Potential Application of Muscle Precursor Cells from Male Specific-Pathogen-Free (SPF) Chicken Embryos in In Vitro Agriculture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Reagents and Media
2.3. Preparation of Experimental Embryos
2.4. Microbial Testing Using the SS MC-Media Pad
2.5. Isolation and Culture of Myogenic Precursor Cells (MPCs)
2.6. Cell Culture
2.7. Sex Determination
2.8. Myotube Fusion Index (MFI)
2.9. Confirmation of Skeletal Muscle Stem Cell Ability
2.10. Real-Time-Quantitative PCR (RT-qPCR)
2.11. Statistical Analysis
3. Results
3.1. Microbial Contamination Testing
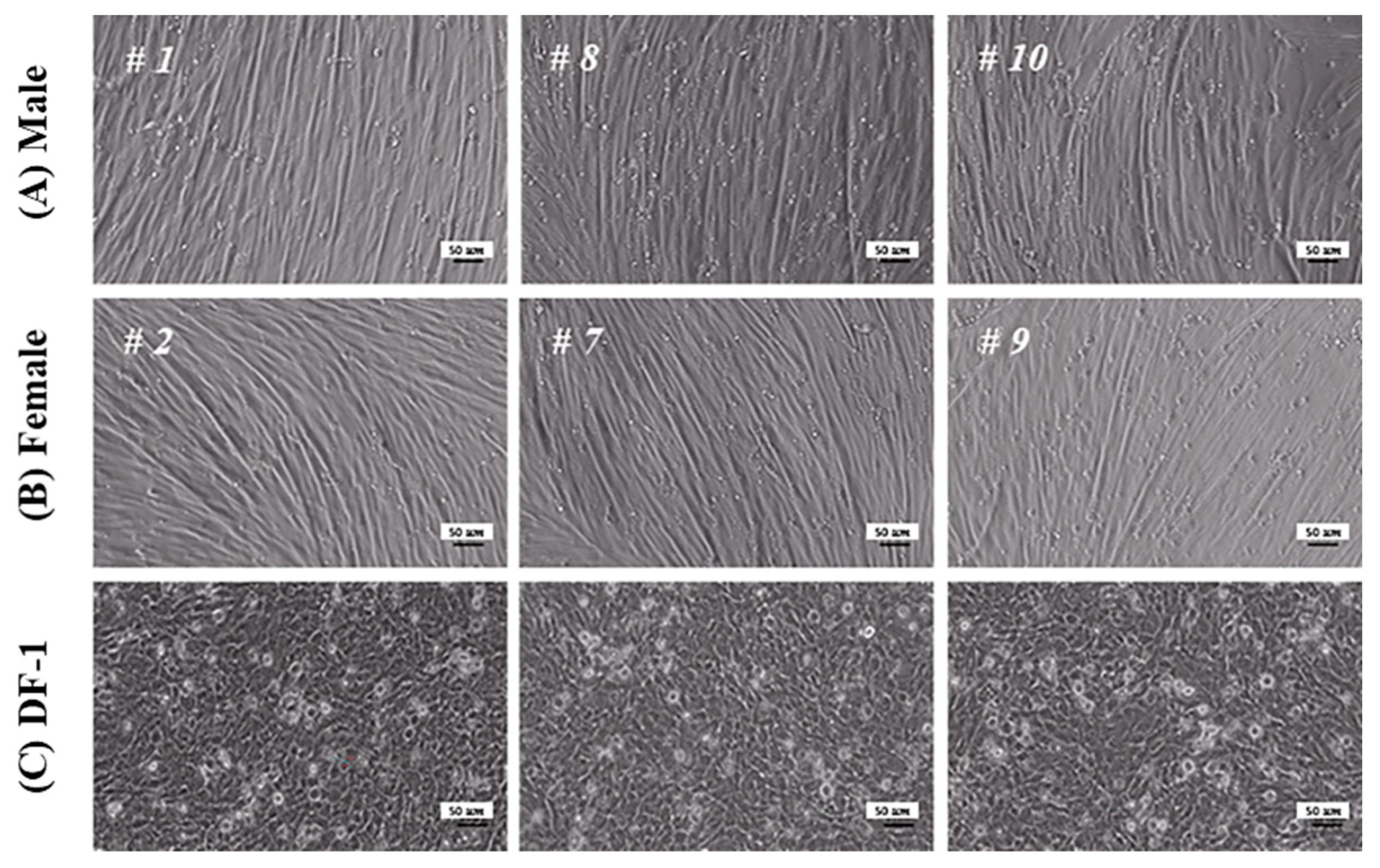
3.2. Morphology and Sex Determination of Chicken Embryos
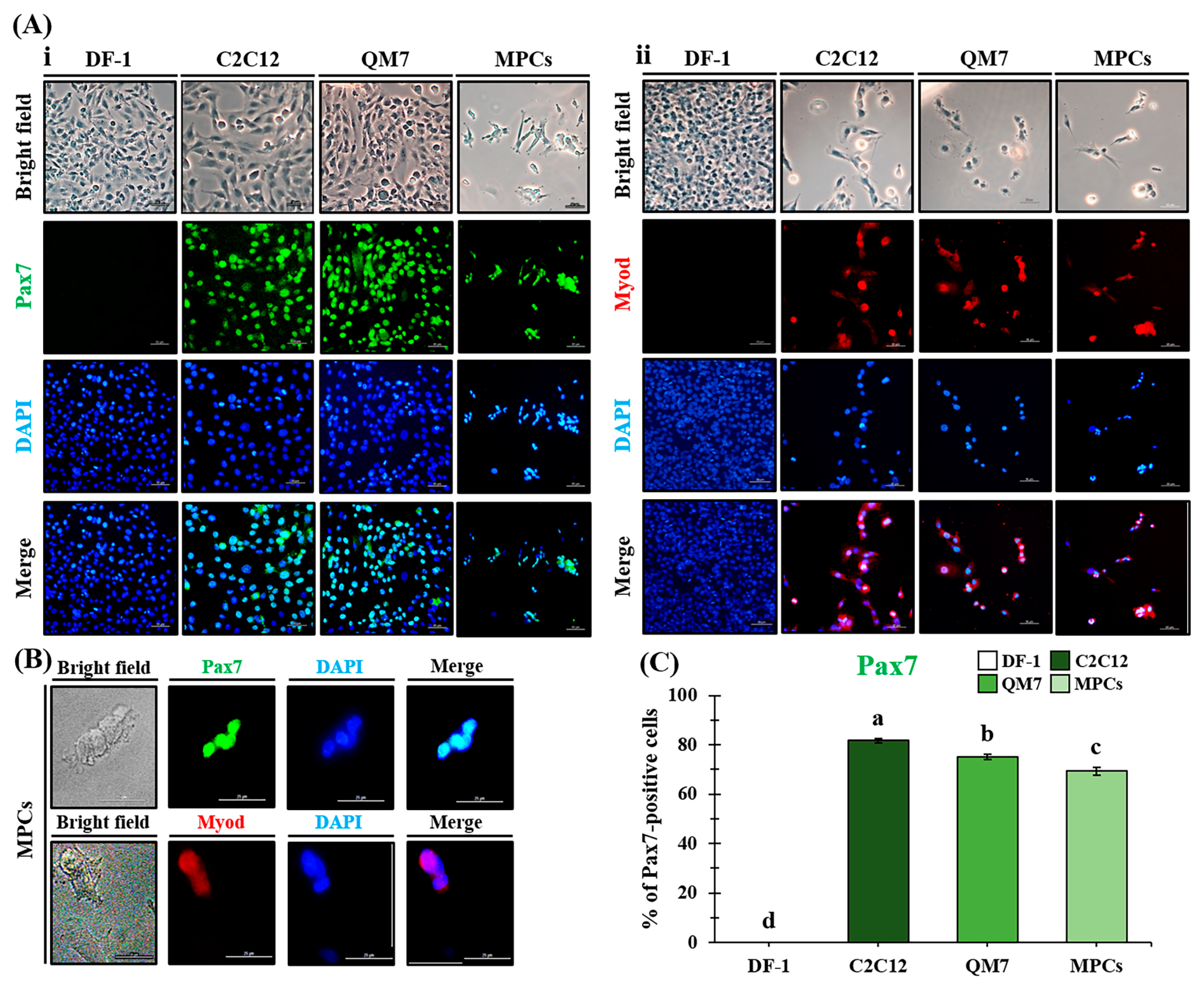
3.3. Characterization of MPCs
3.4. Confirmation of Skeletal Muscle Stem Cell Factors—PAX7 and MYOD
3.5. Genes Involved in the Growth and Differentiation of MPCs
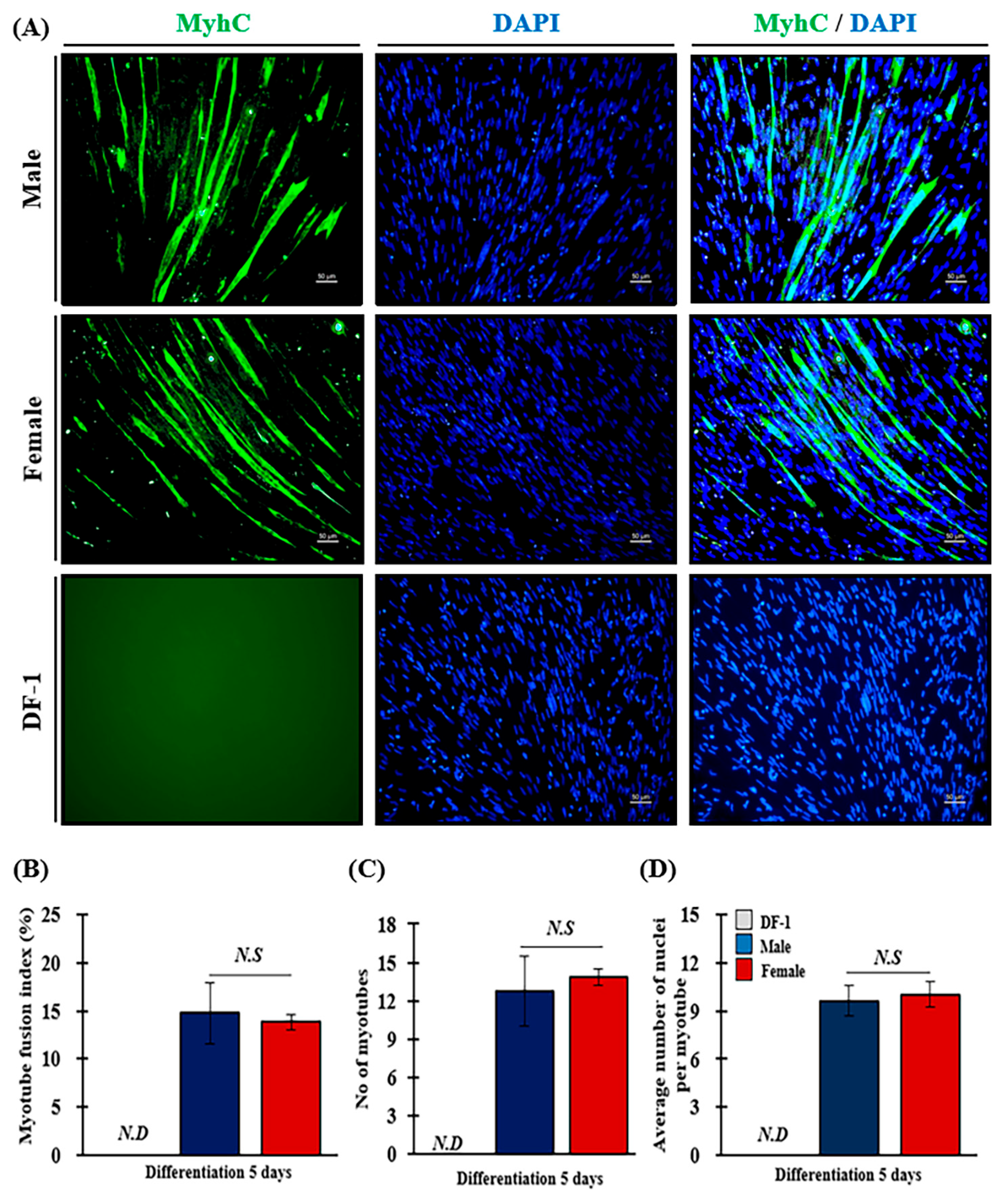
3.6. Effect of Sex on Myotube Fusion Index (MFI) of MPCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Thind, S.S.; Kaur, A. In vitro meat production system: Why and how? J. Food Sci. Technol. 2015, 52, 7599–7607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Arye, T.; Levenberg, S. Tissue engineering for clean meat production. Front. Sustain. Food Syst. 2019, 3, 46. [Google Scholar] [CrossRef]
- Schmidinger, K. Worldwide Alternatives to Animal Derived Foods-Overview and Evaluation Models: Solution to Global Problems Caused by Livestock; University of Natural Resources and Life Sciences: Vienna, Austria, 2012. [Google Scholar]
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Slade, P. If you build it, will they eat it? Consumer preferences for plant-based and cultured meat burgers. Appetite 2018, 125, 428–437. [Google Scholar]
