Genetic Diversity Analysis of the Red Swamp Crayfish Procambarus clarkii in Three Cultured Populations Based on Microsatellite Markers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source
2.2. Genomic DNA Extraction
2.3. Obtaining Microsatellite Primers and PCR Amplification
2.4. Data Analysis
3. Results
3.1. Genetic Diversity of Three P. clarkii Culture Populations
3.2. Hardy–Weinberg Equilibrium Analysis
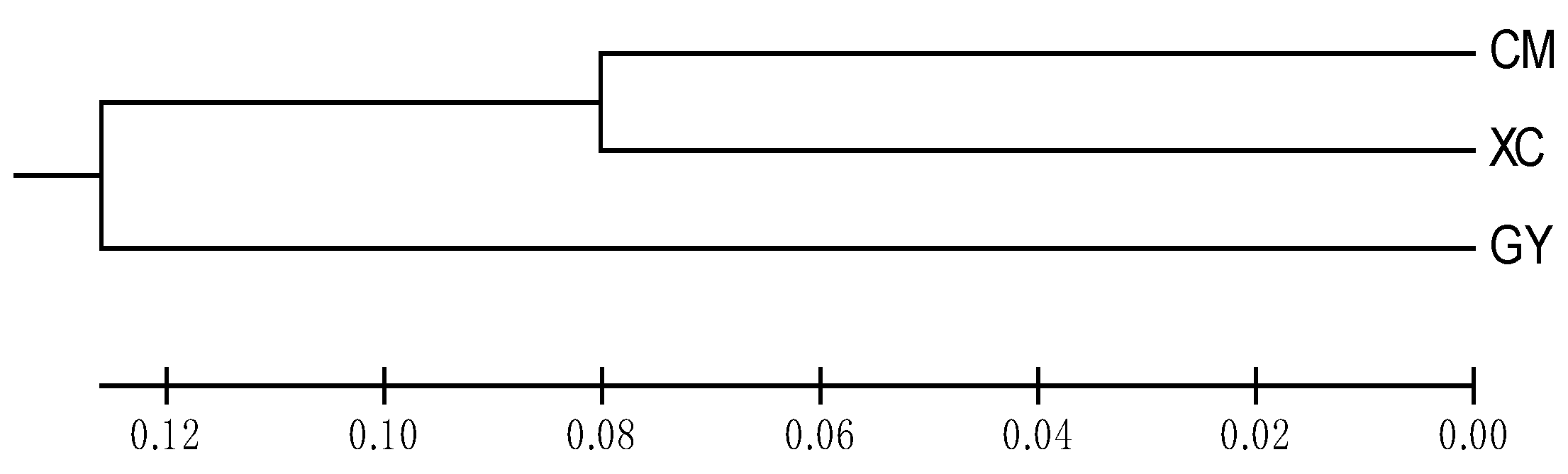
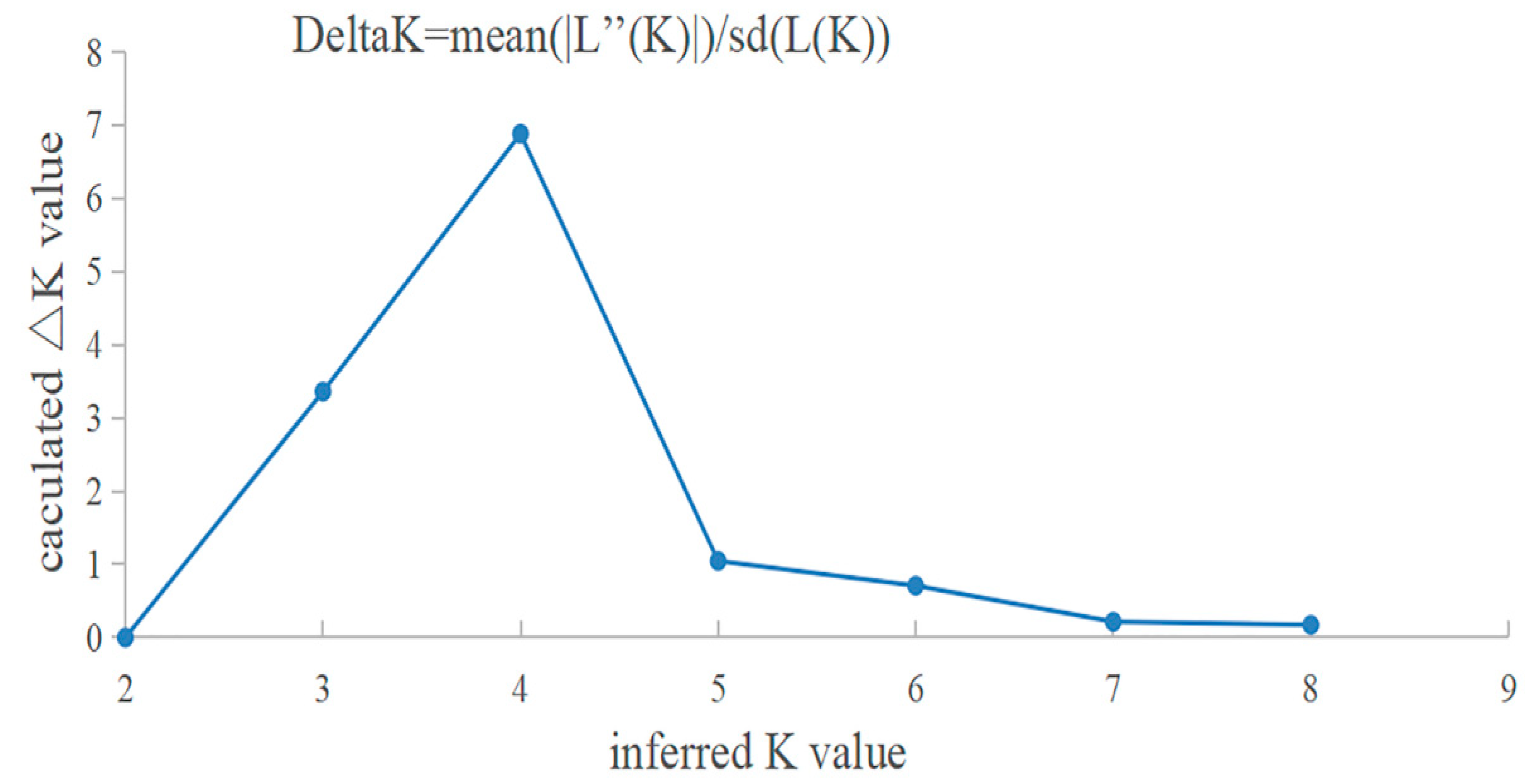
3.3. Genetic Structure and Differentiation
4. Discussion
4.1. Genetic Diversity of Microsatellite Loci and Cultured Populations of P. clarkii
4.2. Genetic Structure and Differentiation of the Three Cultured P. clarkii Populations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.J.; Chen, C.; Tan, Q.S. Ontogenic changes in the digestive enzyme activities and the effect of different starvation duration on the digestive enzyme activities of larval red swamp crayfish (Procambarus clarkii). Aquac. Res. 2018, 49, 676–683. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, J.; Jin, H.; Xu, W.J.; Zou, J.X.; Shi, L.B.; Zhang, X.Y.; Wang, Y.; Hong, F.F.; Nie, Z.H.; et al. Morphological multi-variation analysis of different geographic populations of Procambarus clarkii. J. Nanchang Univ. (Nat. Sci.) 2016, 40, 188–196. [Google Scholar]
- Zhang, H.J.; Yao, X.M.; Ding, Y.F.; Xu, Z.; Liang, R.N.; Zhang, Y.; Wu, Y.L.; Li, B.Q.; Guan, B. Pl3k signaling pathways modulated white spot syndrome virus (WSSV) replication in Procambarus clarkii. Fish Shellfish. Immunol 2018, 76, 279–286. [Google Scholar] [CrossRef]
- Wu, F.; Gu, Z.M.; Chen, X.R.; Yu, X.; Lu, X.; Zhang, L.; Wen, H.; Tian, J. Effect of lipid sources on growth performance, muscle composition, haemolymph biochemical indices and digestive enzyme activities of red swamp crayfish (Procambarus clarkii). Aquac. Nutr. 2021, 27, 1996–2006. [Google Scholar] [CrossRef]
- Yu, X.J.; Hao, X.J.; Dang, Z.Q.; Yang, L.K.; Wang, X.G.; Zhang, Y.H.; Cai, J.; Zhang, Y. Chinese crayfish Industry Development Report (2022). China Fish. 2022, 6, 47–54. [Google Scholar]
- Alvanou, M.V.; Apostolidis, A.P.; Lattos, A.; Michaelidis, B.; Giantsis, I.A. The Coding Mitogenome of the Freshwater Crayfish Pontastacus leptodactylus (Decapoda:Astacidea:Astacidae) from Lake Vegoritida, Greece and Its Taxonomic Classification. Genes 2023, 14, 494. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, J.; Yang, Y.; Nie, Z.; Liu, K.; Xu, G. Genetic Diversity and Population Structure Analysis of Chinese Mitten Crab (Eriocheir sinensis) in the Yangtze and Liaohe Rivers. Fishes 2023, 8, 253. [Google Scholar] [CrossRef]
