.8) | 0.13 |
| Nubariyah | 57 | 17 (29.8) | 40 (70.2) | 7.1 (2.1–19.8) | 0.0006 |
| Abu Almatamer | 27 | 3 (11.1) | 24 (88.9) | 24 (5.1–91.9) | <0.0001 |
| Dilinjat | 60 | 29 (48.3) | 31 (51.7) | 3.2 (1.0–8.7) | 0.04 |
| Kafr El-Dawar | 76 | 35 (46.1) | 41 (53.9) | 3.5 (1.2–9.4) | 0.025 |
| Housh Eissa | 23 | 21 (91.3) | 2 (8.7) | 0.3 (0.05–1.7) | 0.22 |
| Variables | Estimated Value | SE | p-Value * | OR | 95% CIOR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Constant | −3.19 | 0.62 | 0.0001 | |||
| Age | 1.20 | 0.38 | 0.002 | 3.32 | 1.57 | 7.04 |
| Sex | 0.19 | 0.59 | 0.74 | 1.22 | 0.38 | 3.89 |
| Breed | 0.52 | 0.24 | 0.03 | 1.69 | 1.05 | 2.72 |
| Location | −0.04 | 0.06 | 0.49 | 0.96 | 0.85 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwally, S.; Bkear, N.; Samir, M.; Hamada, R.; Elshafey, B.; Batiha, G.; Almanaa, T.N.; Sobhy, K.; Badr, Y. The First Serological Detection and Risk Factors Analysis of Akabane Virus in Egyptian Cattle. Animals 2023, 13, 1849. https://doi.org/10.3390/ani13111849
Metwally S, Bkear N, Samir M, Hamada R, Elshafey B, Batiha G, Almanaa TN, Sobhy K, Badr Y. The First Serological Detection and Risk Factors Analysis of Akabane Virus in Egyptian Cattle. Animals. 2023; 13(11):1849. https://doi.org/10.3390/ani13111849
Chicago/Turabian StyleMetwally, Samy, Nabil Bkear, Marwa Samir, Rania Hamada, Besheer Elshafey, Gaber Batiha, Taghreed N. Almanaa, Kamel Sobhy, and Yassien Badr. 2023. "The First Serological Detection and Risk Factors Analysis of Akabane Virus in Egyptian Cattle" Animals 13, no. 11: 1849. https://doi.org/10.3390/ani13111849