- Yablonka-Reuveni, Z.; Quinn, L.S.; Nameroff, M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev. Biol. 1987, 119, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Swennen, G.N.M.; Messmer, T.; Gagliardi, M.; Molin, D.G.M.; Li, C.; Zhou, G.; Post, M.J. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 2018, 17, 10808. [Google Scholar] [CrossRef] [Green Version]
- Stout, A.J.; Mairliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K., Jr.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Schlemper, A.E.; Da Silva, M.V.; Fonseca, B.B. Chicken embryo: A useful animal model for drug testing? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4828–4839. [Google Scholar] [CrossRef]
- Gast, R.K.; Jones, D.R.; Guraya, R.; Garcia, J.S.; Karcher, D.M. Research Note: Internal organ colonization by Salmonella Enteritidis in experimentally infected layer pullets reared at different stocking densities in indoor cage-free housing. Poult. Sci. 2022, 101, 102104. [Google Scholar] [CrossRef]
- Fridolfsson, A.-K.; Cheng, H.; Copeland, N.G.; Jenkins, N.A.; Liu, H.-C.; Raudsepp, T.; Woodage, T.; Chowdhary, B.; Halverson, J.; Ellegren, H. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc. Natl. Acad. Sci. USA 1998, 95, 8147–8152. [Google Scholar] [CrossRef] [Green Version]
- Tůmová, E.; Chodová, D.; Skřivanová, E.; Laloučková, K.; Šubrtová-Salmonová, H.; Ketta, M.; Machander, V.; Cotozzolo, E. Research Note: The effects of genotype, sex, and feeding regime on performance, carcasses characteristic, and microbiota in chickens. Poult. Sci. 2021, 100, 760–764. [Google Scholar] [CrossRef]
- Chen, Y.; Zajac, J.D.; MacLean, H.E. Androgen regulation of satellite cell function. J. Endocrinol. 2005, 186, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enns, D.L.; Tiidus, P.M. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J. Appl. Physiol. 2008, 104, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Liu, J.; Fan, J.; Li, R.; Li, D.; Yin, J.; Cui, S. Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J. Cell. Physiol. 