- Ren, S.J.; Mather, P.B.; Tang, B.G.; Hurwood, D.A. Levels of genetic diversity and inferred origins of Penaeus vannamei culture resources in China: Implications for the production of a broad synthetic base population for genetic improvement. Aquaculture 2008, 491, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, A.M.; Patrick, D.D.; Matthew, E.A. New markers for new species: Microsatellite loci and the East African cichlids. Trends Ecol. Evol. 2001, 16, 100–107. [Google Scholar]
- Dimitry, A.C.; Bart, H.; Filip, A.M.V. Microsatellites and their genomic distribution, evolution, function and applications: A review with special reference to fish genetics. Aquaculture 2006, 255, 1–29. [Google Scholar]
- Sun, S. Family Tracing and Genetic Parameter Estimation Based on Microsatellite Markers in Turbot. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Chen, W.W. Scieening of Sex-Related Microsatellite Markers in Tilapia and Their Application in Breeding of All-Male Tilapia. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2019. [Google Scholar]
- Zhang, X.A.; Kong, W.P.; Wang, X.T.; Zhang, J.; Liu, L.P.; Wang, W.X.; Liu, Y.R.; Wang, X.; Zhang, H.F.; Deng, Q.X. Genetic diversity analysis of 34 fig varieties (Ficus carica L.) based on ISSR molecular marker. Genet. Resour. Crop Evol. 2020, 67, 913–921. [Google Scholar] [CrossRef]
- Patil, P.G.; Jamma, S.M.; Singh, N.V.; Bohra, A.; Parashuram, S.; Injal, A.S.; Gargade, V.A.; Chakranarayan, M.G.; Salutgi, U.D.; Babu, K.D.; et al. Assessment of genetic diversity and population structure in pomegranate (Punica granatum L.) using hypervariable SSR markers. Physiol. Mol. Biol. Plants 2020, 26, 1249–1261. [Google Scholar] [CrossRef]
- Kaya, S.; Kabasakal, B.; Erdoğan, A. Geographic Genetic Structure of Alectoris chukar in Türkiye: Post-LGM-Induced Hybridization and Human-Mediated Contaminations. Biology 2023, 12, 401. [Google Scholar] [CrossRef]
- Sunnucks, P. Efficient genetic markers for population biology. Trends Ecol. Evol. 2000, 15, 199–203. [Google Scholar] [CrossRef]
- Liu, Z.J.; Cordes, J.F. DNA marker technologies and their applications in aquaculture genetics. Aquaculture 2004, 238, 1–37. [Google Scholar] [CrossRef]
- Liu, F.; Qu, Y.K.; Geng, C.; Wang, A.M.; Zhang, J.H.; Li, J.F.; Chen, K.J.; Liu, B.; Tian, H.Y.; Yang, W.P.; et al. Analysis of the population structure and genetic diversity of the red swamp crayfish (Procambarus clarkii) in China using SSR markers. Electron. J. Biotechnol. 2020, 47, 59–71. [Google Scholar] [CrossRef]
- Cui, W.T.; Zou, Y.F.; Bai, Z.Y.; Wang, Z.Y.; Li, D.Z. Genetic diversity and structure analysis of Procambarus clarkii stocks in Anhui Province. J. Fish. China 2022, 8, 1–10. [Google Scholar]
- Xing, Z.J.; Jiang, H.C.; Lu, W.; Qian, Z.J.; Yu, H.W.; Li, J.L. Genetic diversity analysis of eight Procambarus clakii stocks in Jiangsu Province based on microsatellites. J. Shanghai Ocean. Univ. 2014, 23, 656–662. [Google Scholar]
- Hu, Y.C. Analysis on Half-Sib Families of Procambrus clarkii and the Parentage Assignment Using Microsatellite Markers. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Tan, Y.F.; Peng, G.H.; Xiong, L.J.; Peng, B.; Wu, Y.B.; Song, C.W.; Bai, X.F. Genetic diversity and structure analysis of 13 red swamp crayfish (Procambrus clarkii) populations in Yangtze River basin. J. Huazhong Agric. Univ. 2020, 39, 33–39. [Google Scholar]
- Huang, X.F.; Tang, Z.S.; Liu, J.D.; Zhang, H.Y.; Zhong, Y.Z.; Lu, Z.F.; Hou, S.J.; Wang, D.P.; Lu, Z.L. Genetic diversity microsatellite analysis of Procambarus clarkii populations in different regions of Guangxi. J. South. Agric. 2020, 51, 437–444. [Google Scholar]
- Glaubitz, J.C. Convert: A user-friendly program to reformat diploid genotypic data for commonly used population genetic software packages. Mol. Ecol. Notes 2004, 4, 309–310. [Google Scholar] [CrossRef]
- Oosterhout, C.V.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Nagy, S.; Poczai, P.; Cernák, I.; Gorji, A.M.; Hegedűs, G.; Taller, J. PICcalc: An online program to calculate Polymorphic Information Content for molecular genetic studies. Biochem. Genet. 2012, 50, 670–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping erro increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, A. An overview of structure: Applications, parameter settings, and supporting software. Front. Genet. 2013, 4, 98. [Google Scholar] [CrossRef] [Green Version]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; LI, M.; Knyaz, C.; Tamura, k. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhou, M.C.; Xu, D.P.; Fang, D.A.; Zhou, Y.F.; Liu, K.; Zhang, M.Y. Genetic diversity of six populations of Siniperca Chuatsi: Analysis based on SSR. Chin. Agric. Sci. Bull. 2020, 36, 147–152. [Google Scholar]