2012, 227, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S.; Chen, B.; An, X. Muscle-derived stem cells: Isolation, characterization, differentiation, and application in cell and gene therapy. Cell Tissue Res. 2010, 340, 549–567. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Martins, P.; Huguenin, J.; Van, T.-N.; Manso, T.; Galindo, T.; Gregoire, F.; Catherinot, L.; Molina, F.; Espeut, J. Simple, sensitive and robust chicken specific sexing assays, compliant with large scale analysis. PLoS ONE 2019, 14, e0213033. [Google Scholar] [CrossRef] [Green Version]
- Stern-Straeter, J.; Bonaterra, G.A.; Kassner, S.S.; Zügel, S.; Hörmann, K.; Kinscherf, R.; Goessler, U.R. Characterization of human myoblast differentiation for tissue-engineering purposes by quantitative gene expression analysis. J. Tissue Eng. Regen. Med. 2011, 5, e197–e206. [Google Scholar] [CrossRef]
- Burt, D.; White, S. Avian genomics in the 21st century. Cytogenet. Genome Res. 2007, 117, 6–13. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Na, K.W.; Byun, K.-H.; Kim, D.H.; Yoon, J.W.; Mizan, M.F.R.; Kang, I.; Ha, S.-D. Isolation and characterization of Salmonella spp. from food and food contact surfaces in a chicken processing factory. Poult. Sci. 2021, 100, 101234. [Google Scholar] [CrossRef]
- Brauer, R.; Chen, P. Influenza Virus Propagation in Embryonated Chicken Eggs. J. Vis. Exp. 2015, 97, 52421. [Google Scholar] [CrossRef] [Green Version]
- Yablonka–Reuveni, Z.; Paterson, B.M. MyoD and Myogenin Expression Patterns in Cultures of Fetal and Adult Chicken Myoblasts. J. Histochem. Cytochem. 2001, 49, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Halevy, O.; Piestun, Y.; Allouh, M.; Rosser, B.W.; Rinkevich, Y.; Reshef, R.; Rozenboim, I.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 2004, 231, 489–502. [Google Scholar] [CrossRef]
- Collins, C.A.; Zammit, P.S.; Ruiz, A.P.; Morgan, J.E.; Partridge, T.A. A Population of Myogenic Stem Cells That Survives Skeletal Muscle Aging. Stem Cells 2007, 25, 885–894. [Google Scholar] [CrossRef]