- Wang, L.; Meng, Z.; Liu, X.C.; Zhang, Y.; Lin, H.R. Genentic diversity and differentiation of the Orange-Spotted Grouper (Epinephelus coioides) between and within cultured stocks and wild populations inferred form microsatellite DNA analysis. Int. J. Mol. Sci. 2011, 12, 4378–4394. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tong, J.G.; Wang, J.R.; Yu, X.M. Development of microsatellite markers and genetic diversity in wild and cultured populations of black carp (Mylopharyngodon piceus) along the Yangtze River. Aquac. Int. 2020, 28, 1867–1882. [Google Scholar] [CrossRef]
- Sun, L.; Yang, G.L.; Wang, J.Y.; Wu, T.T.; Zhang, H.B.; Wu, T.W.; Chen, G.H. Study on genetic diversity of Longfin carp, Koi carp and Longfeng carp by microsatellite markers. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2009, 30, 35–40. [Google Scholar]
- Nussey, D.H.; Wilson, A.J.; Brommer, J. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 2007, 20, 831–844. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Bi, K.; Gu, W.; Wang, W. Sensitive and rapid detection of freshwater crustacean Spiroplasmas by ISRs-sequence-targeted species-specific primers. Eur. Food Res. Technol. 2008, 227, 1733–1737. [Google Scholar] [CrossRef]
- Souza-Shibatta, L.; Kotelok-Diniz, T.; Ferreira, D.G.; Shibatta, O.A.; Sofia, S.H.; Assumpção, L.D.; Pini, S.F.R.; Makrakis, S.; Makrakis, M.C. Genetic Diversity of the Endangered Neotropical Cichlid Fish (Gymnogeophagus setequedas) in Brazil. Front. Genet. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Shi, G.; Sun, Y. Evaluation of genetic diversity in Pampus argenteus ueing SSR markers. Genet. Mol. Res. 2013, 12, 5833–5841. [Google Scholar] [CrossRef]
- Nei, M.; Maruyama, T.; Chakraborty, R. The bottleneck effect and genetic variability in populations. Evolution 1975, 29, 1–10. [Google Scholar] [CrossRef]
- Takezaki, N.; Nei, M. Genetic Distances and Reconstruction of Phylogenetic Trees from Microsatellite DNA. Genetics 1996, 144, 389–399. [Google Scholar] [CrossRef]
- Chang, Y.M.; Liang, L.Q.; Ma, H.T.; He, J.G.; Sun, X.W. Microsatellite analysis of genetic diversity and population structure of Chinese mitten crab (Eriocheir sinensis). J. Genet. Genom. 2008, 35, 171–176. [Google Scholar] [CrossRef]
- Fitzsimmons, N.N. Single paternity of clutches and sperm storage in the promiscuous green turtle (Chelonia mydas). Mol. Ecol. 1998, 7, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wen, B.N.; Liu, H. Microsatellite genetic diversity in different Litopenaeus vannamei breeding populations. J. South. Agric. 2021, 52, 1108–1115. [Google Scholar]
- Xie, L.; Chen, G.L.; Ye, F.L.; Li, Z.M. Genetic diversity of four selected stocks of Litopenaeus vannamei as revealed by SSR marker. J. Guangdong Ocean. Univ. 2009, 29, 5–9. [Google Scholar]
- Chen, N.; Luo, X.; Lu, C.K.; Ke, C.; You, W.W. Effects of artificial selection practices on loss of genetic diversity in the Pacific abalone, Haliotis discun hannai. Aquac. Res. 2017, 48, 4923–4933. [Google Scholar] [CrossRef]
- Cui, W.T.; Zheng, G.D.; Su, X.L.; Li, B.Y.; Zou, S.M. Analysis of microsatellite markers and growth performance of hybrid F2 and two backcross F2 populations of Megalobrama amblycephala♀ and Culter alburnus♂. J. Fish. Sci. China 2020, 27, 613–623. [Google Scholar]
- Antoro, S.; Na-Nakorn, U.; Koedprang, W. Study of Genetic Diversity of Orange-Spotted Grouper, Epinephelus coioides, from Thailand and Indinesia Using Microsatellite Markers. Mar. Biotechnol. 2006, 8, 17–26. [Google Scholar] [CrossRef]
- Sved, J.A. The stability of linked systems of lociwith a smallpopulation size. Genetics 1968, 5, 543–563. [Google Scholar] [CrossRef]
- Liang, Y.X.; Xu, C.X.; Li, Q. Heterosis and genetic diversity of intraspecific hybrids crosses between two selected lines of the Pacific oyster Crassostrea gigas. Aquaculture 2023, 569, 739369. [Google Scholar] [CrossRef]
- Hu, L.P.; Huang, X.T.; Sun, Y.; Mao, J.X.; Wang, X.; Wang, C.D.; Bao, Z.M. Molecular genetic analysis of heterosis in interspecific hybrids of Argopecten purpuratus x A. irradians irradians. Genet. Mol. Res. 2015, 14, 10692–10704. [Google Scholar] [CrossRef]
- Zou, L.; Wang, J.L.; Li, C.W.; Wang, D.W.; Zeng, C.F.; Liu, M.Q.; Liu, L.; Xie, M.; Zeng, M. Genetic Diversity of stay carp Cyprinus carpio Adapted to Paddy Field Conditions. Fish. Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Rumisha, C.; Mdegela, R.H.; Gwakisa, P.S.; Kochzius, M. Genetic diversity and gene flow among the giant mud crab (Scylla serrata) in anthropogenic-polluted mangroves of mainland Tanzania: Implications for conservation. Fish. Res. 2018, 205, 96–104. [Google Scholar] [CrossRef]
- Slatkin, M. Gene Flow and the Geographic Structure of Natural Populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.T.; Ling, J.; Jiang, H.; Wang, H.; Pan, T.S.; Zhou, H.X. Genetic diversity and structure analysis of four Eriocheir sinensis cultured population. Jiangsu Agric. Sci. 2022, 50, 54–59. [Google Scholar]