- Jankowski, M.; Mozdziak, P.; Petitte, J.; Kulus, M.; Kempisty, B. Avian Satellite Cell Plasticity. Animals 2020, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.A.; Seo, K.-M.; Ju, W.S.; Kim, Y.-I.; Wi, H.-Y.; Lee, P. Effect of Serum and Oxygen on the In Vitro Culture of Hanwoo Korean Native Cattle-Derived Skeletal Myogenic Cells Used in Cellular Agriculture. Foods 2023, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, F.E. Myogenic cell lineages. Dev. Biol. 1992, 154, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Yablonka-Reuveni, Z. Myogenesis in the chicken: The onset of differentiation of adult myoblasts is influenced by tissue factors. Basic Appl. Myol. 1995, 5, 33–41. [Google Scholar]
- Da Costa, M.J.; Zaragoza-Santacruz, S.; Frost, T.J.; Halley, J.; Pesti, G.M. Straight-run vs. sex separate rearing for 2 broiler genetic lines Part 1: Live production parameters, carcass yield, and feeding behavior. Poult. Sci. 2017, 96, 2641–2661. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, X.; Cheng, R.; Ansari, A.R.; Elokil, A.A.; Hu, Y.; Chen, Y.; Nafady, A.A.; Liu, H. Sex differences in growth performance are related to cecal microbiota in chicken. Microb. Pathog. 2021, 150, 104710. [Google Scholar] [CrossRef]
- Henry, M.H.; Burke, W.H. Sexual dimorphism in broiler chick embryos and embryonic muscle development in late incubation. Poult. Sci. 1998, 77, 728–736. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Zheng, W.; Bhasin, S. Androgen Receptor in Human Skeletal Muscle and Cultured Muscle Satellite Cells: Up-Regulation by Androgen Treatment. J. Clin. Endocrinol. Metab. 2004, 89, 5245–5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, R.; Toivonen, J.M.; Calvo, A.C.; Miana-Mena, F.J.; Zaragoza, P.; Muñoz, M.J.; Montarras, D.; Osta, R. Sex, fiber-type, and age dependent in vitro proliferation of mouse muscle satellite cells. J. Cell. Biochem. 2011, 112, 2825–2836. [Google Scholar] [CrossRef] [PubMed]



| Antibodies | Dilution Factor | Time | ||
|---|---|---|---|---|
| Name | Information | |||
| Myhc | Primary | Monoclonal anti-myosin (Skeletal, Fast) IgG1 (Sigma-Aldrich, St. Louis, MO, USA) | 1:400 | Overnight |
| Secondary | Goat anti-mouse IgM (Heavy chain) Cross-Absorbed Alexa Fluor™488 (Invitrogen, Waltham, MA, USA) | 1:400 | 1 h | |
| Pax7 | Primary | PAX7 (Rhabdomyosarcoma Marker) Mouse Monoclonal Antibody [Clone PAX7/497] (NeoBiotechnology, Union city, CA, USA) | 1:1000 | Overnight |
| Secondary | Goat anti-mouse IgG H&L (FITC) (Abcam Inc., Cambridge, UK) | 1:1000 | 1 h | |
| Myod | Primary |
MYOD Monoclonal Antibody (5.8A) IgG1κ (Invitrogen, Waltham, MA, USA) | 1:1000 | Overnight |
| Secondary |
Goat anti-mouse IgG (H+L) Alexa Fluor Plus 555 (Invitrogen, Waltham, MA, USA) | 1:1000 | 1 h | |
| Gene | Primer Sequence (5′→3′) | Accession Number | Product Size (bp) |
|---|---|---|---|
| PAX7 (Paired box 7) | F: ATT AGC CGT GTG CTA CGC AT R: AGC CTT CAT CCA GCC TGT TC | NM_205065.1 | 142 |
| MYF5 (Myogenic factor 5) | F: GAG GAA CGC CAT CAG GTA CAT C R: AGT TCT CCA CCT GTT CCC TCA A | NM_001030363.1 | 62 |
| MYOD1 (Myoblast determination protein 1) | F: GCC GCC GAT GAC TTC TAT GA R: CAG GTC CTC GAA GAA GTG CAT | NM_204214.2 | 66 |
| MYOG (Myogenin) | F: CGT GTG CCA CAG CCA ATG R: CCG CCG GAG AGA GAC CTT | NM_204184.1 | 63 |
| MYF6 (Myogenic factor 6) | F: GCG CCA TCA GCT ACA TCG A R: GCA TTT TGT CCT GCT GAT CCA | NM_001030746.1 | 66 |
| MYMK (Myomaker) | F: TGC GCT ATG ACA TCC TGG AGT A R: GGG ACA CCC AGA TGG ACA GA | NM_001318457.1 | 63 |
| MYH1E (Myosin heavy chain 1E) | F: TGG CAC AGT GGA CTA CAA CAT CT R: ACC ATA GGT GGC AAA CAG TAA GG | NM_001013397.2 | 127 |
| TBP (TATA-box binding protein) | F: TTG TGT CCA CGG TGA ATC TTG R: TCG GGC ACG AAG TGC AAT | NM_205103.1 | 62 |