- Yan, C.M.; Zheng, W.; Gao, C.S.; Han, Y.; Li, Z.Q.; Li, X.Y. Analysis of genetic diversity of chum salmon in Tumen River based on microsatellite markers. Chin. J. Fish. 2021, 34, 1–7. [Google Scholar]
- Zhong, Y.Z.; Tang, Z.S.; Huang, L.M.; Wang, D.P.; Lu, Z.L. Genetic diversity of Procambarus clarkii populations based on mitochonrial DNA and microsatellite markers in different areas of Guangxi, China. Mitochondrial DNA Part A 2020, 31, 48–56. [Google Scholar] [CrossRef]
- Luo, Y.T.; Fang, D.A.; Zhou, Y.F.; Xu, D.P.; Peng, Y.X.; Peng, F.; Zhang, G.N.; Liu, K.; You, Y. Genetic diversity of silver carp (Hypophthalmichthys molitrix) in lower reaches of Yangtze River based on microsatellite markers. South China Fish. Sci. 2021, 17, 48–57. [Google Scholar]

| Locus | Primer Sequences | Repeat Motif | Annealing Temperature (°C) | Size (bp) |
|---|---|---|---|---|
| PcLG02 | F:CTCCCCATGCACTCTGGCTCTGT R:TGGCGAATTTTGCCTGTTTCTGTC | (GATA)3GAGAA(GATA)5 | 66 | 216–224 |
| PcLG03 | F:CTCTCCACCAGTCATTTCTT R:AAGCTTACAATAAATATAGATAGAC | (TCTA)20 | 52 | 216–420 |
| PcLG04 | F:TATATCAGTCAATCTGTCCAG R:TCAGTAAGTAGATTGATAGAAGG | (TCTA)3...(TCTA)2...(TCAT)29...(TCTA)2 | 54 | 170–290 |
| PcLG07 | F:CCTCCCACCAGGGTTATCTATTCA R:GTGGGTGTGGCGCTCTTGTT | (TCTA)8 | 63 | 100–160 |
| PcLG08 | F:ACGATAAATGGATAGATGGATGAA R:CCGGGTCTGTCTGTCTGTCA | (GATA)16 | 62 | 148–220 |
| PcLG09 | F:TATGCACCTTTACCTGAAT R:TGTTGGTGTGGTCATCA | (TCTA)14 | 60 | 80–160 |
| PcLG13 | F:CTCTCCTGGCGCTGTTATTTAGC R:TGAAGAGGCAGAGTGAGGATTCTC | (TCTA)12 | 62 | 130–150 |
| PcLG15 | F:GGCGTGACGCCAACGTGTCTT R:GGCTGGCCACTTTGTTAGCCTGAG | (TATC)2TGTC(TATC)17TATT(TATC)3 | 70 | 150–185 |
| PcLG16 | F:CTCGGAATGTCCACCTGAGA R:TCATTATGGATTTTGTCAATCTAT | (TCTA)18TCTC(TATC)3 | 54 | 80–160 |
| PcLG17 | F:GTCGGGAACCTATTTACAGTGTAT R:AAGAGCGAAGAAAGAGATAAAGAT | (TCTA)14 | 57 | 156–190 |
| PcLG27 | F:AATCTTAAGATCATGAAAAAGGTA R:TTTAAGGAACGTATAAGAAAAGAC | (TATC)4CATC(TATC)8 | 57 | 80–150 |
| PcLG28 | F:CTCGGCGAGTTTACTGAAAT R:AGAAGAAAGGGATATAAGGTAAAG | (GATA)22(GA)5 | 60 | 210–270 |
| PcLG29 | F:GAAAGTCATGGGTGTAGGTGTAAC R:TTTTTGGGCTATGTGACGAG | (TATC)9 | 65 | 95–165 |
| PcLG33 | F:TTCGAGGCGTTGCTGATTGTAAGT R:CAAGGAAGCGTATAGCCGGAGTCT | (GT)21 | 68 | 120–180 |
| PcLG37 | F:TAAATAAGTGGCGTGTAAGACGAG R:TAACTAAGCCAGGGTGGTCTCCAG | (CA)4CG(CA)15CG(CA)23 | 66 | 80–180 |
| Locus | Sample Size | Na | Ne | Ho | He | I | PIC |
|---|---|---|---|---|---|---|---|
| PclG02 | 180 | 3 | 2.0436 | 0.6333 | 0.5135 | 0.8033 | 0.4142 |
| PclG03 | 180 | 7 | 2.1141 | 0.4111 | 0.5299 | 0.9818 | 0.4643 |
| PclG04 | 180 | 7 | 2.7825 | 0.3222 | 0.6442 | 1.2597 | 0.5821 |
| PclG07 | 180 | 8 | 2.8531 | 0.6444 | 0.6531 | 1.3044 | 0.5906 |
| PclG08 | 180 | 10 | 3.9589 | 0.5000 | 0.7516 | 1.5920 | 0.7109 |
| PclG09 | 180 | 7 | 4.4690 | 0.3778 | 0.7806 | 1.6326 | 0.7412 |
| PclG13 | 180 | 2 | 1.9387 | 0.5333 | 0.4869 | 0.6773 | 0.3670 |
| PclG15 | 180 | 8 | 3.6594 | 0.9333 | 0.7308 | 1.5382 | 0.6917 |
| PclG16 | 180 | 8 | 5.2427 | 0.6556 | 0.8138 | 1.8259 | 0.7851 |
| PclG17 | 180 | 8 | 3.3109 | 0.5111 | 0.7019 | 1.3933 | 0.6465 |
| PclG27 | 178 | 9 | 4.7374 | 0.6966 | 0.7934 | 1.7041 | 0.7573 |
| PclG28 | 178 | 8 | 3.8303 | 0.6067 | 0.7431 | 1.5229 | 0.6988 |
| PclG29 | 180 | 7 | 5.4619 | 0.6889 | 0.8215 | 1.7646 | 0.7917 |
| PclG33 | 180 | 10 | 3.6751 | 0.9889 | 0.7320 | 1.5846 | 0.6906 |
| PclG37 | 180 | 11 | 4.1422 | 0.2111 | 0.7628 | 1.7496 | 0.7275 |
| mean | 180 | 7.5333 | 3.3147 | 0.5810 | 0.6973 | 1.4243 | 0.6440 |
| Population | Sample Size | Na | Ne | I | Ho | He | PIC |
|---|---|---|---|---|---|---|---|
| CM | 60 | 5.2667 | 3.0448 | 1.2329 | 0.5600 | 0.6440 | 0.5774 |
| GY | 60 | 4.8000 | 2.7969 | 1.1292 | 0.5567 | 0.6166 | 0.5446 |
| XC | 60 | 6.2000 | 3.5470 | 1.3987 | 0.6257 | 0.7086 | 0.6452 |
| mean | 60 | 5.4222 | 3.1296 | 1.2536 | 1.7424 | 0.6564 | 0.5891 |
| Locus | CM | GY | XC |
|---|---|---|---|
| PclG02 | 0.1785 | 0.0330 | 0.6991 |
| PclG03 | −0.1709 * | −0.3022 | −0.2027 |
| PclG04 | −0.0436 * | −0.4306 ** | −0.7793 ** |
| PclG07 | −0.1529 | 0.1689 | 0.0492 * |
| PclG08 | −0.4788 ** | −0.1991 * | −0.2916 ** |
| PclG09 | −0.6903 ** | −0.1618 ** | −0.5092 ** |
| PclG13 | 0.2248 | −0.0068 | 0.0679 |
| PclG15 | 0.1164 | 0.3755 | 0.3949 |
| PclG16 | −0.1054 | −0.1213 * | −0.1883 |
| PclG17 | −0.2058 | −0.2387 | −0.0295 * |
| PclG27 | −0.1360 | −0.1904 | 0.1149 |
| PclG28 | −0.1748 ** | 0.0146 * | −0.2669 ** |
| PclG29 | 0.0328 | −0.3306 ** | −0.0702 |
| PclG33 | 0.7167 ** | 0.2647 | 0.4148 |
| PclG37 | −0.7395 ** | −0.5864 ** | −0.7150 ** |
| mean | −0.1086 | −0.1141 | −0.0875 |
| Locus | Sample Size | Fis | Fit | Fst | Nm |
|---|---|---|---|---|---|
| PclG02 | 180 | −0.3014 | −0.2402 | 0.0470 | 5.0668 |
| PclG03 | 180 | 0.2060 | 0.2199 | 0.0175 | 14.0738 |