| Embryo Description | No. of Total Nuclei | No. of Nuclei in Myotubes | MFI (%) | No. of Myotubes | Ave. of Nuclei in Myotubes | |
|---|---|---|---|---|---|---|
| Male | #1 | 648.13 | 59.00 | 9.38 | 7.63 | 9.41 |
| #8 | 867.63 | 178.50 | 20.29 | 17.00 | 11.36 | |
| #10 | 714.25 | 105.50 | 14.57 | 13.63 | 8.07 | |
| Mean | 743.33 | 114.33 | 14.75 | 12.75 | 9.61 | |
| SEM | 65.01 | 34.78 | 3.15 | 1.84 | 0.95 | |
| Female | #2 | 983.50 | 119.63 | 12.16 | 13.13 | 9.43 |
| #7 | 842.13 | 138.63 | 14.33 | 15.13 | 9.07 | |
| #9 | 815.50 | 134.63 | 14.98 | 13.25 | 11.60 | |
| Mean | 847.04 | 130.96 | 13.82 | 13.83 | 10.03 | |
| SEM | 19.78 | 5.78 | 0.85 | 0.65 | 0.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, W.-S.; Seo, K.; Lee, B.-R.; Park, M.-R.; Lee, M.-G.; Byun, S.-J.; Yang, H.; Kim, Y.; Ock, S.-A. Potential Application of Muscle Precursor Cells from Male Specific-Pathogen-Free (SPF) Chicken Embryos in In Vitro Agriculture. Animals 2023, 13, 1887. https://doi.org/10.3390/ani13111887
Ju W-S, Seo K, Lee B-R, Park M-R, Lee M-G, Byun S-J, Yang H, Kim Y, Ock S-A. Potential Application of Muscle Precursor Cells from Male Specific-Pathogen-Free (SPF) Chicken Embryos in In Vitro Agriculture. Animals. 2023; 13(11):1887. https://doi.org/10.3390/ani13111887
Chicago/Turabian StyleJu, Won-Seok, Kangmin Seo, Bo-Ram Lee, Mi-Ryung Park, Min-Gook Lee, Sung-June Byun, Hyeon Yang, Youngim Kim, and Sun-A Ock. 2023. "Potential Application of Muscle Precursor Cells from Male Specific-Pathogen-Free (SPF) Chicken Embryos in In Vitro Agriculture" Animals 13, no. 11: 1887. https://doi.org/10.3390/ani13111887
APA StyleJu, W.-S., Seo, K., Lee, B.-R., Park, M.-R., Lee, M.-G., Byun, S.-J., Yang, H., Kim, Y., & Ock, S.-A. (2023). Potential Application of Muscle Precursor Cells from Male Specific-Pathogen-Free (SPF) Chicken Embryos in In Vitro Agriculture. Animals, 13(11), 1887. https://doi.org/10.3390/ani13111887