| PclG04 | 180 | 0.4481 | 0.4970 | 0.0886 | 2.5732 |
| PclG07 | 180 | −0.0296 | 0.0078 | 0.0363 | 6.6361 |
| PclG08 | 180 | 0.3030 | 0.3310 | 0.0401 | 5.9784 |
| PclG09 | 180 | 0.4352 | 0.5133 | 0.1383 | 1.5578 |
| PclG13 | 180 | −0.1103 | −0.1015 | 0.0079 | 31.3790 |
| PclG15 | 180 | −0.3111 | −0.2843 | 0.0205 | 11.9627 |
| PclG16 | 180 | 0.1246 | 0.1899 | 0.0746 | 3.1012 |
| PclG17 | 180 | 0.1337 | 0.2677 | 0.1547 | 1.3662 |
| PclG27 | 178 | 0.0480 | 0.1185 | 0.0741 | 3.1260 |
| PclG28 | 178 | 0.1344 | 0.1799 | 0.0526 | 4.5057 |
| PclG29 | 180 | 0.1027 | 0.1567 | 0.0601 | 3.9064 |
| PclG33 | 180 | −0.4691 | −0.3585 | 0.0752 | 3.0736 |
| PclG37 | 180 | 0.6889 | 0.7217 | 0.1055 | 2.1187 |
| mean | 180 | 0.1001 | 0.1623 | 0.0691 | 3.3658 |
| Population | Chongming | Gaoyou | Xuancheng |
|---|---|---|---|
| Chongming | - | 0.1068 (2.0908) | 0.0553 (4.2708) |
| Gaoyou | 0.2560 | - | 0.0927 (2.4469) |
| Xuancheng | 0.1602 | 0.2478 | - |
| Source of Variation | Degree of Freedom (d.f.) | Sum of Squares | Variance Components | Percentage of Variation | F-Statistic (Fst) | p-Value |
|---|---|---|---|---|---|---|
| Among population | 2 | 64.433 | 0.45516 | 8.49 | ||
| Within population | 177 | 868.600 | 4.90734 | 91.51 | ||
| Total | 179 | 933.033 | 5.36250 | 0.08488 | 0.000 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Sun, Y.; Chen, Q.; Wang, M.; Li, Q.; Zhou, W.; Cheng, Y. Genetic Diversity Analysis of the Red Swamp Crayfish Procambarus clarkii in Three Cultured Populations Based on Microsatellite Markers. Animals 2023, 13, 1881. https://doi.org/10.3390/ani13111881
Liu J, Sun Y, Chen Q, Wang M, Li Q, Zhou W, Cheng Y. Genetic Diversity Analysis of the Red Swamp Crayfish Procambarus clarkii in Three Cultured Populations Based on Microsatellite Markers. Animals. 2023; 13(11):1881. https://doi.org/10.3390/ani13111881
Chicago/Turabian StyleLiu, Jiaqing, Yunfei Sun, Qianqian Chen, Miaomiao Wang, Qin Li, Wenzong Zhou, and Yongxu Cheng. 2023. "Genetic Diversity Analysis of the Red Swamp Crayfish Procambarus clarkii in Three Cultured Populations Based on Microsatellite Markers" Animals 13, no. 11: 1881. https://doi.org/10.3390/ani13111881
APA StyleLiu, J., Sun, Y., Chen, Q., Wang, M., Li, Q., Zhou, W., & Cheng, Y. (2023). Genetic Diversity Analysis of the Red Swamp Crayfish Procambarus clarkii in Three Cultured Populations Based on Microsatellite Markers. Animals, 13(11), 1881. https://doi.org/10.3390/ani13111881





